Reviewing the Significance of Blood–Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment
Abstract
1. Introduction
2. The Correlation between the Structure and Function of the BBB in Both Normal and Demyelinating Brains
3. Metabolic Changes in BBB Cells
4. Microvasculature: The Involvement of NVU in MS Pathology
5. BBB in Progressive Forms of MS
6. Chronic MS DMTs with Central Effect beyond the BBB
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J.; MSCOI Study Group; European Multiple Sclerosis Platform. New insights into the burden and costs of multiple sclerosis in Europe. Mult. Scler. 2017, 23, 1123–1136. [Google Scholar] [CrossRef]
- Spencer, J.I.; Bell, J.S.; DeLuca, G.C. Vascular pathology in multiple sclerosis: Reframing pathogenesis around the blood-brain barrier. J. Neurol. Neurosurg. Psychiatry 2018, 89, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Titus, H.E.; Chen, Y.; Podojil, J.R.; Robinson, A.P.; Balabanov, R.; Popko, B.; Miller, S.D. Pre-clinical and Clinical Implications of “Inside-Out” vs. “Outside-In” Paradigms in Multiple Sclerosis Etiopathogenesis. Front. Cell Neurosci. 2020, 14, 599717. [Google Scholar] [CrossRef] [PubMed]
- Goodin, D.S.; Reder, A.T.; Bermel, R.A.; Cutter, G.R.; Fox, R.J.; John, G.R.; Lublin, F.D.; Luccinetti, C.F.; Miller, A.E.; Pelletier, D.; et al. Relapses in multiple sclerosis: Relationship to disability. Mult. Scler. Relat. Disord. 2016, 6, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Leray, E.; Yaouanq, J.; Le Page, E.; Coustans, M.; Laplaud, D.; Oger, J.; Edan, G. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010, 133, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Macías-Islas, M.A.; Flores-Alvarado, L.J.; Mireles-Ramírez, M.A.; González-Renovato, E.D.; Hernández-Navarro, V.E.; Sánchez-López, A.L.; Alatorre-Jiménez, M.A. Role of the Blood-Brain Barrier in Multiple Sclerosis. Arch. Med. Res. 2014, 45, 687–697. [Google Scholar] [CrossRef]
- Miller, D.H.; Grossman, R.I.; Reingold, S.C.; McFarland, H.F. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain 1998, 121, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Shimitzu, F.; Nishihara, H.; Kanda, T. Blood–brain barrier dysfunction in immunomediated neurological diseases. Immunol. Med. 2018, 41, 120–128. [Google Scholar] [CrossRef][Green Version]
- Xiao, M.; Xiao, Z.J.; Yang, B.; Lan, Z.; Fang, F. Blood-Brain Barrier: More Contributor to Disruption of Central Nervous System Homeostasis Than Victim in Neurological Disorders. Front. Neurosci. 2020, 14, 764. [Google Scholar] [CrossRef]
- Chow, B.W.; Gu, C. The molecular constituents of the blood-brain barrier. Trends Neurosci. 2015, 38, 598–608. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and cell biology of the blood-brain barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef] [PubMed]
- Haseloff, R.F.; Dithmer, S.; Winkler, L.; Wolburg, H.; Blasig, I.E. Transmembrane proteins of the tight junctions at the blood–brain barrier: Structural and functional aspects. Semin. Cell Dev. Biol. 2015, 38, 16–25. [Google Scholar] [CrossRef]
- Engelhardt, B. T Cell migration into the central nervous system during health and disease: Different molecular keys allow access to different central nervous system compartments. Clin. Exp. Neurimmunol. 2010, 1, 79–93. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012, 12, 623–635. [Google Scholar] [CrossRef]
- Odoardi, F.; Sie, C.; Streyl, K.; Ulaganathan, V.K.; Schläger, C.; Lodygin, D.; Heckelsmiller, K.; Nietfeld, E.; Ellwart, J.; Kilnkert, E.E.F.; et al. T cells become licensed in the lung to enter the central nervous system. Nature 2012, 488, 675–679. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef]
