Natural Compounds Attenuate Denervation-Induced Skeletal Muscle Atrophy
Abstract
:1. Introduction
2. Maxillofacial Problems Caused by Muscle Atrophy
3. Difference in Gene Expression between Facial and Other Muscles
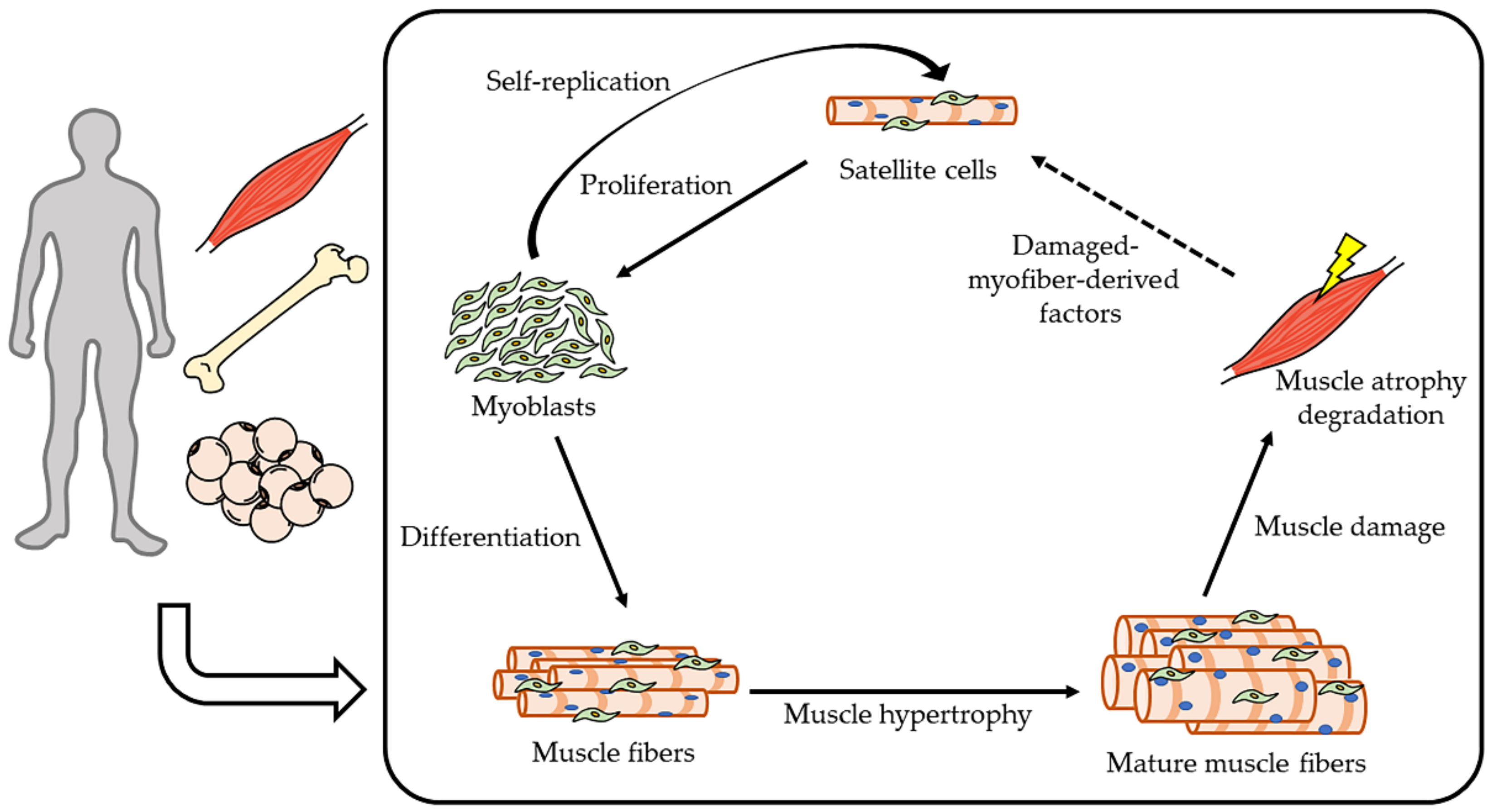
4. Molecular Mechanism of Muscle Homeostasis
4.1. Intracellular Signaling of Skeletal Muscle Anabolism
4.2. Intracellular Signaling of Skeletal Muscle Catabolism
5. Denervation Animal Model
6. Natural Compounds (Effective Foods against Denervation-Induced Skeletal Muscle Atrophy)
6.1. Royal Jelly (RJ)
6.2. Geranylgeraniol (GGOH)
6.3. Soybeans
6.4. Polyphenol
6.5. Vitamins
6.6. Capsaicin
| References | Species | Natural Compounds | Phenotype, Intervention/ Key Findings |
|---|---|---|---|
| Niu K et al. [97] | mice | Royal jelly (RJ) | C57BL/6J mice, aged mice/ Suppression of decrease muscle weight and grip strength Increase the regeneration of injured muscles and the serum insulin-like growth factor-1 (IGF-1) |
| Takahashi Y et al. [98] | mice | RJ | ICR mice, training/ RJ induces mitochondrial adaptation with endurance training by AMP-activated protein kinase (AMPK) activation |
| Shirakawa T et al. [99] | mice | RJ | C57BL/6J mice, denervation/ Suppression decrease muscle fiber size by oral administration |
| Okumura N et al. [100] | mice | RJ | Genetically heterogeneous mice, aged mice/ Motor function Increase fiber size Increase proliferation and differentiation |
| Takikawa M et al. [108] | mice | 10H2DA | C57BL/6J mice, oral adnimistration/ Stimulated phosphorylation of AMPK Glucose transporter type 4 (Glut4) translocation to the plasma membrane |
| Miyawaki A et al. [111] | mice | Geranyl-geraniol (GGOH) | C57BL/6J mice, denervation/ Suppression decrease muscle fiber size and expression of atrogin-1 |
| Hashimoto R et al. [139] | Humans | Soy protein | High and low physical activity, food intake/ Increase skeletal muscle mass in low activity human |
| Kitajima Y et al. [140] | mice | soymilk | C57BL/6 mice, ovariectomized mice/ Muscle fiber hypertrophy Increase grip strength |
| Abe T et al. [142] | mice | Soy glycinin | C57BL/6J mice, denervation/ Increase fiber diameter Suppression expression of muscle atrogene via IGF-1 signaling |
| Nikawa T et al. [143] | mice | Soy protein and whey protein | C57BL/6 mice, denervation/ Suppression muscle atrophy |
| Tachibana N et al. [144] | mice | Soy protein and red bell pepper juice | C57BL/6J mice, denervation/ Suppression of muscle atrophy and decrease atrogenes |
| Kakigi R et al. [145] | Humans | Whey protein | Male, Food intake, resistance exercise/ Mammalian target of rapamycin (mTOR) signaling activate |
| Tabata S et al. [146] | mice | isoflavones | ICR mice, denervation/ Suppression muscle atrophy Decrease in apoptosis-dependent signaling |
| Hirasaka K et al. [147] | mice | Soy isoflavones | C57BL/6J mice, denervation/ Resistance to muscle atrophy Suppression of acetylcholine receptor disorders in denervating atrophic muscles |
| Aubertin-Leheudre M et al. [149] | Humans | isoflavones | Sarcopenic-obese women, food intake/ Increase fat-free mass and muscle mass index |
| Mukai R et al. [152] | mice | 8-Prenylnarin-genin | C57/BL6 mice, denervation/suppress muscle atrophy Increased phosphorylation of Akt Suppression expression of Atrogin-1 |
| Mukai R et al. [158] | mice | quercetin | C57BL/6J mice, tail suspension/ Suppression decrease muscle weight and express ubiquitin ligase |
| Mukai R et al. [159] | mice | quercetin | C57BL/6 mice, denervation/ Suppression muscle atrophy Decrease Reactive oxygen species (ROS) Increased phosphorylation of Akt |
| Saito K et al. [163] | Humans | Vitamin C | Women, 70-84 years old/ Plasma vitamin C levels are positively correlated with grip strength, length of time standing on one leg with eyes open, and walking speed |
| Takisawa S et al. [164] | mice | Vitamin C | SPM30 knockout mice/ Muscle atrophy due to vitamin C deficiency, and recovery of muscle mass after vitamin C supplementation |
| Makanae Y et al. [165] | rats | Vitamin C | Wistar rats, overload/ suppression muscle hypertrophy on overload by administration of vitamin C |
| Ceglia L et al. [166] | Humans | Vitamin D | Mobility-limited, vitamin D-insufficient women/ Increase muscle fiber by supplemental vitamin D |
| Endo I et al. [167] | mice | Vitamin D | Vitamin D receptor (VDR) deletion mice/ Muscle fiber contraction by deletion of VDR |
| Visser M et al. [168] | Humans | Vitamin D | 55–85 years old/ In humans with low serum vitamin D, lower grip test and tend to low appendicular skeletal muscle mass |
| Servais S et al. [171] | rats | Vitamin E | Wistar rats, hindlimb-suspend/ Suppression of muscle atrophy and decrease atrogenes |
| Ikemoto M et al. [172] | rats | Vitamin E | Wistar rats, tail suspension/ Supplemental vitamin E does not show effect of suppression muscle atrophy |
| Ito N et al. [173] | mice | capsaicin | Denervation, hindlimb suspension/ Suppression of muscle atrophy by capsaicin injected intramuscularly |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MyHC | myosin heavy chain |
| Myf5 | myogenic factor 5 |
| MyoD | myoblast determination protein 1 |
| Pax7 | paired box protein 7 |
| Mesp1 | mesoderm posterior 1 |
| ISl1 | insulin gene enhancer protein |
| IGF-1 | insulin-like growth factor 1 |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mammalian target of rapamycin complex 1 |
| mTORC2 | mammalian target of rapamycin complex 2 |
| PI3K | phosphoinositide 3-kinase |
| AMP | adenosine monophosphate |
| AMPK | adenosine monophosphate -activated protein kinase |
| eIF4E | eukaryotic translation initiation factor 4E |
| 4EBP1 | eukaryotic translation initiation factor 4E-binding protein 1 |
| S6K1 | ribosomal protein S6 kinase-1 |
| IL-6 | interleukin-6 |
| TGF-β | transforming growth factor β |
| FGF | fibroblast growth factor |
| JAK2 | janus kinase 2 |
| STAT3 | signal transducer and activator of transcription 3 |
| MuRF-1 | muscle ring finger 1 |
