A Short-Type Peptidoglycan Recognition Protein 1 (PGRP1) Is Involved in the Immune Response in Asian Corn Borer, Ostrinia furnacalis (Guenée)
Abstract
:1. Introduction
2. Results
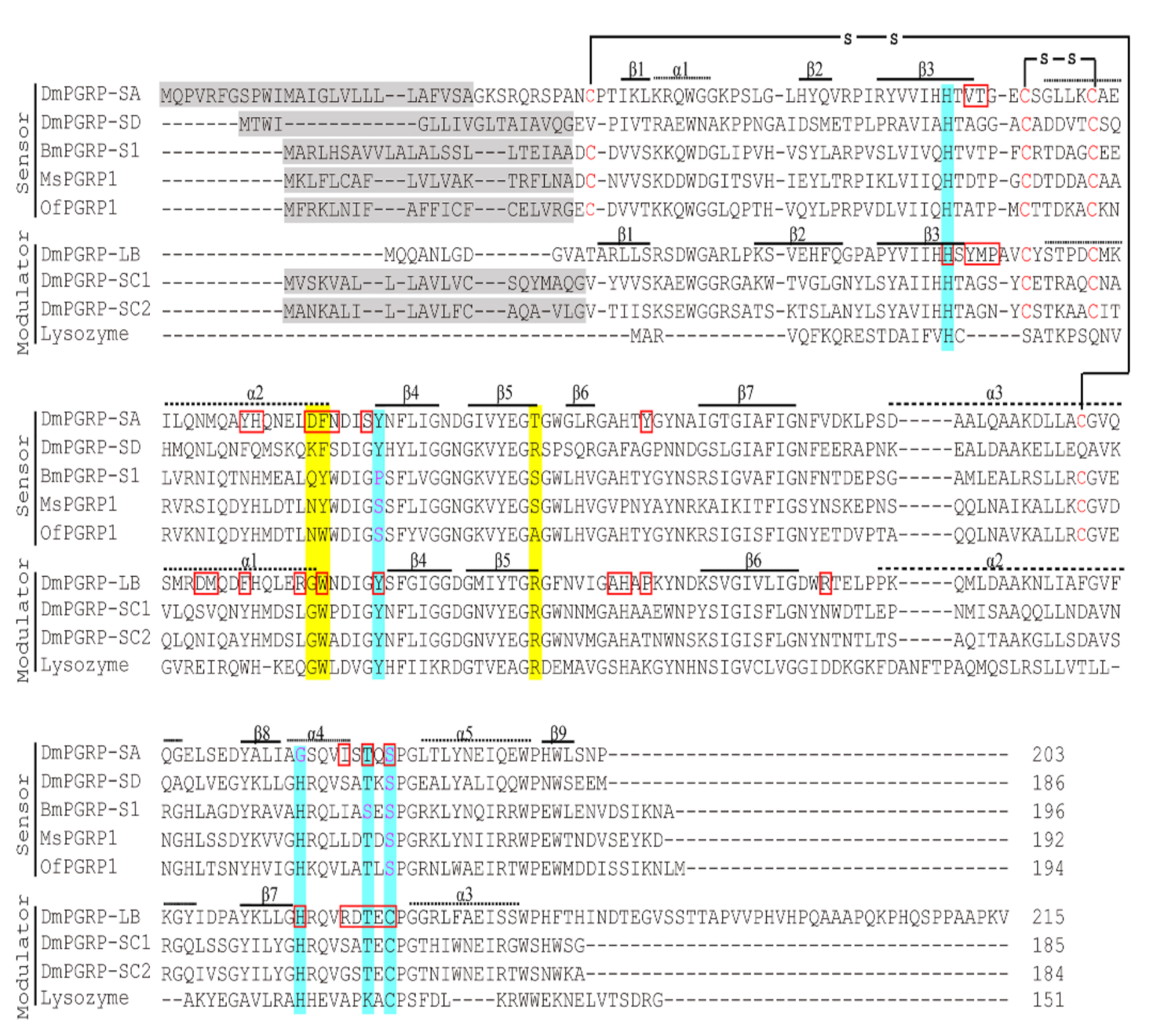
2.1. cDNA Cloning and Sequence Analysis of O. furnacalis PGRP1
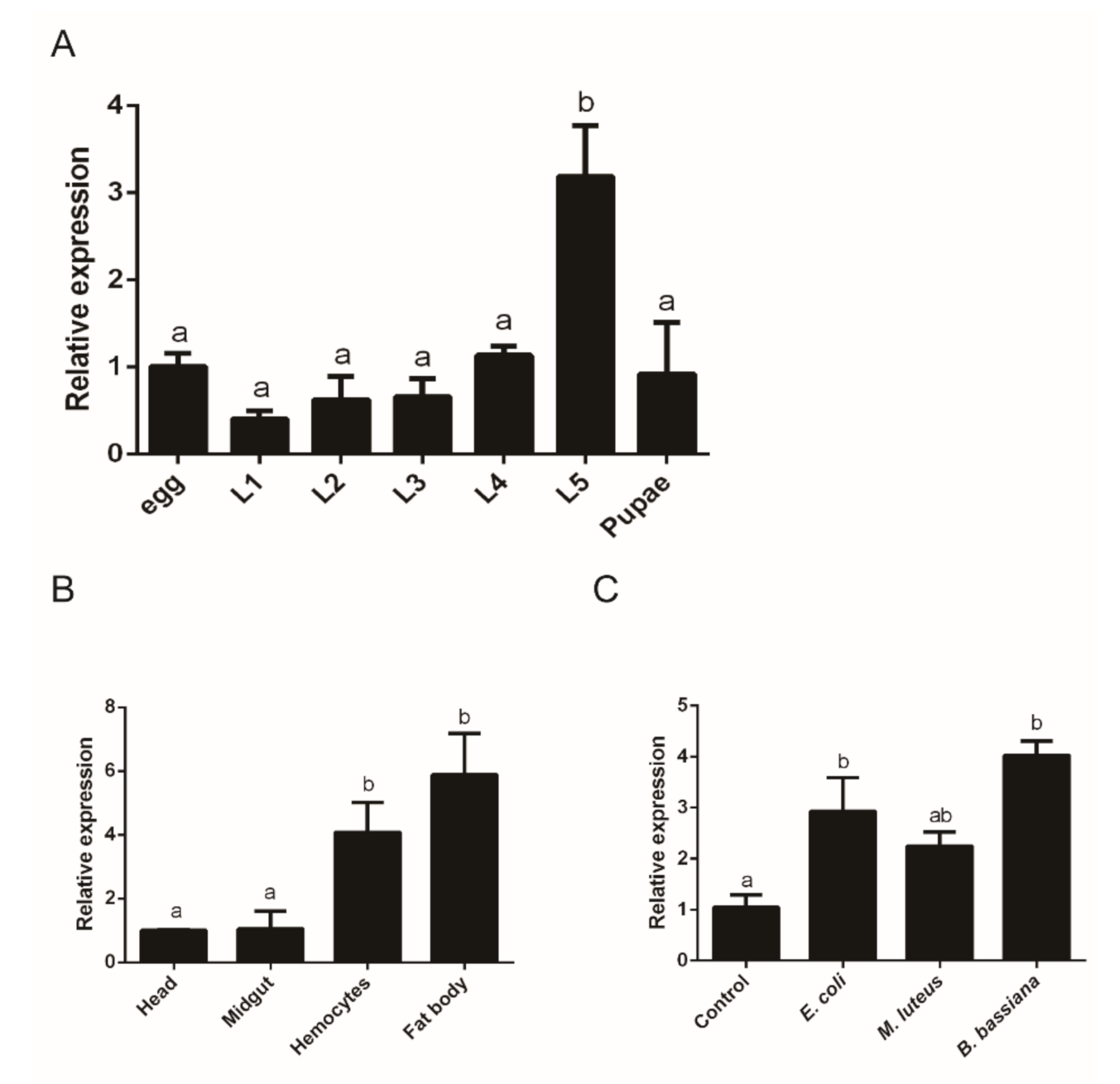
2.2. Expression Profiles of O. furnacalis PGRP1
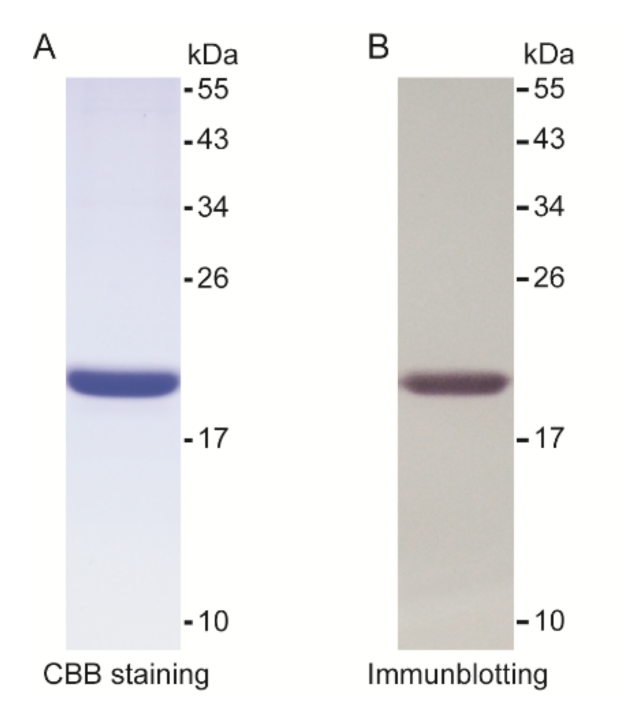
2.3. Production of Recombinant PGRP1 (rPGRP1)
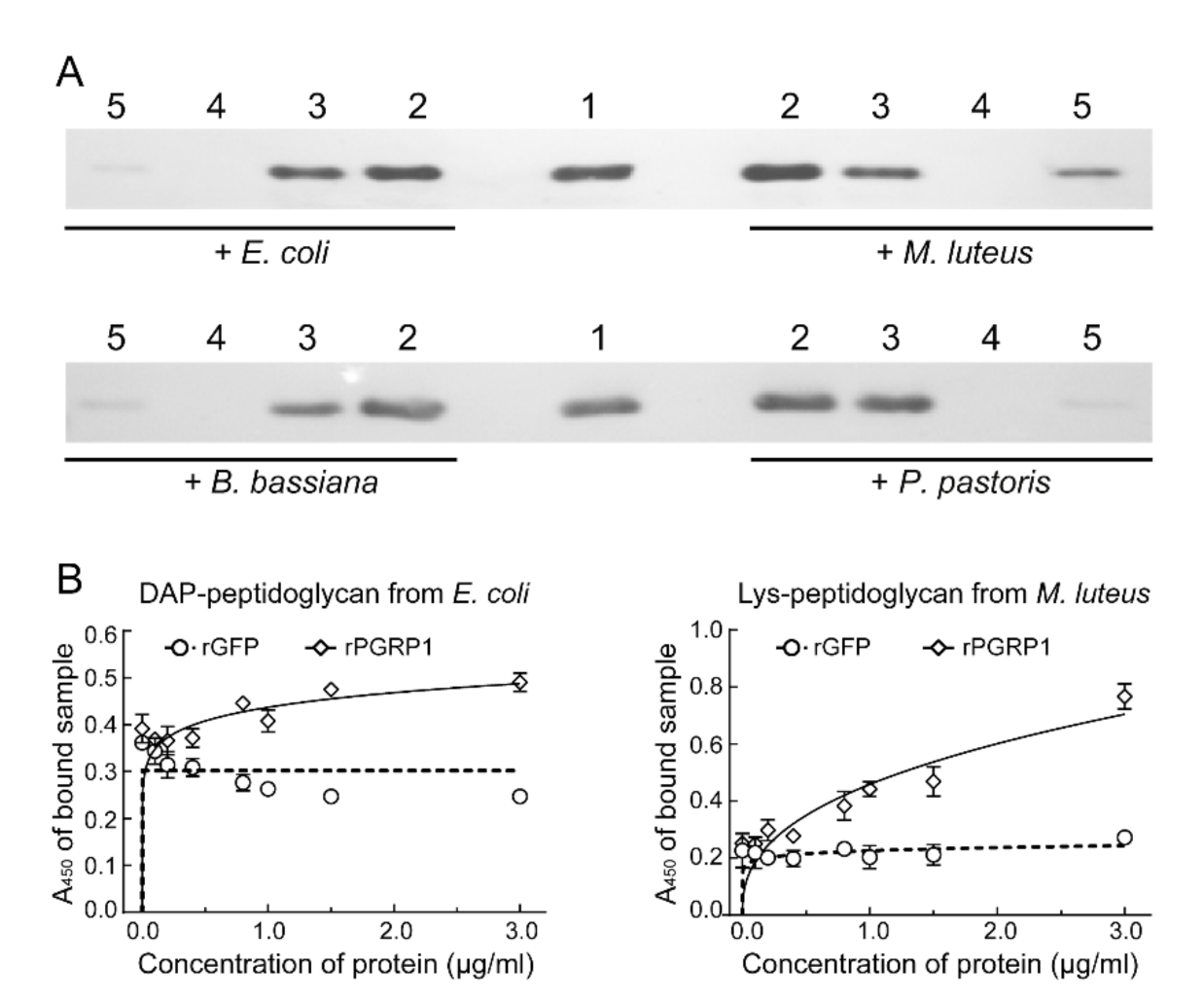
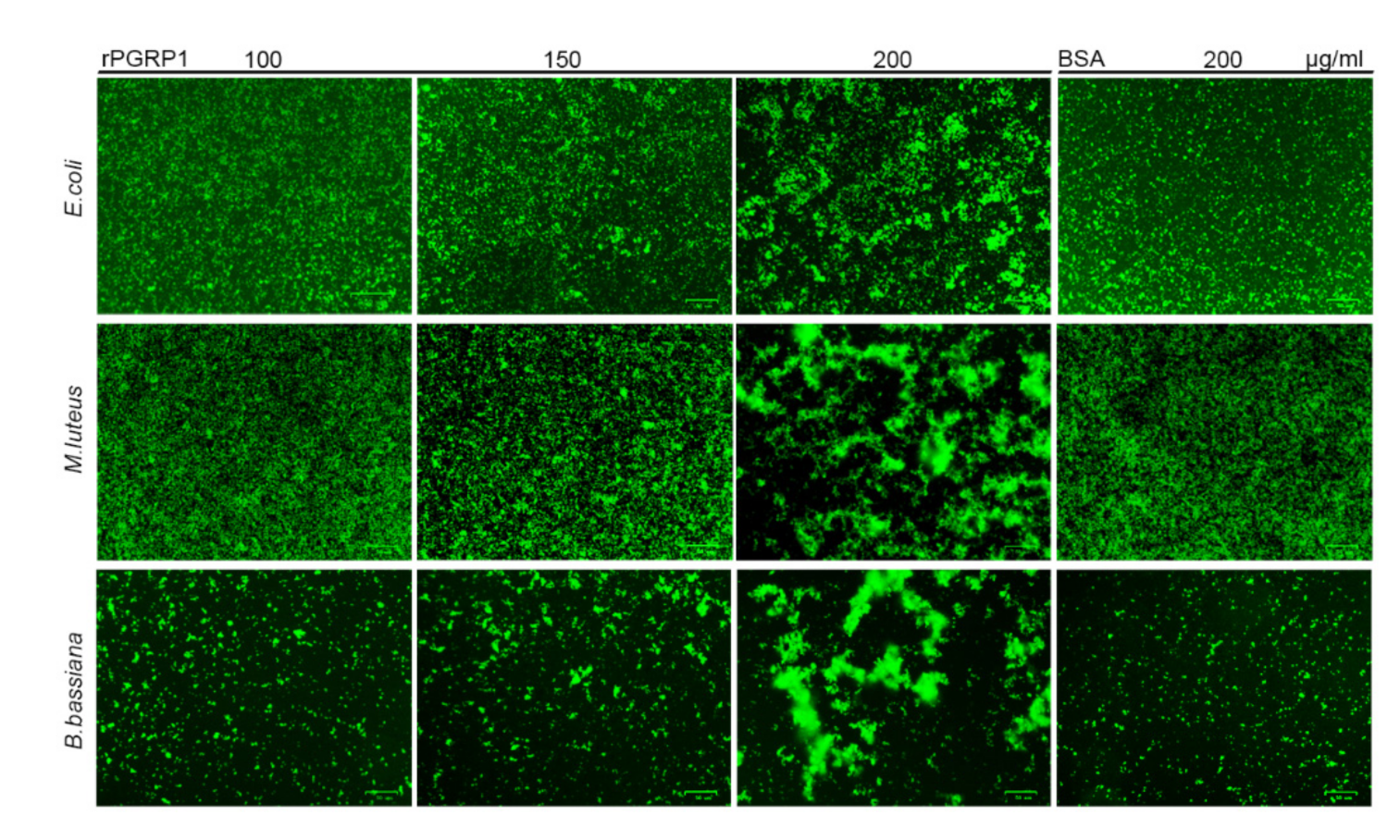
2.4. O. furnacalis PGRP1 Bound to Bacteria and Fungi and Caused Agglutination
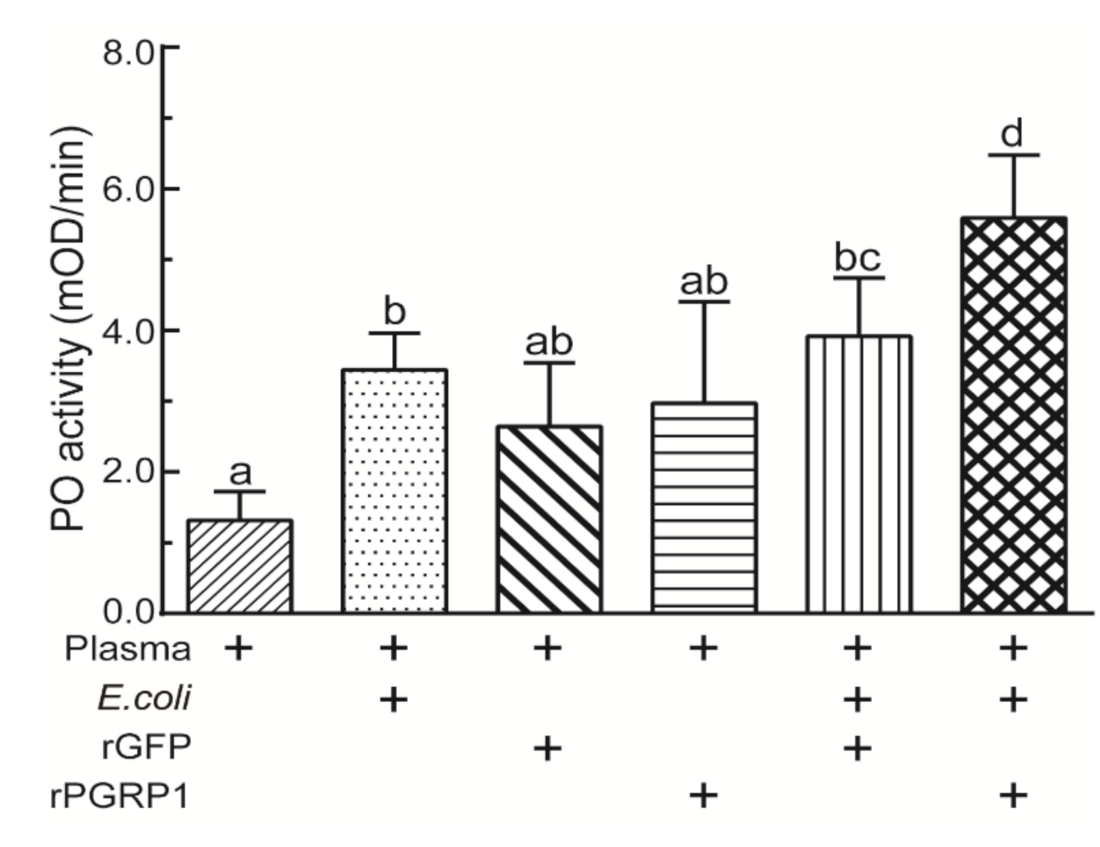
2.5. Recombinant PGRP1 Increased PO Activity of O. furnacalis Plasma
2.6. O. furnacalis PGRP1 was Related to the Expression of AMP Genes
3. Discussion
4. Materials and Methods
4.1. Biological Materials
4.2. Sequence Analysis and Comparison
4.3. Expression Profile Analysis of O. furnacalis PGRP1
4.4. Production and Purification of Recombinant PGRP1 (rPGRP1)
4.5. Binding of rPGRP1 to Different Microorganisms
4.6. Binding of rPGRP1 to Peptidoglycans
4.7. Microbial Agglutination Assay
4.8. Stimulation of PPO Activation by rPGRP1 in O. furnacalis Larval Plasma
4.9. Analysis of the Expression of Antimicrobial Peptide Genes after Injection of rPGRP1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kingsolver, M.B.; Hardy, R.W. Making connections in insect innate immunity. Proc. Natl. Acad. Sci. USA 2012, 109, 18639–18640. [Google Scholar] [CrossRef] [Green Version]
