Analogs of a Natural Peptaibol Exert Anticancer Activity in Both Cisplatin- and Doxorubicin-Resistant Cells and in Multicellular Tumor Spheroids
Abstract
:1. Introduction
2. Results and Discussion
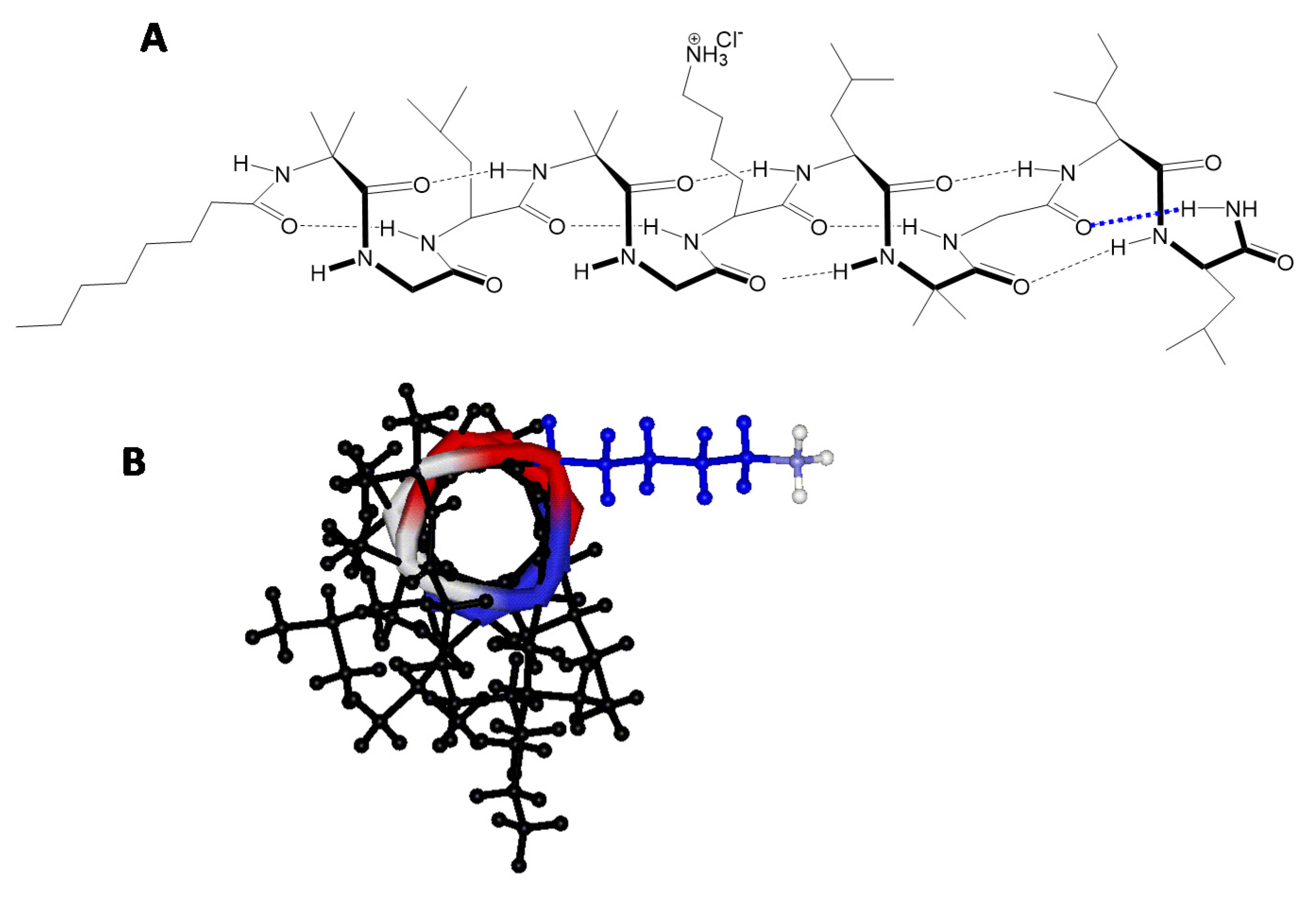
2.1. Peptide Synthesis
2.2. Peptaibols Are Stable in Serum and Resistant to Enzymatic Digestion
2.3. In Vitro Cytotoxicity of Peptaibols in cHL and OvCa Cells
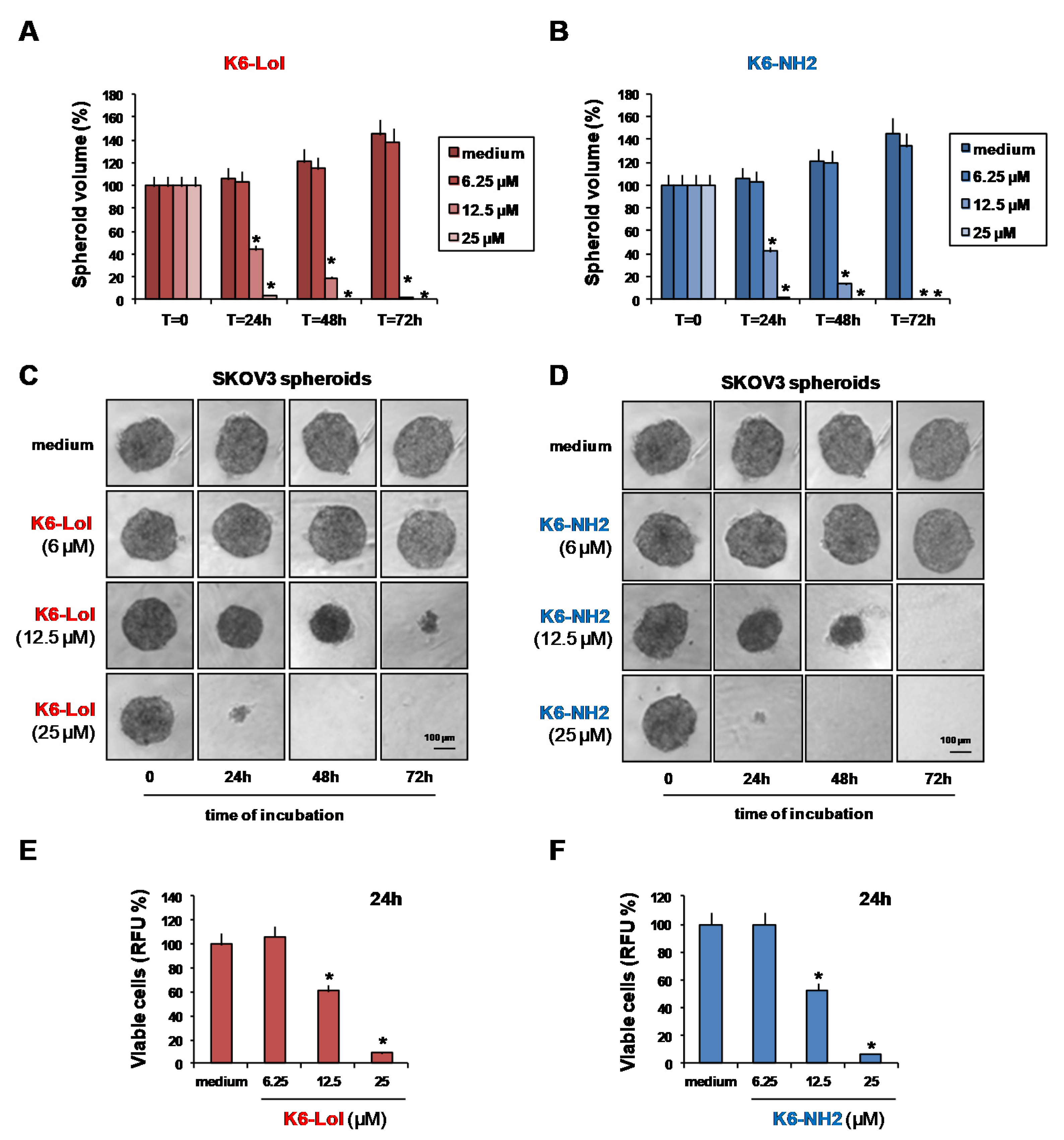
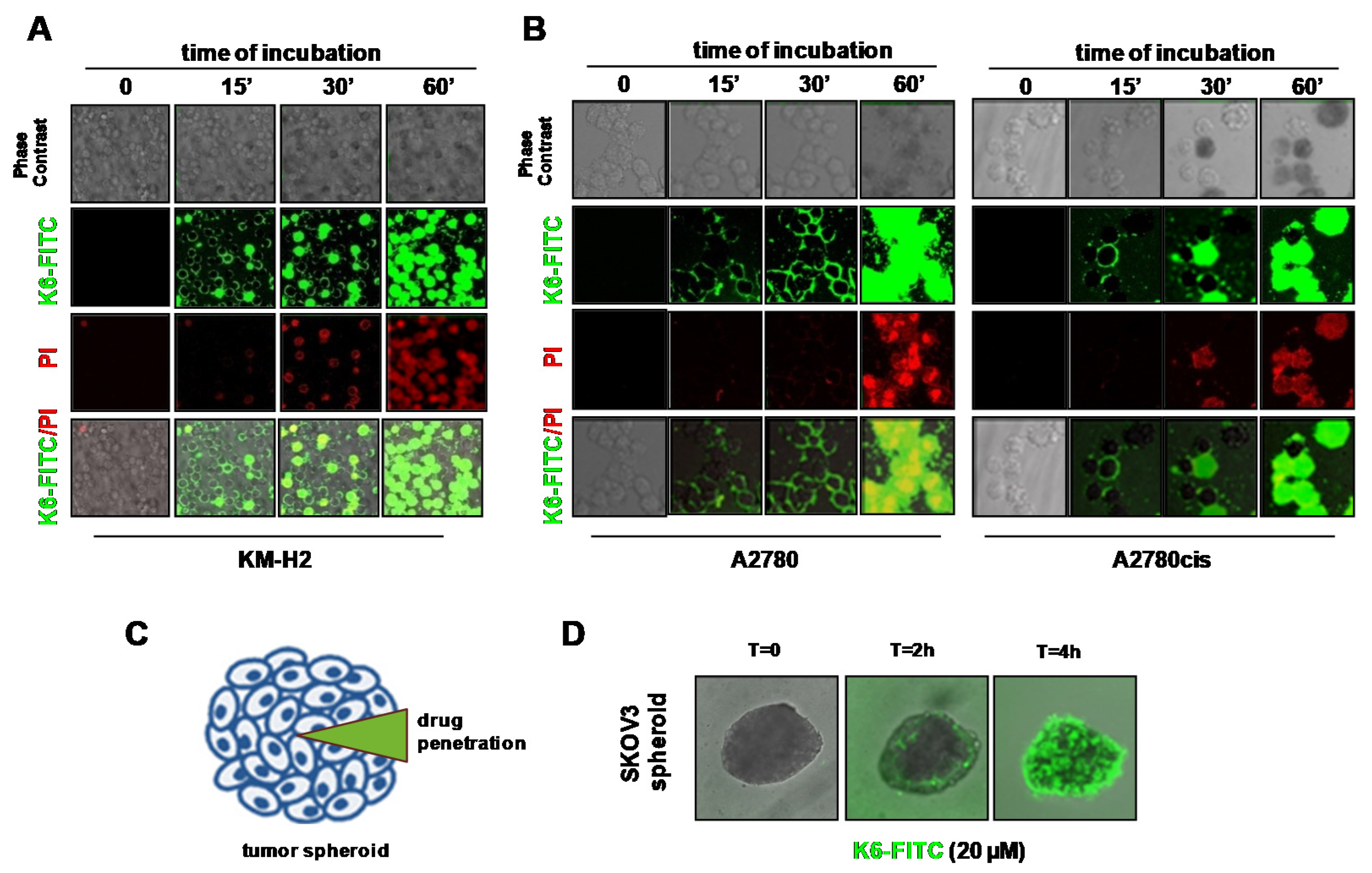
2.4. Peptaibols Deeply Penetrate and Kill SKOV3-MCTSs
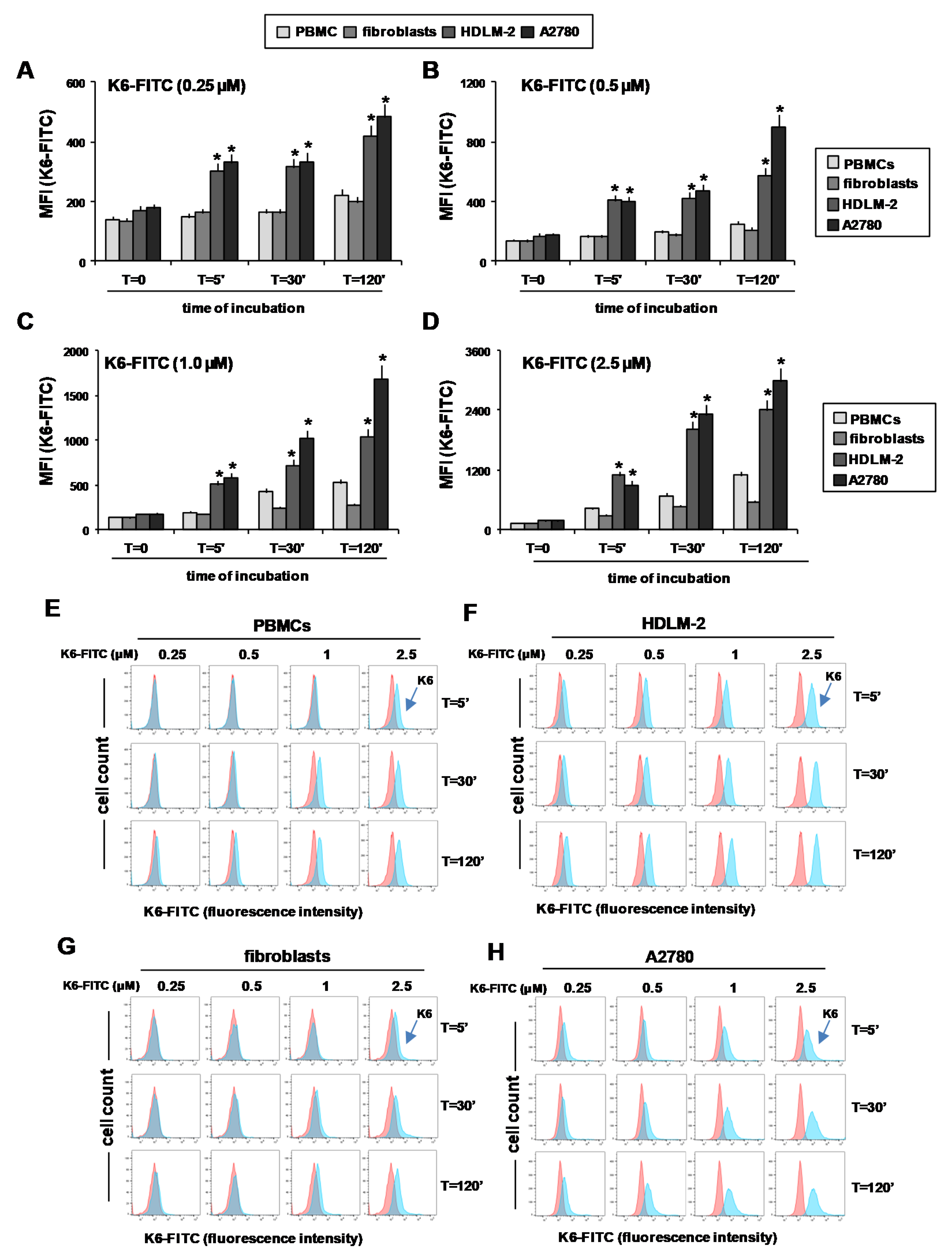
2.5. Peptaibol Uptake by HRS and OvCa Cells
2.6. Peptaibols Activity in Healthy Cells
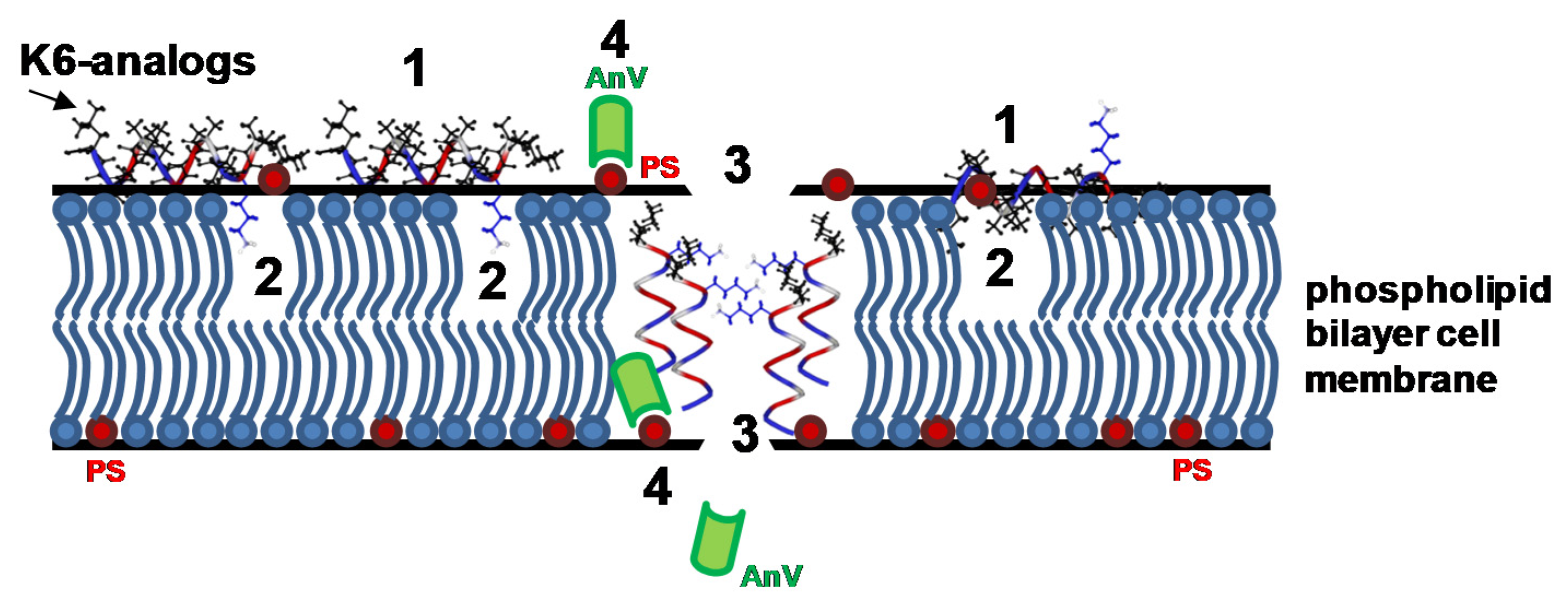
2.7. Peptaibols Cause Cell Membrane Permeabilization in Tumor Cells
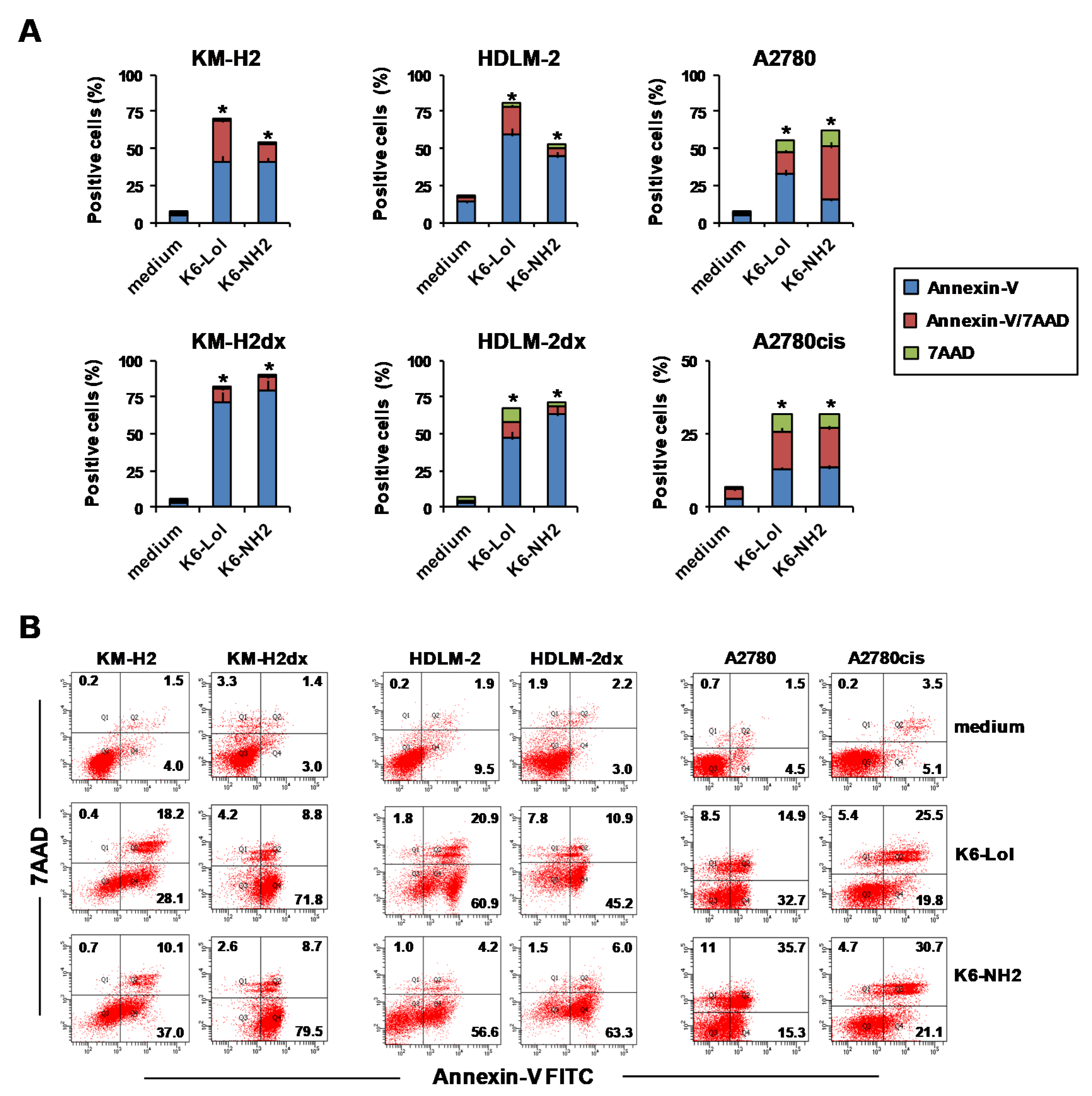
2.8. Peptaibols Induce Phosphatidylserine Exposure wihout Caspase Activation
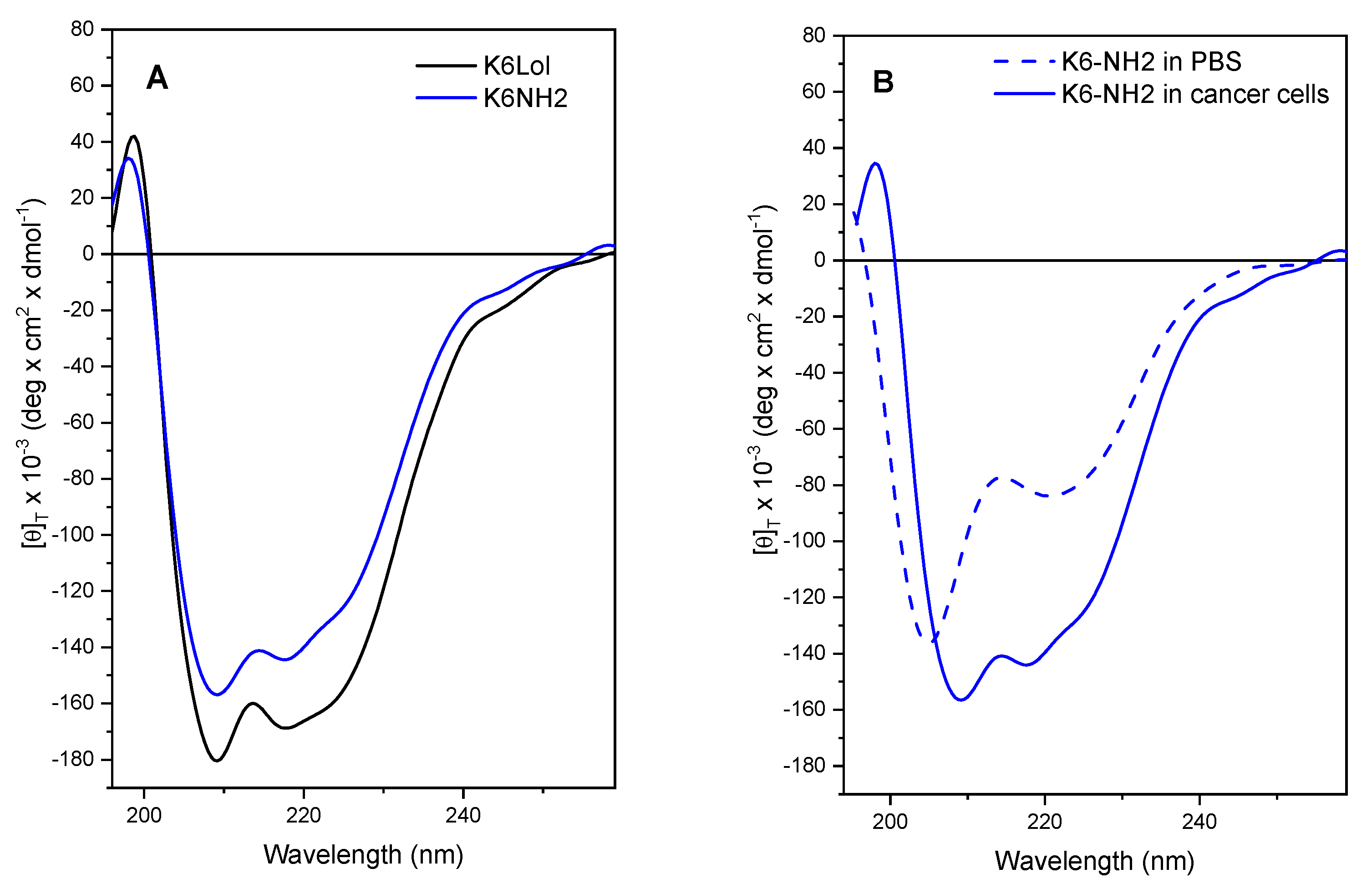
2.9. Peptaibols Maintain a Stable Helical Structure in the Presence of Cancer Cells
3. Materials and Methods
3.1. Materials
3.2. Peptide Synthesis
3.3. Serum Stability Assays
3.4. Proteolytic Stability Assays
3.5. Circular Dichroism (CD)
3.6. Cell Culture
3.7. Cellular Assays
3.8. K6- FITC Uptake and Annexin-V Assay
3.9. Calcein AM Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lau, J.L.; Dunn, M.K. Therapeutic Peptides: Historical Perspectives, Current Development Trends, and Future Directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Sorolla, A.; Wang, E.; Golden, E.; Duffy, C.; Henriques, S.T.; Redfern, A.D.; Blancafort, P. Precision Medicine by Designer Interference Peptides: Applications in Oncology and Molecular Therapeutics. Oncogene 2020, 39, 1167–1184. [Google Scholar] [CrossRef] [Green Version]