| Foxo | forkhead box O |
| NF-ĸB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| TNF-α | Tumor necrosis factor α |
| JNK | c-jun N-terminal kinase |
| PGC1-α | peroxisome proliferator-activated receptor gamma coactivator 1 |
| Cbl-b | casitas B-lineage lymphoma-b |
| IRS-1 | insulin receptor substrate-1 |
| ActRIIB | activin type II B receptors |
| CDK2 | cyclin-dependent kinase 2 |
| RJ | royal jelly |
| 10H2DA | trans-10-hydroxy-2-decenoic acid |
| 10HDAA | 10-hydroxydecanoic acid |
| pRJ | protease-treated royal jelly |
| HDAC | histone deacetylase |
| GLUT4 | glucose transporter type 4 |
| GGOH | geranylgeraniol |
| GRAS | generally recognized as safe |
| GGPP | geranylgeranylpyrophosphate |
| LPS | lipopolysaccharide |
| ER | estrogen receptors |
| ROS | reactive oxygen species |
| TRPV1 | transient receptor potential vanilloid 1 |
| VDR | Vitamin D receptor |
References
- Rosenberg, I. Sarcopenia: Diagnosis and Mechanisms Sarcopenia: Origins and Clinical Relevance. Clin. Geriatr. Med. 2011, 27, 337–339. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine. 2019, 86, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; Raina, P. Sarcopenia: New definitions, same limitations. Age Ageing 2019, 48, 613–614. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, J.; Yang, M.; Hao, Q.; Luo, L.; Dong, B. Prevalence of Sarcopenia and Associated Factors in Chinese Community-Dwelling Elderly: Comparison Between Rural and Urban Areas. J. Am. Med. Dir. Assoc. 2015, 16, e1–e1003. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J. Gerontol. Ser. A. 2018, 73, 1661–1667. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Moss, M.L.; Salentijn, L. The primary role of functional matrices in facial growth. Am. J. Orthod. 1969, 55, 566–577. [Google Scholar] [CrossRef]
- Howland, J.P.; Brodie, A.G. Pressures exerted by the buccinator muscle. Angle Orthod. 1966, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S.; Rocancourt, D.; Cossu, G.; Buckingham, M. Redefining the Genetic Hierarchies Controlling Skeletal Myogenesis: Pax-3 and Myf-5 Act Upstream of MyoD. Cell 1997, 89, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.G.; Jerome-Majewska, L.A.; Papaioannou, V.E. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum. Mol. Genet. 2004, 13, 2829–2840. [Google Scholar] [CrossRef]
- Rios, A.C.; Marcelle, C. Head Muscles: Aliens Who Came in from the Cold? Dev. Cell. 2009, 16, 779–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avis, V. The relation of the temporal muscle to the form of the coronoid process. Am. J. Phys. Anthropol. 1959, 17, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.; Miller, A.J.; Okano, T.; Hatcher, D.; Yamaguchi, T.; Kobayashi, H.; Shibasaki, Y. Cortical bone mineral density in asymmetrical mandibles: A three-dimensional quantitative computed tomography study. Eur. J. Orthod. 2001, 23, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Becht, M.P.; Mah, J.; Martin, C.; Razmus, T.; Gunel, E.; Ngan, P. Evaluation of masseter muscle morphology in different types of malocclusions using cone beam computed tomography. Int. Orthod. 2014, 12, 32–48. [Google Scholar] [CrossRef]
- Avis, V. The significance of the angle of the mandible: An experimental and comparative study. Am. J. Phys. Anthropol. 1961, 19, 55–61. [Google Scholar] [CrossRef]
- Park, C.; Park, K.; Kim, J. Growth effects of botulinum toxin type A injected unilaterally into the masseter muscle of developing rats. J. Zhejiang Univ. B. 2015, 16, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Gedrange, T.; Büttner, C.; Schneider, M.; Lauer, G.; Mai, R.; Oppitz, R.; Harzer, W. Change of mRNA amount of myosin heavy chain in masseter muscle after orthognathic surgery of patients with malocclusion. J. Cranio-Maxillofac. Surg. 2006, 34, 110–115. [Google Scholar] [CrossRef]
- Breuel, W.; Krause, M.; Schneider, M.; Harzer, W. Genetic stretching factors in masseter muscle after orthognathic surgery. Br. J. Oral Maxillofac. Surg. 2013, 51, 530–535. [Google Scholar] [CrossRef]
- Oukhai, K.; Maricic, N.; Schneider, M.; Harzer, W.; Tausche, E. Developmental myosin heavy chain mRNA in masseter after orthognathic surgery: A preliminary study. J. Cranio Maxillofac. Surg. 2011, 39, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Proffit, W.R.; Phillips, C.; Dann, C.; Turvey, T.A. Stability after surgical-orthodontic correction of skeletal Class III malocclusion. I. Mandibular setback. Int. J. Adult Orthodon. Orthognath. Surg. 1991, 6, 7–18. [Google Scholar] [PubMed]
- Ayoub, A.F.; Trotman, C.A.; Stirrups, D.R.; Wilmot, J.J. Stability of bimaxillary osteotomy following surgical correction of class II skeletal deformities: A two-centre study. Br. J. Oral Maxillofac. Surg. 1997, 35, 107–115. [Google Scholar] [CrossRef]
- Hunt, N.P.; Cunningham, S.J. The influence of orthognathic surgery on occlusal force in patients with vertical facial deformities. Int. J. Oral Maxillofac. Surg. 1997, 26, 87–91. [Google Scholar] [CrossRef]
- Harzer, W.; Worm, M.; Gedrange, T.; Schneider, M.; Wolf, P. Myosin heavy chain mRNA isoforms in masseter muscle before and after orthognathic surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, 486–490. [Google Scholar] [CrossRef]
- Boyd, S.B.; Gonyea, W.J.; Legan, H.L.; Bell, W.H. Masseter muscle adaptation following surgical correction of vertical maxillary excess. J. Oral Maxillofac. Surg. 1989, 47, 953–962. [Google Scholar] [CrossRef]
- Maricic, N.; Stieler, E.; Gedrange, T.; Schneider, M.; Tausche, E.; Harzer, W. MGF- and myostatin-mRNA regulation in masseter muscle after orthognathic surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 487–492. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Mammalian Skeletal Muscle Fiber Type Transitions. Int. Rev. Cytol. 1997, 170, 143–223. [Google Scholar] [CrossRef]
- Roy, R.R.; Talmadge, R.J.; Hodgson, J.A.; Oishi, Y.; Baldwin, K.M.; Edgerton, V.R. Differential response of fast hindlimb extensor and flexor muscles to exercise in adult spinalized cats. Muscle Nerve 1999, 22, 230–241. [Google Scholar] [CrossRef]