- Ifergan, I.; Kebir, H.; Alvarez, J.I.; Marceau, G.; Bernard, M.; Bourbonnière, L.; Poirier, J.; Duquette, P.; Talbot, P.J.; Kebir, V.; et al. Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on 4 integrin. Brain 2011, 134, 3560–3577. [Google Scholar] [CrossRef]
- Hernández-Pedro, N.Y.; Espinosa-Ramirez, G.; de la Cruz, V.P.; Pineda, B.; Sotelo, J. Initial immunopathogenesis of multiple sclerosis: Innate immune response. Clin. Dev. Immunol. 2013, 2013, 413465. [Google Scholar] [CrossRef]
- Breuer, J.; Korpos, E.; Hannocks, M.J.; Schneider-Hohendorf, T.; Song, J.; Zondles, L.; Herich, S.; Flanagan, K.; Korn, T.; Zarbock, A.; et al. Blockade of MCAM/CD146 impedes CNS infiltration of T cells over the choroid plexus. J. Neuroinflammation 2018, 15, 236. [Google Scholar] [CrossRef]
- Carrithers, M.D.; Visintin, I.; Kang, S.J.; Janeway, C.A., Jr. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain 2000, 123, 1092–1101. [Google Scholar] [CrossRef]
- Kleine, T.O.; Benes, L. Immune surveillance of the human central nervous system (CNS): Different migration pathways of immune cells through the blood–brain barrier and blood–cerebrospinal fluid barrier in healthy persons. Cytometry 2006, 69A, 147–151. [Google Scholar] [CrossRef]
- Marchetti, L.; Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2020, 2, H1–H18. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, E.; Choi, E.Y.; Chavakis, T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb. Haemost. 2009, 102, 191–197. [Google Scholar] [CrossRef]
- Mitroulis, I.; Alexaki, V.I.; Kourtzelis, I.; Ziogas, A.; Hajishengallis, G.; Chavakis, T. Leukocyte integrins: Role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol. Ther. 2015, 147, 123–135. [Google Scholar] [CrossRef]
- Carman, C.V. Mechanisms for transcellular diapedesis: Probing and pathfinding by ‘invadosome-like protrusions’. J. Cell Sci. 2009, 122, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Wolburg-Buchholz, K.; Engelhardt, B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta. Neuropathologica 2005, 109, 181–190. [Google Scholar] [CrossRef]
- Winger, R.C.; Koblinski, J.E.; Kanda, T.; Ransohoff, R.M.; Muller, W.A. Rapid remodeling of tight junctions during paracellular diapedesis in a human model of the blood-brain barrier. J. Immunol. 2014, 193, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Balasa, R.; Barcutean, L.; Balasa, A.; Motataianu, A.; Roman-Filip, C.; Manu, D. The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum. Immunol. 2020, 81, 237–243. [Google Scholar] [CrossRef]
- Johnson, A.K.; Gross, P.M. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993, 7, 678–686. [Google Scholar] [CrossRef]
- Restorick, S.M.; Durant, L.; Kalra, S.; Hassan-Smith, G.; Rahbone, E.; Douglas, M.R.; Curnow, S.J. The CCR6+ Th cells in the cerebrospinal fluid of persons with multiple sclerosis are dominated by pathogenic non-classic Th1 cells and GM-CSF-only secreting Th cells. Brain Behav. Immun. 2017, 64, 71–79. [Google Scholar] [CrossRef]
- Reboldi, A.; Coisne, C.; Baumjohann, D.; Benvenuto, F.; Bottinelli, D.; Lira, S.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B.; Sallusto, F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009, 10, 514–523. [Google Scholar] [CrossRef]
- Schulz, M.; Engelhardt, B. The circumventricular organs participate in the immunopathogenesis of experimental autoimmune encephalomyelitis. Cerebrospinal Fluid Res. 2005, 2, 8. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Treaba, A.C.; Balasa, R.; Podoleanu, D.M.; Simu, I.P.; Buruian, M.M. Cerebral lesions of multiple sclerosis: Is gadolinium always irreplaceable in assessing lesion activity? Diagn. Interv. Radiol. 2014, 20, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. Multiple sclerosis: A two-stage disease. Nat. Immunol. 2001, 2, 762–764. [Google Scholar] [CrossRef]