- Kanost, M.R.; Jiang, H.B. Clip-domain serine proteases as immune factors in insect hemolymph. Curr. Opin. Insect Sci. 2015, 11, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, D.; Garcia, B.L.; Kanost, M.R. Initiating protease with modular domains interacts with beta-glucan recognition protein to trigger innate immune response in insects. Proc. Natl. Acad. Sci. USA 2015, 112, 13856–13861. [Google Scholar] [CrossRef] [Green Version]
- Basbous, N.; Coste, F.; Leone, P.; Vincentelli, R.; Royet, J.; Kellenberger, C.; Roussel, A. The Drosophila peptidoglycan-recognition protein LF interacts with peptidoglycan-recognition protein LC to downregulate the Imd pathway. EMBO Rep. 2011, 12, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Steiner, H. Peptidoglycan recognition proteins: On and off switches for innate immunity. Immunol. Rev. 2004, 198, 83–96. [Google Scholar] [CrossRef]
- Watson, F.L.; Puttmann-Holgado, R.; Thomas, F.; Lamar, D.L.; Hughes, M.; Kondo, M.; Rebel, V.I.; Schmucker, D. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science 2005, 309, 1874–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christophides, G.K.; Vlachou, D.; Kafatos, F.C. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol. Rev. 2004, 198, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Ning, M.; Li, J.; Zhang, P.; Wang, L.; Xu, S.; Zhong, Y.; Wang, Z.; Song, Q.; Li, B. A C-type lectin with dual-CRD from Tribolium castaneum is induced in response to bacterial challenge. Pest. Manag. Sci. 2020, 76, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Royet, J.; Dziarski, R. Peptidoglycan recognition proteins: Pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 2007, 5, 264–277. [Google Scholar] [CrossRef]
- Chaput, C.; Boneca, I.G. Peptidoglycan detection by mammals and flies. Microbes Infect. 2007, 9, 637–647. [Google Scholar] [CrossRef]