- Harel, I.; Nathan, E.; Tirosh-Finkel, L.; Zigdon, H.; Guimarães-Camboa, N.; Evans, S.M.; Tzahor, E. Distinct Origins and Genetic Programs of Head Muscle Satellite Cells. Dev. Cell. 2009, 16, 822–832. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, K.; Kitajima, Y.; Seko, D.; Tsuchiya, Y.; Ono, Y. The body region specificity in murine models of muscle regeneration and atrophy. Acta Physiol. 2021, 231, 1–9. [Google Scholar] [CrossRef]
- Gojo, K.; Abe, S.; Ide, Y. Characteristics of Myofibres in the Masseter Muscle of Mice during Postnatal Growth Period. Anat. Histol. Embryol. 2002, 31, 105–112. [Google Scholar] [CrossRef]
- Mascarello, F.; Toniolo, L.; Cancellara, P.; Reggiani, C.; Maccatrozzo, L. Expression and identification of 10 sarcomeric MyHC isoforms in human skeletal muscles of different embryological origin. Diversity and similarity in mammalian species. Ann. Anat. 2016, 207, 9–20. [Google Scholar] [CrossRef]
- Schiaffino, S. Muscle fiber type diversity revealed by anti-myosin heavy chain antibodies. FEBS J. 2018, 285, 3688–3694. [Google Scholar] [CrossRef]
- Stedman, H.H.; Kozyak, B.W.; Nelson, A.; Thesier, D.M.; Su, L.T.; Low, D.W.; Bridges, C.R.; Shrager, J.B.; Minugh-Purvis, N.; Mitchell, M.A. Myosin gene mutation correlates with anatomical changes in the human lineage. Nature 2004, 428, 415–418. [Google Scholar] [CrossRef]
- Ono, Y.; Boldrin, L.; Knopp, P.; Morgan, J.E.; Zammit, P.S. Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev. Biol. 2010, 337, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Guertin, D.A.; Stevens, D.M.; Thoreen, C.C.; Burds, A.A.; Kalaany, N.Y.; Moffat, J.; Brown, M.; Fitzgerald, K.J.; Sabatini, D.M. Ablation in Mice of the mTORC Components raptor, rictor, or mLST8 Reveals that mTORC2 Is Required for Signaling to Akt-FOXO and PKCα, but Not S6K1. Dev. Cell. 2006, 11, 859–871. [Google Scholar] [CrossRef] [Green Version]
- Shimobayashi, M.; Hall, M.N. Multiple amino acid sensing inputs to mTORC1. Cell Res. 2016, 26, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, S.; Peterson, T.R.; Sabatini, D.M. Regulation of the mTOR Complex 1 Pathway by Nutrients, Growth Factors, and Stress. Mol. Cell. 2010, 40, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Bazgir, B.; Fathi, R.; Valojerdi, M.R.; Mozdziak, P.; Asgari, A. Satellite cells contribution to exercise mediated muscle hypertrophy and repair. Cell J. 2016, 18, 473–484. [Google Scholar] [CrossRef]
- Hawke, T.J.; Garry, D.J. Myogenic satellite cells: Physiology to molecular biology. J. Appl. Physiol. 2001, 91, 534–551. [Google Scholar] [CrossRef]
- Kurosaka, M.; Machida, S. Interleukin-6-induced satellite cell proliferation is regulated by induction of the JAK2/STAT3 signalling pathway through cyclin D1 targeting. Cell Prolif. 2013, 46, 365–373. [Google Scholar] [CrossRef]
- Weissman, A.M.; Shabek, N.; Ciechanover, A. The predator becomes the prey: Regulating the ubiquitin system by ubiquitylation and degradation. Nat. Rev. Mol. Cell Biol. 2011, 12, 605–620. [Google Scholar] [CrossRef]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kai, Y.; Mizukami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H.; et al. Skeletal Muscle FOXO1 (FKHR) Transgenic Mice Have Less Skeletal Muscle Mass, Down-regulated Type I (Slow Twitch/Red Muscle) Fiber Genes, and Impaired Glycemic Control. J. Biol. Chem. 2004, 279, 41114–41123. [Google Scholar] [CrossRef] [Green Version]
- Bodine, S.C. Identification of Ubiquitin Ligases Required for Skeletal Muscle Atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Frantz, J.D.; Tawa, N.E.; Melendez, P.A.; Oh, B.; Lidov, H.G.W.; Hasselgren, P.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. IKKβ/NF-κB Activation Causes Severe Muscle Wasting in Mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Dogra, C.; Changoua, H.; Wedhas, N.; Qin, X.; Wergedal, J.E.; Kumar, A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J. 2007, 21, 1857–1869. [Google Scholar] [CrossRef] [Green Version]
- Draznin, B. Molecular mechanisms of insulin resistance: Serine phosphorylation of insulin receptor substrate-1 and increased expression of p85 α: The two sides of a coin. Diabetes 2006, 55, 2392–2397. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef] [Green Version]
- Suzue, N.; Nikawa, T.; Onishi, Y.; Yamada, C.; Hirasaka, K.; Ogawa, T.; Furochi, H.; Kosaka, H.; Ishidoh, K.; Gu, H.; et al. Ubiquitin Ligase Cbl-b Downregulates Bone Formation Through Suppression of IGF-I Signaling in Osteoblasts During Denervation. J. Bone Miner. Res. 2006, 21, 722–734. [Google Scholar] [CrossRef] [Green Version]
- Nakao, R.; Hirasaka, K.; Goto, J.; Ishidoh, K.; Yamada, C.; Ohno, A.; Okumura, Y.; Nonaka, I.; Yasutomo, K.; Baldwin, K.M.; et al. Ubiquitin Ligase Cbl-b Is a Negative Regulator for Insulin-Like Growth Factor 1 Signaling during Muscle Atrophy Caused by Unloading. Mol. Cell. Biol. 2009, 29, 4798–4811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Lee, S.-J. Regulation of Muscle Mass by Myostatin. Annu. Rev. Cell Dev. Biol. 2004, 20, 61–86. [Google Scholar] [CrossRef]
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.C.; Hadj Sassi, A.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. 2014, 71, 4361–4371. [Google Scholar] [CrossRef]
- MacDonald, E.M.; Andres-Mateos, E.; Mejias, R.; Simmers, J.L.; Mi, R.; Park, J.-S.; Ying, S.; Hoke, A.; Lee, S.-J.; Cohn, R.D. Denervation atrophy is independent from Akt and mTOR activation and is not rescued by myostatin inhibition. Dis. Model. Mech. 2014, 7, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Grossman, E.J.; Roy, R.R.; Talmadge, R.J.; Zhong, H.; Edgerton, V.R. Effects of inactivity on myosin heavy chain composition and size of rat soleus fibers. Muscle Nerve 1998, 21, 375–389. [Google Scholar] [CrossRef]