- Lucchinetti, C.F.; Popescu, B.F.; Bunyan, R.F.; Moll, N.M.; Roemer, S.F.; Scheithauer, B.W.; Giannini, C.; Weigand, S.D.; Mandrekar, J.; Ransohoff, R.M. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 2011, 365, 2188–2197. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.P.; Modvig, S.; Simonsen, H.J.; Fredriksen, J.L.; Larsson, H.B.W. Permeability of the blood-brain barrier predicts conversion from optic neuritis to multiple sclerosis. Brain 2015, 138, 2571–2583. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.A.; Henson, S.M.; Loiola, R.A.; Mercurio, S.; Colamatteo, A.; Maniscalco, G.T.; De Rosa, V.; McArthur, S.; Solito, E. Immuno-metabolic impact of the multiple sclerosis patients’ sera on endothelial cells of the blood-brain barrier. J. Neuroinflammation 2020, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Hanley, N.; Campbel, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Corthals, A.P. Multiple sclerosis is not a disease of the immune system. Q. Rev. Biol. 2011, 86, 287–321. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Macias-Islas, M.A.; Pacheco-Moises, F.P.; Cruz-Ramos, J.A.; Sustersik, S.; Barba, E.A.; Aguayo, A. Oxidative stress is increased in serum from Mexican patients with relapsing-remitting multiple sclerosis. Dis. Markers 2009, 26, 35–39. [Google Scholar] [CrossRef]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, D.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Balasa, R.; Maier, S.; Barcutean, L.; Stoian, A.; Motataianu, A. The direct deleterious effect of Th17 cells in the nervous system compartment in multiple sclerosis and experimental autoimmune encephalomyelitis: One possible link between neuroinflammation and neurodegeneration. Rev. Rom. Med. Lab 2020, 28, 9–18. [Google Scholar] [CrossRef]
- Lopes Pinheiro, M.A.; Kooij, G.; Mizee, M.R.; Kamermans, A.; Enzmann, G.; Lyck, R.; Schwaninger, M.; Engelhardt, B.; de Vries, H.E. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim. Biophy. Acta 2016, 1862, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.; Lassmann, H.; Turnbull, D.M. Mitochondria and disease progression in multiple sclerosis. Neuropathol. App. Neurobiol. 2008, 34, 577–589. [Google Scholar] [CrossRef] [PubMed]
- D’haeseleer, M.; Cambron, M.; Vanopdenbosch, L.; De Keyser, J. Vascular aspects of multiple sclerosis. Lancet Neurol. 2011, 10, 657–666. [Google Scholar] [CrossRef]
- Troletti, C.D.; de Goede, P.; Kamermans, A.; de Vries, H. Molecular alterations of the blood–brain barrier under inflammatory conditions: The role of endothelial to mesenchymal transition. Biochim. Biophy. Acta 2016, 1862, 452–460. [Google Scholar] [CrossRef]
- Zeiser, R. Immune modulatory effects of statins. Immunology 2018, 154, 69–75. [Google Scholar] [CrossRef]
- Youssef, S.; Stüve, O.; Patarroyo, C.; Ruiz, P.J.; Rodosevich, J.L.; Hur, M.E.; Bravo, M.; Mitchell, D.J.; Sobel, R.A.; Steinman, L.; et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002, 420, 78–84. [Google Scholar] [CrossRef]
- Stanislaus, R.; Singh, A.K.; Singh, I. Lovastatin treatment decreases mononuclear cell infiltration into the CNS of Lewis rats with experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2001, 66, 155–162. [Google Scholar] [CrossRef]
- Paintlia, A.S.; Paintlia, M.K.; Singh, A.K.; Singh, I. Modulation of RhoRocksignaling pathway protects oligodendrocytes against cytokine toxicity via PPAR-dependent mechanism. Glia 2013, 61, 1500–1517. [Google Scholar] [CrossRef]
- Pihl-Jensen, G.; Tsakiri, A.; Frederiksen, J.L. Statin Treatment in Multiple Sclerosis: A Systematic Review and Meta-Analysis. CNS Drugs 2015, 29, 277–291. [Google Scholar] [CrossRef]