- Mellroth, P.; Karlsson, J.; Steiner, H. A scavenger function for a Drosophila peptidoglycan recognition protein. J. Biol. Chem. 2003, 278, 7059–7064. [Google Scholar] [CrossRef] [Green Version]
- Guan, R.J.; Mariuzza, R.A. Peptidoglycan recognition proteins of the innate immune system. Trends Microbiol. 2007, 15, 127–134. [Google Scholar] [CrossRef]
- Yoshida, H.; Kinoshita, K.; Ashida, M. Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 1996, 271, 13854–13860. [Google Scholar] [CrossRef] [Green Version]
- Werner, T.; Liu, G.; Kang, D.; Ekengren, S.; Steiner, H.; Hultmark, D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 13772–13777. [Google Scholar] [CrossRef] [Green Version]
- Christophides, G.K.; Zdobnov, E.; Barillas-Mury, C.; Birney, E.; Blandin, S.; Blass, C.; Brey, P.T.; Collins, F.H.; Danielli, A.; Dimopoulos, G.; et al. Immunity-related genes and gene families in Anopheles gambiae. Science 2002, 298, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Ishibashi, J.; Fujita, K.; Nakajima, Y.; Sagisaka, A.; Tomimoto, K.; Suzuki, N.; Yoshiyama, M.; Kaneko, Y.; Iwasaki, T.; et al. A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem. Mol. Biol. 2008, 38, 1087–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Y.; Cao, X.; Gunaratna, R.T.; Chen, Y.R.; Blissard, G.; Kanost, M.R.; Jiang, H. Phylogenetic analysis and expression profiling of the pattern recognition receptors: Insights into molecular recognition of invading pathogens in Manduca sexta. Insect Biochem. Mol. Biol. 2015, 62, 38–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, H.; Kato, D.; Minakuchi, C.; Tanaka, T.; Yokoi, K.; Miura, K. Peptidoglycan recognition protein genes and their roles in the innate immune pathways of the red flour beetle, Tribolium castaneum. J. Invertebr. Pathol. 