- Borisov, A.B.; Dedkov, E.I.; Carlson, B.M. Interrelations of myogenic response, progressive atrophy of muscle fibers, and cell death in denervated skeletal muscle. Anat. Rec. 2001, 264, 203–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, K.W.; Jung, Y.K.; Kim, J.S.; Park, J.S.; Hah, Y.S.; Kim, S.J.; Yoo, J.-I. Rodent model of muscular atrophy for sarcopenia study. J. Bone Metab. 2020, 27, 97–110. [Google Scholar] [CrossRef]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 Coordinately Activates Protein Degradation by the Autophagic/Lysosomal and Proteasomal Pathways in Atrophying Muscle Cells. Cell Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Aweida, D.; Rudesky, I.; Volodin, A.; Shimko, E.; Cohen, S. GSK3-β promotes calpain-1–mediated desmin filament depolymerization and myofibril loss in atrophy. J. Cell Biol. 2018, 217, 3698–3714. [Google Scholar] [CrossRef] [Green Version]
- Wall, P.D.; Devor, M.; Inbal, R.; Scadding, J.W.; Schonfeld, D.; Seltzer, Z.; Tomkiewicz, M.M. Autotomy following peripheral nerve lesions: Experimental anesthesia dolorosa. Pain 1979, 7, 103–113. [Google Scholar] [CrossRef]
- Weinreb, M.; Rodan, G.A.; Thompson, D.D. Immobilization-related bone loss in the rat is increased by calcium deficiency. Calcif. Tissue Int. 1991, 48, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment—Facts and numbers. J. Cachexia. Sarcopenia Muscle. 2020, 11, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.P.; Edgerton, V.R.; Grindeland, R.E. Influence of spaceflight on rat skeletal muscle. J. Appl. Physiol. 1988, 65, 2318–2325. [Google Scholar] [CrossRef]
- Bialek, P.; Morris, C.; Parkington, J.; St. Andre, M.; Owens, J.; Yaworsky, P.; Seeherman, H.; Jelinsky, S.A. Distinct protein degradation profiles are induced by different disuse models of skeletal muscle atrophy. Physiol. Genom. 2011, 43, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Inoki, K.; Lee, M.; Wright, E.; Khuong, A.; Khuong, A.; Sugiarto, S.; Garner, M.; Paik, J.; DePinho, R.A.; et al. mTORC1 Promotes Denervation-Induced Muscle Atrophy Through a Mechanism Involving the Activation of FoxO and E3 Ubiquitin Ligases. Sci. Signal. 2014, 7, ra18. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, D.N.; Mesnard-Hoaglin, N.A.; Batka, R.J.; Haulcomb, M.M.; Miller, W.M.; Jones, K.J. Facial Nerve Axotomy in Mice: A Model to Study Motoneuron Response to Injury. J. Vis. Exp. 2015, 23, e52382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, B.E.; Irving, D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J. Neurol. Sci. 1977, 34, 213–219. [Google Scholar] [CrossRef]
- Oliveira, A.; Vaz, C. The role of sarcopenia in the risk of osteoporotic hip fracture. Clin. Rheumatol. 2015, 34, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.L.; Hale, L.; Grant, A.M.; Herbison, P.; Goulding, A. Osteoporosis and gait and balance disturbances in older sarcopenic obese New Zealanders. Osteoporos. Int. 2010, 21, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Leung, J.; Woo, J. Incremental Predictive Value of Sarcopenia for Incident Fracture in an Elderly Chinese Cohort: Results From the Osteoporotic Fractures in Men (MrOs) Study. J. Am. Med. Dir. Assoc. 2014, 15, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Matsumoto, T.; Aoyagi, Y.; Tanaka, S.; Okadome, J.; Morisaki, K.; Shirabe, K.; Maehara, Y. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J. Vasc. Surg. 2015, 61, 945–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia Exacerbates Obesity-Associated Insulin Resistance and Dysglycemia: Findings from the National Health and Nutrition Examination Survey III. Earnest CP, ed. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef] [PubMed]
- Tolea, M.; Galvin, J. Sarcopenia and impairment in cognitive and physical performance. Clin. Interv. Aging. 2015, 10, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Stene, G.B.; Helbostad, J.L.; Amundsen, T.; Sørhaug, S.; Hjelde, H.; Kaasa, S.; Grønberg, B.H. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol. 2015, 54, 340–348. [Google Scholar] [CrossRef]
- Harada, K.; Ida, S.; Baba, Y.; Ishimoto, T.; Kosumi, K.; Tokunaga, R.; Izumi, D.; Ohuchi, M.; Nakamura, K.; Kiyozumi, Y.; et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis. Esophagus 2016, 29, 627–633. [Google Scholar] [CrossRef]
- Thoresen, L.; Frykholm, G.; Lydersen, S.; Ulveland, H.; Baracos, V.; Birdsell, L.; Falkmer, U. The association of nutritional assessment criteria with health-related quality of life in patients with advanced colorectal carcinoma. Eur. J. Cancer Care 2012, 21, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Kitajima, Y.; Masumoto, H.; Ono, Y. Damaged Myofiber-Derived Metabolic Enzymes Act as Activators of Muscle Satellite Cells. Stem Cell Rep. 2020, 15, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [Green Version]
- Khazaei, M.; Ansarian, A.; Ghanbari, E. New Findings on Biological Actions and Clinical Applications of Royal Jelly: A Review. J. Diet Suppl. 2018, 15, 757–775. [Google Scholar] [CrossRef]
- Inoue, S.; Koya-Miyata, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly prolongs the life span of C3H/HeJ mice: Correlation with reduced DNA damage. Exp. Gerontol. 2003, 38, 965–969. [Google Scholar] [CrossRef]
- Honda, Y.; Fujita, Y.; Maruyama, H.; Araki, Y.; Ichihara, K.; Sato, A.; Kojima, T.; Tanaka, M.; Nozawa, Y.; Ito, M.; et al. Lifespan-Extending Effects of Royal Jelly and Its Related Substances on the Nematode Caenorhabditis elegans. PLoS ONE 2011, 6, e23527. [Google Scholar] [CrossRef] [Green Version]
- Kamakura, M.; Mitani, N.; Fukuda, T.; Fukushima, M. Antifatigue Effect of Fresh Royal Jelly in Mice. J. Nutr. Sci. Vitaminol. 2001, 47, 394–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef]