- Moiola, M.; Memeo, M.G.; Quadrelli, P. Stapled Peptides-A Useful Improvement for Peptide-Based Drugs. Molecules 2019, 24, 3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, X.; Jin, K. A Gold Mine for Drug Discovery: Strategies to Develop Cyclic Peptides into Therapies. Med. Res. Rev. 2020, 40, 753–810. [Google Scholar] [CrossRef] [PubMed]
- Albertini, B.; Mathieu, V.; Iraci, N.; Van Woensel, M.; Schoubben, A.; Donnadio, A.; Greco, S.M.L.; Ricci, M.; Temperini, A.; Blasi, P.; et al. Tumor Targeting by Peptide-Decorated Gold Nanoparticles. Mol. Pharm. 2019, 16, 2430–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalzini, A.; Bergamini, C.; Biondi, B.; De Zotti, M.; Panighel, G.; Fato, R.; Peggion, C.; Bortolus, M.; Maniero, A.L. The Rational Search for Selective Anticancer Derivatives of the Peptide Trichogin GA IV: A Multi-Technique Biophysical Approach. Sci. Rep. 2016, 6, 24000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Liu, D.; Yang, Y. Anti-Cancer Peptides: Classification, Mechanism of Action, Reconstruction and Modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- De Zotti, M.; Sella, L.; Bolzonello, A.; Gabbatore, L.; Peggion, C.; Bortolotto, A.; Elmaghraby, I.; Tundo, S.; Favaron, F. Targeted Amino Acid Substitutions in a Trichoderma Peptaibol Confer Activity against Fungal Plant Pathogens and Protect Host Tissues from Botrytis Cinerea Infection. Int. J. Mol. Sci. 2020, 21, 7521. [Google Scholar] [CrossRef]