2015, 132, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, V.; Vignal, C.; Duvic, B.; Boneca, I.G.; Hoffmann, J.A.; Royet, J. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLos Pathog. 2006, 2, 139–147. [Google Scholar] [CrossRef]
- Zaidman-Remy, A.; Herve, M.; Poidevin, M.; Pili-Floury, S.; Kim, M.S.; Blanot, D.; Oh, B.H.; Ueda, R.; Mengin-Lecreulx, D.; Lemaitre, B. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity 2006, 24, 463–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royet, J.; Gupta, D.; Dziarski, R. Peptidoglycan recognition proteins: Modulators of the microbiome and inflammation. Nat. Rev. Immunol. 2011, 11, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Kurata, S. Peptidoglycan recognition proteins in Drosophila immunity. Dev. Comp. Immunol 2014, 42, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.M.; Wang, Y.H.; Yang, Q.T.; Wang, X.L.; Wang, M.; Raikhel, A.S.; Zou, Z. Regulation of antimicrobial peptides by juvenile hormone and its receptor, Methoprene-tolerant, in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2020, 128, 103509. [Google Scholar] [CrossRef]
- Bischoff, V.; Vignal, C.; Boneca, I.G.; Michel, T.; Hoffmann, J.A.; Royet, J. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat. Immunol. 2004, 5, 1175–1180. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, J.; De Mandal, S.; Li, S.; Zheng, Z.; Jin, F.; Xu, X. An immune-responsive PGRP-S1 regulates the expression of antibacterial peptide genes in diamondback moth, Plutella xylostella (L.). Int. J. Biol. Macromol. 2020, 142, 114–124. [Google Scholar] [CrossRef]