- Chataway, J.; Schuerer, N.; Alsanousi, A.; Chan, D.; MacManus, D.; Hunter, K.; Anderson, V.; Bangham, C.R.M.; Clegg, S.; Nielsen, C.; et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): A randomised, placebo-controlled, phase 2 trial. Lancet 2013, 383, 2213–2221. [Google Scholar] [CrossRef]
- Williams, E.; John, N.A.; Blackstone, J.; Brownlee, W.; Frost, C.; Greenwood, J.; Chataway, J. MS-STAT2: A phase 3 trial of high dose simvastatin in secondary progressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, e13.1-e13. [Google Scholar] [CrossRef]
- Leech, S.; Kirk, J.; Plumb, J.; McQuaid, S. Persistent endothelial abnormalities and blood-brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol. Appl. Neurobiol. 2007, 33, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Frischer, J.M.; Bramow, S.; Dal-Bianco, A.; Luccinetti, C.F.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Lassmann, H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009, 132, 1175–1189. [Google Scholar] [CrossRef]
- Yates, R.L.; Esiri, M.M.; Palace, J.; Jacobs, B.; Perera, R.; DeLuca, G.C. Fibrin(ogen) and neurodegeneration in the progressive multiple sclerosis cortex. Ann. Neurol. 2017, 82, 259–270. [Google Scholar] [CrossRef]
- Dastagir, A.; Healy, B.C.; Chua, A.S.; Chitnis, T.; Weiner, H.L.; Bakshi, R.; Tauhid, S. Brain and spinal cord MRI lesions in primary progressive vs. relapsingremitting multiple sclerosis. eNeurologicalSci 2018, 12, 42–46. [Google Scholar] [CrossRef]
- Kappos, L.; Butzkueven, H.; Wiendl, H.; Spelman, T.; Pellegrini, F.; Chen, Y.; Dong, Q.; Koendgen, H.; Belachew, S.; Trojano, M.; et al. Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult. Scler. 2018, 24, 963–973. [Google Scholar] [CrossRef]
- Londono, A.C.; Mora, C.A. Evidence of disease control: A realistic concept beyond NEDA in the treatment of multiple sclerosis. F1000Research 2017, 6, 566. [Google Scholar] [CrossRef]
- Elliott, C.; Belachew, S.; Wolinsky, J.S.; Hauser, S.L.; Kappos, L.; Barkhof, F.; Bernasconi, C.; Fecker, J.; Model, F.; Wei, W.; et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 2019, 142, 2787–2799. [Google Scholar] [CrossRef]
- University of California, San Francisco MS-EPIC Team; Cree, B.A.C.; Hollenbach, J.A.; Bove, R.; Kirkish, G.; Sacco, S.; Caverzasi, E.; Bischof, A.; Gundel, T.; Zhu, A.H.; et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann. Neurol. 2019, 85, 653–666. [Google Scholar]
- Scott, L.J. Siponimod: A Review in Secondary Progressive Multiple Sclerosis. CNS Drugs 2020, 34, 1191–1200. [Google Scholar] [CrossRef]
- Balașa, R.; Maier, S.; Voidazan, S.; Hutanu, A.; Bajko, Z.; Motataianu, A.; Brandusa, T.; Tiu, C. Assessment of interleukin-17A, interleukin-10 and transforming growth factor-beta 1 serum titers in relapsing remitting multiple sclerosis patients treated with Avonex, possible biomarkers for treatment response. CNS Neurol. Disord. Drug Targets 2017, 16, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Barcutean, L.I.; Romaniuc, A.; Maier, S.; Bajko, Z.; Motataianu, A.; Hutanu, A.; Simu, I.; Andone, S.; Balasa, R. Clinical and Serological Biomarkers of Treatment’s Response in Multiple Sclerosis Patients Treated Continuously with Interferonβ-1b for More than a Decade. CNS Neurol. Disord. Drug Targets 2018, 17, 780–792. [Google Scholar] [CrossRef]
- La Mantia, L.; Vacchi, L.; Di Pietrantonj, C.; Ebers, G.; Rovaris, M.; Fredrikson, S.; Filip-pini, G. Interferon beta for secondary progressive multiple sclerosis. Cochrane Database Syst. Rev. 2012, 1, CD005181. [Google Scholar] [CrossRef]
- Kraus, J.; Oschmann, P. The impact of interferon-β treatment on the blood–brain barrier. Drug Discov. Today 2006, 11, 755–762. [Google Scholar] [CrossRef]