- Lundquist, J.T.; Satterfield, A.D.; Pelletier, J.C. Mild and Adaptable Silver Triflate-Assisted Method for Trityl Protection of Alcohols in Solution with Solid-Phase Loading Applications. Org. Lett. 2006, 8, 3915–3918. [Google Scholar] [CrossRef]
- De Zotti, M.; Biondi, B.; Peggion, C.; Formaggio, F.; Park, Y.; Hahm, K.-S.; Toniolo, C. Trichogin GA IV: A Versatile Template for the Synthesis of Novel Peptaibiotics. Org. Biomol. Chem. 2012, 10, 1285–1299. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA: A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The Untapped Potential of Ascites in Ovarian Cancer Research and Treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A Three-Dimensional Microenvironment Alters Protein Expression and Chemosensitivity of Epithelial Ovarian Cancer Cells in Vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in Ovarian Cancer Therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, R.L.; Monk, B.J.; Sood, A.K.; Herzog, T.J. Latest Research and Treatment of Advanced-Stage Epithelial Ovarian Cancer. Nat. Rev. Clin. Oncol. 2013, 10, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Ansell, S.M. Hodgkin Lymphoma: A 2020 Update on Diagnosis, Risk-Stratification, and Management. Am. J. Hematol. 2020, 95, 978–989. [Google Scholar] [CrossRef]
- Connors, J.M.; Cozen, W.; Steidl, C.; Carbone, A.; Hoppe, R.T.; Flechtner, H.-H.; Bartlett, N.L. Hodgkin Lymphoma. Nat. Rev. Dis. Primers. 2020, 6, 61. [Google Scholar] [CrossRef]