- Schmidt, R.L.; Trejo, T.R.; Plummer, T.B.; Platt, J.L.; Tang, A.H. Infection-induced proteolysis of PGRP-LC controls the IMD activation and melanization cascades in Drosophila. FASEB J. 2008, 22, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Sumathipala, N.; Jiang, H. Involvement of Manduca sexta peptidoglycan recognition protein-1 in the recognition of bacteria and activation of prophenoloxidase system. Insect Biochem. Mol. Biol. 2010, 40, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.W.; Je, B.R.; Piao, S.; Inamura, S.; Fujimoto, Y.; Fukase, K.; Kusumoto, S.; Soderhall, K.; Ha, N.C.; Lee, B.L. A synthetic peptidoglycan fragment as a competitive inhibitor of the melanization cascade. J. Biol. Chem. 2006, 281, 7747–7755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Ren, M.; Liu, X.; Xia, H.; Chen, K. Identification and characterization of novel short-type BmPGRP-S4 from the silkworm, Bombyx mori, involved in innate immunity. Zeitschrift für Naturforschung C 2020, 75, 13–21. [Google Scholar] [CrossRef]
- Mellroth, P.; Steiner, H. PGRP-SB1: An N-acetylmuramoyl L-alanine amidase with antibacterial activity. Biochem. Biophys. Res. Commun. 2006, 350, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, C.; He, Y.; Jiang, H.; Lu, Z. A short-type peptidoglycan recognition protein from the silkworm: Expression, characterization and involvement in the prophenoloxidase activation pathway. Dev. Comp. Immunol. 2014, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Afidchao, M.M.; Musters, C.J.; de Snoo, G.R. Asian corn borer (ACB) and non-ACB pests in GM corn (Zea mays. L.) in the Philippines. Pest. Manag. Sci. 2013, 69, 792–801. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, D.; Zhou, F.; Wang, G.; An, C. Identification of immunity-related genes in Ostrinia furnacalis against entomopathogenic fungi by RNA-seq analysis. PLoS ONE 2014, 9, e86436. [Google Scholar]