- Liu, J.-R.; Yang, Y.-C.; Shi, L.-S.; Peng, C.-C. Antioxidant Properties of Royal Jelly Associated with Larval Age and Time of Harvest. J. Agric. Food Chem. 2008, 56, 11447–11452. [Google Scholar] [CrossRef]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly Inhibits the Production of Proinflammatory Cytokines by Activated Macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Vittek, J. Effect of Royal Jelly on serum lipids in experimental animals and humans with atherosclerosis. Experientia 1995, 51, 927–935. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Grzech, I. Gas chromatographic–mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. J. Chromatogr. B. 2012, 885–886, 109–116. [Google Scholar] [CrossRef]
- Townsend, G.F.; Brown, W.H.; Felauer, E.E.; Hazlett, B. Studies on the in vitro antitumor activity of fatty acids. IV. The esters of acids closely related to 10-hydroxy-2-decenoic acids from royal jelly against transplantable mouse leukemia. Can. J. Biochem. Physiol. 1961, 39, 1765–1770. [Google Scholar] [CrossRef]
- Maeda, T.; Kuroda, H.; Motoyoshi, K. Effects of royal jelly and 10-hydroxy decenoic acid on the sebaceous glands of hamster ear. Jpn. J. Dermatol. 1988, 98, 469–475. [Google Scholar]
- Koya-Miyata, S.; Okamoto, I.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Identification of a Collagen Production-promoting Factor from an Extract of Royal Jelly and Its Possible Mechanism. Biosci. Biotechnol. Biochem. 2004, 68, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.S.; Novak, A.F.; Taber, S. 10-Hydroxy-Dgr2-Decenoic Acid, an Antibiotic Found in Royal Jelly. Science 1959, 130, 452–453. [Google Scholar] [CrossRef]
- Ito, S.; Nitta, Y.; Fukumitsu, H.; Soumiya, H.; Ikeno, K.; Nakamura, T.; Furukawa, S. Antidepressant-Like Activity of 10-Hydroxy-Trans-2-Decenoic Acid, a Unique Unsaturated Fatty Acid of Royal Jelly, in Stress-Inducible Depression-Like Mouse Model. Evid. Based Complement. Altern. Med. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, K.; Guo, H.; Guo, Y.; Ebihara, S.; Asada, M.; Ohrui, T.; Furukawa, K.; Ichinose, M.; Yanai, K.; Kudo, Y.; et al. Royal Jelly Prevents the Progression of Sarcopenia in Aged Mice In Vivo and In Vitro. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1482–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Hijikata, K.; Seike, K.; Nakano, S.; Banjo, M.; Sato, Y.; Takahashi, K.; Hatta, H. Effects of Royal Jelly Administration on Endurance Training-Induced Mitochondrial Adaptations in Skeletal Muscle. Nutrients 2018, 10, 1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirakawa, T.; Miyawaki, A.; Matsubara, T.; Okumura, N.; Okamoto, H.; Nakai, N.; Rojasawasthien, T.; Morikawa, K.; Inoue, A.; Goto, A.; et al. Daily Oral Administration of Protease-Treated Royal Jelly Protects Against Denervation-Induced Skeletal Muscle Atrophy. Nutrients 2020, 12, 3089. [Google Scholar] [CrossRef]
- Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T. Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice. Nutrients 2018, 10, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleszka, R. Epigenetic integration of environmental and genomic signals in honey bees: The critical interplay of nutritional, brain and reproductive networks. Epigenetics 2008, 3, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Spannhoff, A.; Kim, Y.K.; Raynal, N.J.-M.; Gharibyan, V.; Su, M.; Zhou, Y.; Li, J.; Castellano, S.; Sbardella, G.; Issa, J.J.; et al. Histone deacetylase inhibitor activity in royal jelly might facilitate caste switching in bees. EMBO Rep. 2011, 12, 238–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montesano, A.; Luzi, L.; Senesi, P.; Terruzzi, I. Modulation of Cell Cycle Progression by 5-Azacytidine Is Associated with Early Myogenesis Induction in Murine Myoblasts. Int. J. Biol. Sci. 2013, 9, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Hupkes, M.; Jonsson, M.K.B.; Scheenen, W.J.; Rotterdam, W.; Sotoca, A.M.; Someren, E.P.; Heyden, M.A.G.; Veen, T.A.; Ravestein-van Os, R.I.; Bauerschmidt, S.; et al. Epigenetics: DNA demethylation promotes skeletal myotube maturation. FASEB J. 2011, 25, 3861–3872. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.L.; Zhang, W.; Iwaniuk, M.; Grilli, E.; Stahl, C.H. Dietary tributyrin, an HDAC inhibitor, promotes muscle growth through enhanced terminal differentiation of satellite cells. Physiol. Rep. 2018, 6, e13706. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, R.; Tesfaye, D.; Tholen, E.; Looft, C.; Hölker, M.; Schellander, K.; Cinar, M.U. Sulforaphane causes a major epigenetic repression of myostatin in porcine satellite cells. Epigenetics 2012, 7, 1379–1390. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.S.; Gao, J.; Han, D.; Holloszy, J.O.; Nolte, L.A. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am. J. Physiol. Metab. 2002, 282, E18–E23. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Kumagai, A.; Hirata, H.; Soga, M.; Yamashita, Y.; Ueda, M.; Ashida, H.; Tsuda, T. 10-Hydroxy-2-decenoic acid, a unique medium-chain fatty acid, activates 5’-AMP-activated protein kinase in L6 myotubes and mice. Mol. Nutr. Food Res. 2013, 57, 1794–1802. [Google Scholar] [CrossRef]
- Muraguchi, T.; Okamoto, K.; Mitake, M.; Ogawa, H.; Shidoji, Y. Polished rice as natural sources of cancer-preventing geranylgeranoic acid. J. Clin. Biochem. Nutr. 2011, 49, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, T.; Urata, M.; Nakajima, T.; Fukuzaki, M.; Masuda, R.; Yoshimoto, Y.; Addison, W.N.; Nakatomi, C.; Morikawa, K.; Zhang, M.; et al. Geranylgeraniol-induced Myogenic Differentiation of C2C12 Cells. In Vivo 2018, 32, 1427–1431. [Google Scholar] [CrossRef] [Green Version]
- Miyawaki, A.; Rojasawasthien, T.; Hitomi, S.; Aoki, Y.; Urata, M.; Inoue, A.; Matsubara, T.; Morikawa, K.; Habu, M.; Tominaga, K.; et al. Oral Administration of Geranylgeraniol Rescues Denervation-induced Muscle Atrophy via Suppression of Atrogin-1. In Vivo 2020, 34, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judge, A.R.; Koncarevic, A.; Hunter, R.B.; Liou, H.; Jackman, R.W.; Kandarian, S.C. Role for IκBα, but not c-Rel, in skeletal muscle atrophy. Am. J. Physiol. Physiol. 2007, 292, C372–C382. [Google Scholar] [CrossRef] [Green Version]