- Tavano, R.; Malachin, G.; De Zotti, M.; Peggion, C.; Biondi, B.; Formaggio, F.; Papini, E. Comparison of Bactericidal and Cytotoxic Activities of Trichogin Analogs. Data Brief 2016, 6, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, M.; Musaimi, O.A.; Collins, J.M.; Albericio, F.; de la Torre, B.G. Cleaving Protected Peptides from 2-Chlorotrityl Chloride Resin. Moving Away from Dichloromethane. Green Chem. 2020, 22, 2840–2845. [Google Scholar] [CrossRef]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial Peptides—Advances in Development of Therapeutic Applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A View on Drug Resistance in Cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shnaider, P.V.; Ivanova, O.M.; Malyants, I.K.; Anufrieva, K.S.; Semenov, I.A.; Pavlyukov, M.S.; Lagarkova, M.A.; Govorun, V.M.; Shender, V.O. New Insights into Therapy-Induced Progression of Cancer. Int. J. Mol. Sci. 2020, 21, 7872. [Google Scholar] [CrossRef] [PubMed]
- Greaves, W.; Xiao, L.; Sanchez-Espiridion, B.; Kunkalla, K.; Dave, K.S.; Liang, C.S.; Singh, R.R.; Younes, A.; Medeiros, L.J.; Vega, F. Detection of ABCC1 Expression in Classical Hodgkin Lymphoma Is Associated with Increased Risk of Treatment Failure Using Standard Chemotherapy Protocols. J. Hematol. Oncol. 2012, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Damia, G.; Broggini, M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers 2019, 11, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Herrera, A.F.; Hou, J.; Chen, L.; Wu, J.; Guo, Y.; Synold, T.W.; Ngo, V.N.; Puverel, S.; Mei, M.; et al. Inhibition of MDR1 Overcomes Resistance to Brentuximab Vedotin in Hodgkin Lymphoma. Clin. Cancer Res. 2020, 26, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, R.; Llaneza, D.C.; Landen, C.N. Emerging Therapeutics to Overcome Chemoresistance in Epithelial Ovarian Cancer: A Mini-Review. Int. J. Mol. Sci. 2017, 18, 2171. [Google Scholar] [CrossRef] [Green Version]