- Dziarski, R.; Gupta, D. The peptidoglycan recognition proteins (PGRPs). Genome Biol. 2006, 7, 232. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Byun, M.J.; Oh, B.H. Crystal structure of peptidoglycan recognition protein LB from Drosophila melanogaster. Nat. Immunol. 2003, 4, 787–793. [Google Scholar] [CrossRef]
- Yang, D.; Su, Z.; Qiao, C.; Zhang, Z.; Wang, J.; Li, F.; Liu, X. Identification and characterization of two peptidoglycan recognition proteins with zinc-dependent antibacterial activity from the cotton bollworm, Helicoverpa armigera. Dev. Comp. Immunol. 2013, 39, 343–351. [Google Scholar] [CrossRef]
- Yang, P.J.; Zhan, M.Y.; Ye, C.; Yu, X.Q.; Rao, X.J. Molecular cloning and characterization of a short peptidoglycan recognition protein from silkworm Bombyx mori. Insect Mol. Biol. 2017, 26, 665–676. [Google Scholar] [CrossRef]
- Yao, F.; Li, Z.; Zhang, Y.; Zhang, S. A novel short peptidoglycan recognition protein in amphioxus: Identification, expression and bioactivity. Dev. Comp. Immunol. 2012, 38, 332–341. [Google Scholar] [CrossRef]
- Swaminathan, C.P.; Brown, P.H.; Roychowdhury, A.; Wang, Q.; Guan, R.J.; Silverman, N.; Goldman, W.E.; Boons, G.J.; Mariuzza, R.A. Dual strategies for peptidoglycan discrimination by peptidoglycan recognition proteins (PGRPs). Proc. Natl. Acad. Sci. USA 2006, 103, 684–689. [Google Scholar] [CrossRef] [Green Version]