- Gammeren, D.; Damrauer, J.S.; Jackman, R.W.; Kandarian, S.C. The IκB kinases IKKα and IKKβ are necessary and sufficient for skeletal muscle atrophy. FASEB J. 2009, 23, 362–370. [Google Scholar] [CrossRef]
- Mourkioti, F.; Kratsios, P.; Luedde, T.; Song, Y.; Delafontaine, P.; Adami, R.; Parente, V.; Bottinelli, R.; Pasparakis, M.; Rosenthal, N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J. Clin. Investig. 2006, 116, 2945–2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Shai, M.; Carmeli, E.; Ljubuncic, P.; Reznick, A.Z. Exercise and immobilization in aging animals: The involvement of oxidative stress and NF-κB activation. Free Radic. Biol. Med. 2008, 44, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, M.G.; Kandarian, S.C.; Pacelli, F.; Doglietto, G.B.; Bossola, M. Expression of NF-κB and IκB proteins in skeletal muscle of gastric cancer patients. Eur. J. Cancer. 2010, 46, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Kandarian, S.C.; Jackman, R.W. Identification of Genes that Elicit Disuse Muscle Atrophy via the Transcription Factors p50 and Bcl-3. PLoS ONE 2011, 6, e16171. [Google Scholar] [CrossRef] [Green Version]
- Giriwono, P.E.; Shirakawa, H.; Ohsaki, Y.; Hata, S.; Kuriyama, H.; Sato, S.; Goto, T.; Komai, M. Dietary supplementation with geranylgeraniol suppresses lipopolysaccharide-induced inflammation via inhibition of nuclear factor-κB activation in rats. Eur. J. Nutr. 2013, 52, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Giriwono, P.E.; Shirakawa, H.; Ohsaki, Y.; Sato, S.; Aoyama, Y.; Ho, H.-J.; Goto, T.; Komai, M. Geranylgeraniol Suppresses the Expression of IRAK1 and TRAF6 to Inhibit NFκB Activation in Lipopolysaccharide-Induced Inflammatory Responses in Human Macrophage-Like Cells. Int. J. Mol. Sci. 2019, 20, 2320. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.; Shirakawa, H.; Yoshida, R.; Ito, A.; Maeda, M.; Goto, T.; Komai, M. Geranylgeraniol enhances testosterone production via the cAMP/protein kinase A pathway in testis-derived I-10 tumor cells. Biosci. Biotechnol. Biochem. 2016, 80, 791–797. [Google Scholar] [CrossRef] [Green Version]
- Carson, J.A.; Manolagas, S.C. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone 2015, 80, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Hourdé, C.; Jagerschmidt, C.; Clément-Lacroix, P.; Vignaud, A.; Ammann, P.; Butler-Browne, G.S.; Ferry, A. Androgen replacement therapy improves function in male rat muscles independently of hypertrophy and activation of the Akt/mTOR pathway. Acta Physiol. 2009, 195, 471–482. [Google Scholar] [CrossRef]
- Pires-Oliveira, M.; Maragno, A.L.G.C.; Parreiras-e-Silva, L.T.; Chiavegatti, T.; Gomes, M.D.; Godinho, R.O. Testosterone represses ubiquitin ligases atrogin-1 and Murf-1 expression in an androgen-sensitive rat skeletal muscle in vivo. J. Appl. Physiol. 2010, 108, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Tikkanen, M.J.; Nikkilä, E.A. Current pharmacologic treatment of elevated serum cholesterol. Circulation 1987, 76, 529–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illingworth, D.R.; Sexton, G.J. Hypocholesterolemic effects of mevinolin in patients with heterozygous familial hypercholesterolemia. J. Clin. Investig. 1984, 74, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Hoeg, J.M.; Maher, M.B.; Zech, L.A.; Bailey, K.R.; Gregg, R.E.; Lackner, K.J.; Fojo, S.S.; Anchors, M.A.; Bojanovski, M.; Sprecher, D.L.; et al. Effectiveness of mevinolin on plasma lipoprotein concentrations in type II hyperlipoproteinemia. Am. J. Cardiol. 1986, 57, 933–939. [Google Scholar] [CrossRef]
- Grundy, S.M.; Vega, G.L. Influence of mevinolin on metabolism of low density lipoproteins in primary moderate hypercholesterolemia. J. Lipid Res. 1985, 26, 1464–1475. [Google Scholar] [CrossRef]
- Paul, D.; Thompson, M.D.; Clarkson, P.; Richard, H.K. Statin-Associated Myopathy. JAMA 2003, 289, 1681–1690. [Google Scholar] [CrossRef]
- Pasternak, R.C.; Smith, S.C.; Bairey-Merz, C.N.; Grundy, S.M.; Cleeman, J.I.; Lenfant, C.; Hon, F. ACC/AHA/NHLBI Clinical Advisory on Statins. Circulation 2002, 106, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.M.; Davidson, M.H.; Jacobson, T.A.; Guyton, J.R. Final Conclusions and Recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am. J. Cardiol. 2006, 97, 89C–94C. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, S. Statin induced myotoxicity. Eur. J. Intern. Med. 2012, 23, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Selvarajah, S.; Schneider, E.B. Muscular effects of statins in the elderly female: A review. Clin. Interv. Aging. 2013, 8, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Nishimoto, T.; Tozawa, R.; Amano, Y.; Wada, T.; Imura, Y.; Sugiyama, Y. Comparing myotoxic effects of squalene synthase inhibitor, T-91485, and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors in human myocytes. Biochem. Pharmacol. 2003, 66, 2133–2139. [Google Scholar] [CrossRef]
- Matzno, S.; Yamauchi, T.; Gohda, M.; Ishida, N.; Katsuura, K.; Hanasaki, Y.; Tokunaga, T.; Itoh, H.; Nakamura, N. Inhibition of cholesterol biosynthesis by squalene epoxidase inhibitor avoids apoptotic cell death in L6 myoblasts. J. Lipid Res. 1997, 38, 1639–1648. [Google Scholar] [CrossRef]
- Baba, T.T.; Nemoto, T.K.; Miyazaki, T.; Oida, S. Simvastatin suppresses the differentiation of C2C12 myoblast cells via a Rac pathway. J. Muscle Res. Cell Motil. 2008, 29, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Cao, P.; Hanai, J.; Tanksale, P.; Imamura, S.; Sukhatme, V.P.; Lecker, S.H. Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect. FASEB J. 2009, 23, 2844–2854. [Google Scholar] [CrossRef] [Green Version]
- Report of the Subdivision on Resources, The Council for Science and Technology, Ministry of Education, Culture, Sports, Science and Technology, Japan. Standard Tables of Food Composition In Japan—2020—(Eighth Revised Edition). Available online: https://www.mext.go.jp/content/20201225-mxt_kagsei-mext_01110_011.pdf (accessed on 21 June 2021).