- Aronson, M.R.; Dahl, E.S.; Halle, J.A.; Simonson, A.W.; Gogal, R.A.; Glick, A.B.; Aird, K.M.; Medina, S.H. Re-Engineering Antimicrobial Peptides into Oncolytics Targeting Drug-Resistant Ovarian Cancers. Cell. Mol. Bioeng. 2020, 13, 447–461. [Google Scholar] [CrossRef]
- Luo, X.; Teng, Q.-X.; Dong, J.-Y.; Yang, D.-H.; Wang, M.; Dessie, W.; Qin, J.-J.; Lei, Z.-N.; Wang, J.-Q.; Qin, Z.; et al. Antimicrobial Peptide Reverses ABCB1-Mediated Chemotherapeutic Drug Resistance. Front. Pharmacol. 2020, 11, 1208. [Google Scholar] [CrossRef]
- Celegato, M.; Borghese, C.; Casagrande, N.; Mongiat, M.; Kahle, X.U.; Paulitti, A.; Spina, M.; Colombatti, A.; Aldinucci, D. Preclinical Activity of the Repurposed Drug Auranofin in Classical Hodgkin Lymphoma. Blood 2015, 126, 1394–1397. [Google Scholar] [CrossRef] [Green Version]
- Francisco, J.A.; Cerveny, C.G.; Meyer, D.L.; Mixan, B.J.; Klussman, K.; Chace, D.F.; Rejniak, S.X.; Gordon, K.A.; DeBlanc, R.; Toki, B.E.; et al. CAC10-VcMMAE, an Anti-CD30-Monomethyl Auristatin E Conjugate with Potent and Selective Antitumor Activity. Blood 2003, 102, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.; Ackland, M.L.; Ahmed, N.; Rice, G.E. Multicellular Spheroids in Ovarian Cancer Metastases: Biology and Pathology. Gynecol. Oncol. 2009, 113, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, A.; Afify, H.; Salih, Z.; Kelly, M.; Said, N. Role of Tumor Microenvironment in the Pathobiology of Ovarian Cancer: Insights and Therapeutic Opportunities. Cancer Med. 2018, 7, 5047–5056. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D Tumor Spheroids as in Vitro Models to Mimic in Vivo Human Solid Tumors Resistance to Therapeutic Drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.; Neal, A.S.; Moatamed, N.A.; Memarzadeh, S. Exploring the Potential of Drug Response Assays for Precision Medicine in Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 305. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug Delivery to Solid Tumors: The Predictive Value of the Multicellular Tumor Spheroid Model for Nanomedicine Screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef] [Green Version]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid Composition of the Cancer Cell Membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Zweytick, D.; Lohner, K. Membrane-Active Host Defense Peptides--Challenges and Perspectives for the Development of Novel Anticancer Drugs. Chem. Phys. Lipids 2011, 164, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Ramachander, T.V.N. Peptaibols: Antimicrobial Peptides from Fungi. In Bioactive Natural Products in Drug Discovery; Singh, J., Meshram, V., Gupta, M., Eds.; Springer: Singapore, 2020; pp. 713–730. ISBN 9789811513947. [Google Scholar]
- Salnikov, E.S.; De Zotti, M.; Bobone, S.; Mazzuca, C.; Raya, J.; Siano, A.S.; Peggion, C.; Toniolo, C.; Stella, L.; Bechinger, B. Trichogin GA IV Alignment and Oligomerization in Phospholipid Bilayers. ChemBioChem 2019, 20, 2141–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Watson, B.G.; Mattoo, S.; Rochet, J.-C. Calcein Release Assay to Measure Membrane Permeabilization by Recombinant Alpha-Synuclein. Bio. Protoc. 2020, 10, e3690. [Google Scholar] [CrossRef] [PubMed]