- Guan, R.; Roychowdhury, A.; Ember, B.; Kumar, S.; Boons, G.; Mariuzza, R.A. Structural basis for peptidoglycan binding by peptidoglycan recognition proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 17168–17173. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Cao, X.; Li, X.; Wang, Y.; Boons, G.; Deng, J.; Jiang, H. The three-dimensional structure and recognition mechanism of Manduca sexta peptidoglycan recognition protein-1. Insect Biochem. Mol. 2019, 108, 44–52. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, M.S.; Kim, H.E.; Yano, T.; Oshima, Y.; Aggarwal, K.; Goldman, W.E.; Silverman, N.; Kurata, S.; Oh, B.H. Structural basis for preferential recognition of diaminopimelic acid-type peptidoglycan by a subset of peptidoglycan recognition proteins. J. Biol. Chem. 2006, 281, 8286–8295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, P.; Bischoff, V.; Kellenberger, C.; Hetru, C.; Royet, J.; Roussel, A. Crystal structure of Drosophila PGRP-SD suggests binding to DAP-type but not lysine-type peptidoglycan. Mol. Immunol. 2008, 45, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Garver, L.S.; Wu, J.; Wu, L.P. The peptidoglycan recognition protein PGRP-SC1a is essential for Toll signaling and phagocytosis of Staphylococcus aureus in Drosophila. Proc. Natl. Acad. Sci. USA 2006, 103, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charroux, B.; Rival, T.; Narbonne-Reveau, K.; Royet, J. Bacterial detection by Drosophila peptidoglycan recognition proteins. Microbes Infect. 2009, 11, 631–636. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, X.; Cai, S.; Zhang, S.; Luo, H.; Wu, C.; Zhang, R.; Zhang, J. A novel peptidoglycan recognition protein involved in the prophenoloxidase activation system and antimicrobial peptide production in Antheraea pernyi. Dev. Comp. Immunol. 2018, 86, 78–85. [Google Scholar] [CrossRef]