- Hashimoto, R.; Sakai, A.; Murayama, M.; Ochi, A.; Abe, T.; Hirasaka, K.; Ohno, A.; Teshima-Kondo, S.; Yanagawa, H.; Yasui, N.; et al. Effects of dietary soy protein on skeletal muscle volume and strength in humans with various physical activities. J. Med. Investig. 2015, 62, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitajima, Y.; Ogawa, S.; Egusa, S.; Ono, Y. Soymilk Improves Muscle Weakness in Young Ovariectomized Female Mice. Nutrients 2017, 9, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samoto, M.; Maebuchi, M.; Miyazaki, C.; Kugitani, H.; Kohno, M.; Hirotsuka, M.; Kito, M. Abundant proteins associated with lecithin in soy protein isolate. Food Chem. 2007, 102, 317–322. [Google Scholar] [CrossRef]
- Abe, T.; Kohno, S.; Yama, T.; Ochi, A.; Suto, T.; Hirasaka, K.; Ohno, A.; Teshima-Kondo, S.; Okumura, Y.; Oarada, M.; et al. Soy Glycinin Contains a Functional Inhibitory Sequence against Muscle-Atrophy-Associated Ubiquitin Ligase Cbl-b. Int. J. Endocrinol. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikawa, T.; Hashimoto, R.; Nakao, R.; Uchida, T.; Ninomiya, M.; Kimori, Y.; Maita, A.; Tetsuno, A.; Ida, K.; Kishimoto, H.; et al. Effects of Dietary Soy Protein and Whey Protein on Denervation-induced Skeletal Muscle Atrophy. Soy Protein Res. Jpn. 2017, 20, 8–12. [Google Scholar]
- Tachibana, N.; Fukao, M.; Irie, T.; Irisawa, Y.; Shirono, H.; Oarada, M.; Nikawa, T.; Fukaya, T. A Diet Including Red Bell Pepper Juice and Soy Protein Suppress Physiological Markers of Muscle Atrophy in Mice. J. Nutr. Sci. Vitaminol. 2020, 66, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Kakigi, R.; Yoshihara, T.; Ozaki, H.; Ogura, Y.; Ichinoseki-Sekine, N.; Kobayashi, H.; Naito, H. Whey protein intake after resistance exercise activates mTOR signaling in a dose-dependent manner in human skeletal muscle. Eur. J. Appl. Physiol. 2014, 114, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Tabata, S.; Aizawa, M.; Kinoshita, M.; Ito, Y.; Kawamura, Y.; Takebe, M.; Pan, W.; Sakuma, K. The influence of isoflavone for denervation-induced muscle atrophy. Eur. J. Nutr. 2019, 58, 291–300. [Google Scholar] [CrossRef]
- Hirasaka, K.; Maeda, T.; Haruna, M.; Abe, T.; Ochi, A.; Ohno-Maita, A.; Teshima-Kondo, S.; Taniyama, S.; Tachibana, K.; Nikawa, T. Effects of Isoflavones Derived from Soy Beans on Muscle Atrophy. Soy Protein Res. Jpn. 2014, 17, 150–155. [Google Scholar]
- Hirasaka, K.; Maeda, T.; Ikeda, C.; Haruna, M.; Kohno, S.; Abe, T.; Ochi, A.; Mukai, R.; Oarada, M.; Eshima-Kondo, S.; et al. Isoflavones Derived from Soy Beans Prevent MuRF1-Mediated Muscle Atrophy in C2C12 Myotubes through SIRT1 Activation. J. Nutr. Sci. Vitaminol. 2013, 59, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Aubertin-Leheudre, M.; Lord, C.; Khalil, A.; Dionne, I.J. Six months of isoflavone supplement increases fat-free mass in obese–sarcopenic postmenopausal women: A randomized double-blind controlled trial. Eur. J. Clin. Nutr. 2007, 61, 1442–1444. [Google Scholar] [CrossRef]
- Harris, D.M.; Besselink, E.; Henning, S.M.; Go, V.L.W.; Heber, D. Phytoestrogens Induce Differential Estrogen Receptor Alpha- or Beta-Mediated Responses in Transfected Breast Cancer Cells. Exp. Biol. Med. 2005, 230, 558–568. [Google Scholar] [CrossRef]
- Seko, D.; Fujita, R.; Kitajima, Y.; Nakamura, K.; Imai, Y.; Ono, Y. Estrogen Receptor β Controls Muscle Growth and Regeneration in Young Female Mice. Stem Cell Rep. 2020, 15, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Horikawa, H.; Fujikura, Y.; Kawamura, T.; Nemoto, H.; Nikawa, T.; Terao, J. Prevention of Disuse Muscle Atrophy by Dietary Ingestion of 8-Prenylnaringenin in Denervated Mice. PLoS ONE 2012, 7, e45048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Shahrani, M.; Heales, S.; Hargreaves, I.; Orford, M. Oxidative Stress: Mechanistic Insights into Inherited Mitochondrial Disorders and Parkinson’s Disease. J. Clin. Med. 2017, 6, 100. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Zuo, L. Redox Roles of Reactive Oxygen Species in Cardiovascular Diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef] [Green Version]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Horio, Y. Regulation of FOXOs and p53 by SIRT1 Modulators under Oxidative Stress. PLoS ONE 2013, 8, e73875. [Google Scholar] [CrossRef]
- Mukai, R.; Nakao, R.; Yamamoto, H.; Nikawa, T.; Takeda, E.; Terao, J. Quercetin Prevents Unloading-Derived Disused Muscle Atrophy by Attenuating the Induction of Ubiquitin Ligases in Tail-Suspension Mice. J. Nat. Prod. 2010, 73, 1708–1710. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Matsui, N.; Fujikura, Y.; Matsumoto, N.; Hou, D.; Kanzaki, N.; Shibata, H.; Horikawa, M.; Iwasa, K.; Hirasaka, K.; et al. Preventive effect of dietary quercetin on disuse muscle atrophy by targeting mitochondria in denervated mice. J. Nutr. Biochem. 2016, 31, 67–76. [Google Scholar] [CrossRef]