- Musaimi, O.A.; de la Torre, B.G.; Albericio, F. Greening Fmoc/TBu Solid-Phase Peptide Synthesis. Green Chem. 2020, 22, 996–1018. [Google Scholar] [CrossRef]
- Capuano, A.; Bucciotti, F.; Farwell, K.D.; Tippin Davis, B.; Mroske, C.; Hulick, P.J.; Weissman, S.M.; Gao, Q.; Spessotto, P.; Colombatti, A.; et al. Diagnostic Exome Sequencing Identifies a Novel Gene, EMILIN1, Associated with Autosomal-Dominant Hereditary Connective Tissue Disease. Hum. Mutat. 2016, 37, 84–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Casagrande, N.; Celegato, M.; Borghese, C.; Mongiat, M.; Colombatti, A.; Aldinucci, D. Preclinical Activity of the Liposomal Cisplatin Lipoplatin in Ovarian Cancer. Clin. Cancer Res. 2014, 20, 5496–5506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghese, C.; Casagrande, N.; Corona, G.; Aldinucci, D. Adipose-Derived Stem Cells Primed with Paclitaxel Inhibit Ovarian Cancer Spheroid Growth and Overcome Paclitaxel Resistance. Pharmaceutics 2020, 12, 401. [Google Scholar] [CrossRef] [PubMed]



| Cell Line | Compound (IC50) 1 | |||
|---|---|---|---|---|
| K6-Lol (µM) | K6-NH2 (µM) | Cisplatin (µM) | Doxorubicin (nM) | |
| HDLM-2 | 12.6 (1.1) | 13.2 (1.2) | 1.3 (0.1) | 40.5 (3.7) |
| HDLM-2dx | 11.9 (1.0) | 12.6 (1.0) | 4.0 (0.4) | 368.1 (34.1) |
| KM-H2 | 7.5 (0.7) | 7.8 (0.6) | 0.5 (0.0) | 22.1 (1.9) |
| KM-H2dx | 7.2 (0.6) | 8.2 (0.7) | 1.2 (0.1) | 202.4 (19.8) |
| L-1236 | 11.7 (1.0) | 9.4 (1.1) | 0.8 (0.1) | 46.4 (4.1) |
| L-428 | 8.9 (0.9) | 9.5 (1.0) | 0.5 (0.1) | 18.4 (1.4) |
| A2780 | 7.9 (0.8) | 8.5 (0.9) | 1.3 (0.1) | 20.4 (1.8) |
| A2780cis | 9.1 (0.8) | 9.0 (0.9) | 10.4 (0.9) | 115.5 (10.4) |