- An, C.; Budd, A.; Kanost, M.R.; Michel, K. Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes. Cell. Mol. Life Sci. 2011, 68, 1929–1939. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.; Zhou, F.; Liu, Y.; Hong, F.; Wang, G.; An, C. Ostrinia furnacalis serpin-3 regulates melanization cascade by inhibiting a prophenoloxidase-activating protease. Insect Biochem. Mol. Biol. 2015, 61, 53–61. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, D.; Ji, J.; Zhang, S.; Liu, J.; An, C. A Short-Type Peptidoglycan Recognition Protein 1 (PGRP1) Is Involved in the Immune Response in Asian Corn Borer, Ostrinia furnacalis (Guenée). Int. J. Mol. Sci. 2021, 22, 8198. https://doi.org/10.3390/ijms22158198
Shen D, Ji J, Zhang S, Liu J, An C. A Short-Type Peptidoglycan Recognition Protein 1 (PGRP1) Is Involved in the Immune Response in Asian Corn Borer, Ostrinia furnacalis (Guenée). International Journal of Molecular Sciences. 2021; 22(15):8198. https://doi.org/10.3390/ijms22158198
Chicago/Turabian StyleShen, Dongxu, Jiayue Ji, Shasha Zhang, Jiahui Liu, and Chunju An. 2021. "A Short-Type Peptidoglycan Recognition Protein 1 (PGRP1) Is Involved in the Immune Response in Asian Corn Borer, Ostrinia furnacalis (Guenée)" International Journal of Molecular Sciences 22, no. 15: 8198. https://doi.org/10.3390/ijms22158198
APA StyleShen, D., Ji, J., Zhang, S., Liu, J., & An, C. (2021). A Short-Type Peptidoglycan Recognition Protein 1 (PGRP1) Is Involved in the Immune Response in Asian Corn Borer, Ostrinia furnacalis (Guenée). International Journal of Molecular Sciences, 22(15), 8198. https://doi.org/10.3390/ijms22158198