- Nakamura, S. Chemistry of Reactive Oxygen Species and Antioxidants. J. Nippon Med. Sch. 2013, 9, 164–169. [Google Scholar]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.-H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8749–8754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaszczak, W.; Barczak, W.; Masternak, J.; Kopczyński, P.; Zhitkovich, A.; Rubiś, B. Vitamin C as a Modulator of the Response to Cancer Therapy. Molecules 2019, 24, 453. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Yokoyama, T.; Yoshida, H.; Kim, H.; Shimada, H.; Yoshida, Y.; Iwasa, H.; Shimizu, Y.; Kondo, Y.; Handa, S.; et al. A Significant Relationship between Plasma Vitamin C Concentration and Physical Performance among Japanese Elderly Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 295–301. [Google Scholar] [CrossRef]
- Takisawa, S.; Funakoshi, T.; Yatsu, T.; Nagata, K.; Aigaki, T.; Machida, S.; Ishigami, A. Vitamin C deficiency causes muscle atrophy and a deterioration in physical performance. Sci. Rep. 2019, 9, 4702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makanae, Y.; Kawada, S.; Sasaki, K.; Nakazato, K.; Ishii, N. Vitamin C administration attenuates overload-induced skeletal muscle hypertrophy in rats. Acta Physiol. 2013, 208, 57–65. [Google Scholar] [CrossRef]
- Ceglia, L.; Niramitmahapanya, S.; da Silva Morais, M.; Rivas, D.A.; Harris, S.S.; Bischoff-Ferrari, H.; Fielding, R.A.; Dawson-Hughes, B. A Randomized Study on the Effect of Vitamin D 3 Supplementation on Skeletal Muscle Morphology and Vitamin D Receptor Concentration in Older Women. J. Clin. Endocrinol. Metab. 2013, 98, E1927–E1935. [Google Scholar] [CrossRef] [Green Version]
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of Vitamin D Receptor Gene in Mice Results in Abnormal Skeletal Muscle Development with Deregulated Expression of Myoregulatory Transcription Factors. Endocrinology 2003, 144, 5138–5144. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.; Deeg, D.J.H.; Lips, P. Low Vitamin D and High Parathyroid Hormone Levels as Determinants of Loss of Muscle Strength and Muscle Mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.-Y.; Bruyère, O. The Effects of Vitamin D on Skeletal Muscle Strength, Muscle Mass, and Muscle Power: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, Y.; Onishi, T.; Miura, S.; Hatazawa, Y.; Kamei, Y. Vitamin D Attenuates FOXO1-Target Atrophy Gene Expression in C2C12 Muscle Cells. J. Nutr. Sci. Vitaminol. 2018, 64, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Servais, S.; Letexier, D.; Favier, R.; Duchamp, C.; Desplanches, D. Prevention of unloading-induced atrophy by vitamin E supplementation: Links between oxidative stress and soleus muscle proteolysis? Free Radic. Biol. Med. 2007, 42, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Ikemoto, M.; Okamura, Y.; Kano, M.; Hirasaka, K.; Tanaka, R.; Yamamoto, T.; Sasa, T.; Ogawa, T.; Sairyo, K.; Kishi, K.; et al. A Relative High Dose of Vitamin E Does Not Attenuate Unweighting-Induced Oxidative Stress and Ubiquitination in Rat Skeletal Muscle. J. Physiol. Anthropol. Appl. Human Sci. 2002, 21, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ruegg, U.T.; Kudo, A.; Miyagoe-Suzuki, Y.; Takeda, S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat. Med. 2013, 19, 101–106. [Google Scholar] [CrossRef] [PubMed]
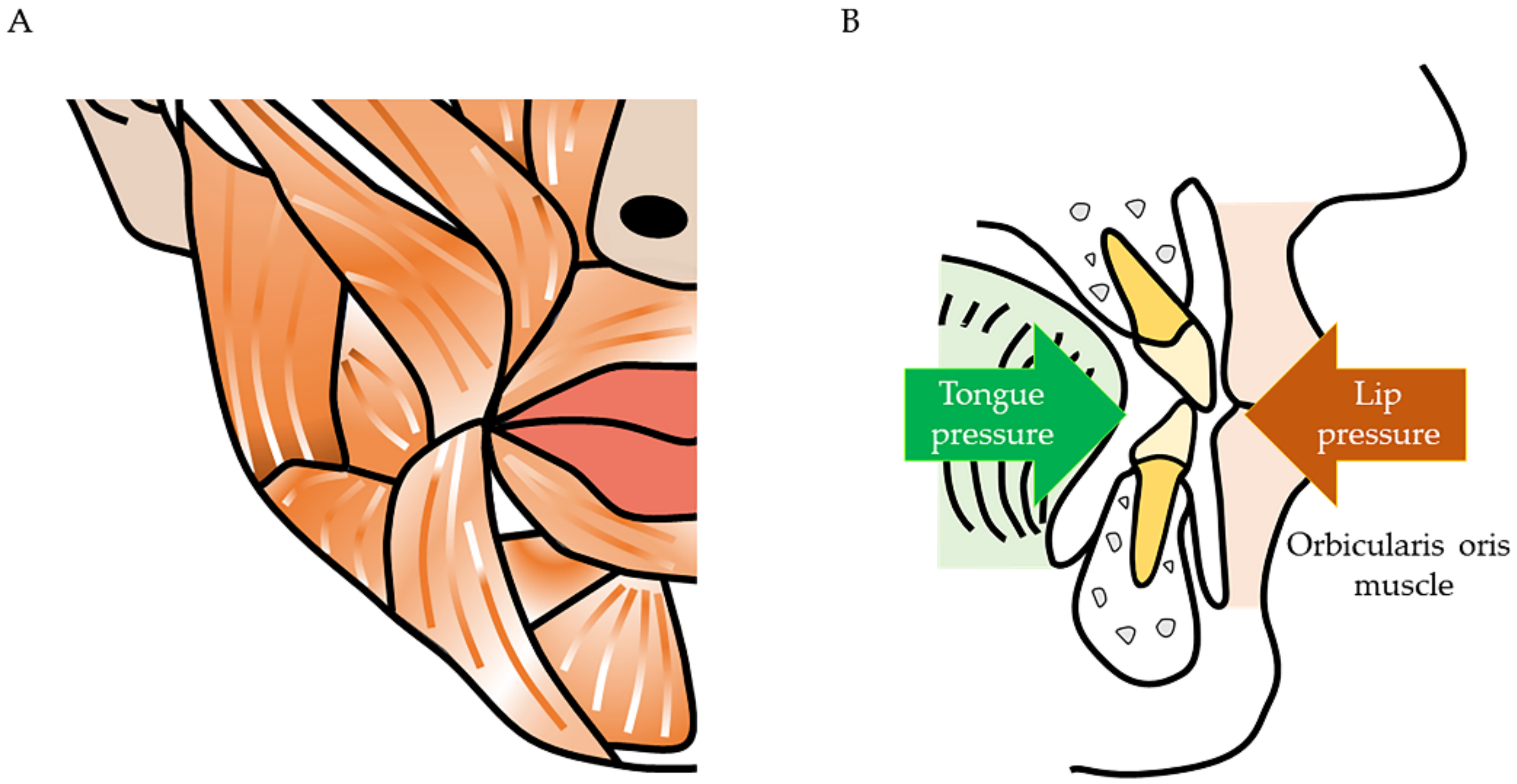

| Genes | Proteins | Characteristics |
|---|---|---|
| MYH 1 | MyHC 2x | Fast type 2x fibers |
| MYH 2 | MyHC 2a | Fast type 2a fibers |
| MYH 3 | MyHC EMB | Developing muscle, Extraocular muscles |
| MYH 4 | MyHC 2b | Fast type 2b fibers |
| MYH 6 | MyHC α | Heart and jaw muscles |
| MYH 7 | MyHC β | Heart and slow muscles, type 1 fibers |
| MYH 7b | MyHC slow tonic | Extraocular muscles |
| MYH 8 | MyHC NEO | Developing muscle, expression in masseter muscles |
| MYH 13 | MyHC EO | Extraocular muscles |
| MYH 15 | MyHC 15 | Extraocular muscles |
| MYH 16 | MyHC 16 | Jaw muscles (in human, translation is blocked) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirakawa, T.; Miyawaki, A.; Kawamoto, T.; Kokabu, S. Natural Compounds Attenuate Denervation-Induced Skeletal Muscle Atrophy. Int. J. Mol. Sci. 2021, 22, 8310. https://doi.org/10.3390/ijms22158310
Shirakawa T, Miyawaki A, Kawamoto T, Kokabu S. Natural Compounds Attenuate Denervation-Induced Skeletal Muscle Atrophy. International Journal of Molecular Sciences. 2021; 22(15):8310. https://doi.org/10.3390/ijms22158310
Chicago/Turabian StyleShirakawa, Tomohiko, Aki Miyawaki, Tatsuo Kawamoto, and Shoichiro Kokabu. 2021. "Natural Compounds Attenuate Denervation-Induced Skeletal Muscle Atrophy" International Journal of Molecular Sciences 22, no. 15: 8310. https://doi.org/10.3390/ijms22158310
APA StyleShirakawa, T., Miyawaki, A., Kawamoto, T., & Kokabu, S. (2021). Natural Compounds Attenuate Denervation-Induced Skeletal Muscle Atrophy. International Journal of Molecular Sciences, 22(15), 8310. https://doi.org/10.3390/ijms22158310