| OVCAR5 | 7.5 (0.6) | 7.5 (0.6) | 7.7 (0.7) | 470 (41.9) |
| SKOV3 | 8.4 (0.8) | 10.5 (1.1) | 7.7 (0.8) | 130.4 (11.7) |
| PBMCs | 7.1 (0.4) | 7.9 (0.4) | nd | nd |
| Fibroblasts | 15.0 (1.4) | 15.5 (1.4) | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casagrande, N.; Borghese, C.; Gabbatore, L.; Morbiato, L.; De Zotti, M.; Aldinucci, D. Analogs of a Natural Peptaibol Exert Anticancer Activity in Both Cisplatin- and Doxorubicin-Resistant Cells and in Multicellular Tumor Spheroids. Int. J. Mol. Sci. 2021, 22, 8362. https://doi.org/10.3390/ijms22168362
Casagrande N, Borghese C, Gabbatore L, Morbiato L, De Zotti M, Aldinucci D. Analogs of a Natural Peptaibol Exert Anticancer Activity in Both Cisplatin- and Doxorubicin-Resistant Cells and in Multicellular Tumor Spheroids. International Journal of Molecular Sciences. 2021; 22(16):8362. https://doi.org/10.3390/ijms22168362
Chicago/Turabian StyleCasagrande, Naike, Cinzia Borghese, Laura Gabbatore, Laura Morbiato, Marta De Zotti, and Donatella Aldinucci. 2021. "Analogs of a Natural Peptaibol Exert Anticancer Activity in Both Cisplatin- and Doxorubicin-Resistant Cells and in Multicellular Tumor Spheroids" International Journal of Molecular Sciences 22, no. 16: 8362. https://doi.org/10.3390/ijms22168362
APA StyleCasagrande, N., Borghese, C., Gabbatore, L., Morbiato, L., De Zotti, M., & Aldinucci, D. (2021). Analogs of a Natural Peptaibol Exert Anticancer Activity in Both Cisplatin- and Doxorubicin-Resistant Cells and in Multicellular Tumor Spheroids. International Journal of Molecular Sciences, 22(16), 8362. https://doi.org/10.3390/ijms22168362