- Kuruganti, P.A.; Hinojoza, J.R.; Eaton, M.J.; Ehmann, U.K.; Sobel, R.A. Interferon-beta counteracts inflammatory mediatorinduced effects on brain endothelial cell tight junction molecules-implications for multiple sclerosis. J. Neuropathol. Exp. Neurol. 2002, 61, 710–724. [Google Scholar] [CrossRef][Green Version]
- Kraus, J.; Ling, A.K.; Hamm, S.; Voigt, K.; Oschmann, P.; Engelhardt, B. Interferon-beta stabilizes barrier characteristics of brain endothelial cells in vitro. Ann. Neurol. 2004, 56, 192–205. [Google Scholar] [CrossRef]
- Weber, M.S.; Hohlfeld, R.; Zamvil, S.S. Mechanism of action of glatiramer acetate in treatment of multiple sclerosis. Neurotherapeutics 2007, 4, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M. Natalizumab: A new treatment for relapsing remitting multiple sclerosis. Ther. Clin. Risk Manag. 2007, 3, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, C.; Zingaropolia, M.A.; Iannetta, M.; Rodio, D.M.; Altieri, M.; Conte, A.; Vullo, V.; Ciardi, M.R.; Palmara, A.T.; Pietropaolo, V. Which is the best PML risk stratification strategy in natalizumab-treated patients affected by multiple sclerosis? Mult. Scler. Relat. Disord. 2020, 41, 102008. [Google Scholar] [CrossRef] [PubMed]
- Balasa, R.; Ciurba, B.; Voidazan, S.; Simu, I.; Hutanu, A.; Andone, S.; Romaniuc, A.; Motataianu, A.; Maier, S. The matrix metalloproteinases panel in multiple sclerosis patients treated with Natalizumab: A possible answer to Natalizumab non-responders. CNS Neurol. Disord. Drug Targets 2018, 17, 464–472. [Google Scholar] [CrossRef]
- Bălașa, R.; Simu, M.; Voidăzan, S.; Barcutean, L.I.; Bajko, Z.; Hutanu, A.; Simu, I.; Maier, S. Natalizumab changes the peripheral profile of the Th17 panel in MS patients: New mechanisms of action. CNS Neurol. Disord. Drug Targets 2017, 16, 1018–1026. [Google Scholar] [PubMed]
- Kapoor, R.; Ho, P.R.; Campbell, N.; Chang, I.; Deykin, A.; Forrestal, F.; Lucas, N.; Yu, B.; Arnold, D.L.; Freedman, M.S.; et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): A phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018, 17, 405–415. [Google Scholar] [CrossRef]
- Avasarala, J. It’s time for combination therapies in multiple sclerosis. Innov. Clin. Neurosci. 2017, 14, 28–30. [Google Scholar]
- Mehta, D.; Miller, C.; Arnold, D.L.; Bame, E.; Bar-Or, A.; Gold, R.; Hanna, J.; Kappos, L.; Shifang, L.; Matta, A.; et al. Effect of dimethyl fumarate on lymphocytes in RRMS: Implications for clinical practice. Neurology 2019, 92, e1724–e1738. [Google Scholar] [CrossRef]
- Kemmerer, C.L.; Pempeinter, V.; Ruschil, C.; Abdelhak, A.; Scholl, M.; Ziemann, U.; Krumbholz, M.; Hemmer, B.; Kowarik, M.C. Differential effects of disease modifying drugs on peripheral blood B cell subsets: A cross sectional study in multiple sclerosis patients treated with interferon-β, glatiramer acetate, dimethyl fumarate, fingolimod or natalizumab. PLoS ONE 2020, 15, e0235499. [Google Scholar] [CrossRef]
- Mills, E.A.; Ogrodnik, M.A.; Plave, A.; Mao-Draayer, Y. Emerging Understanding of the Mechanism of Action for Dimethyl Fumarate in the Treatment of Multiple Sclerosis. Front. Neurol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Wilms, H.; Sievers, J.; Rickert, U.; Rostami-Yazdi, M.; Mrowietz, U.; Lucius, R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflammation 2010, 7, 30. [Google Scholar] [CrossRef]
- Carlström, K.E.; Ewing, E.; Granqvist, M.; Gyllenberg, A.; Aeinehband, S.; Enoksson, S.R.; Checa, A.; Tejaswi, V.S.B.; Huang, J.; Gomez-Cabrero, D.; et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat. Commun. 2019, 10, 3081. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal 2011, 15, 1607–1638. [Google Scholar] [CrossRef] [PubMed]
- Kihara, Y.; Groves, A.; Rivera, R.R.; Chun, J. Dimethyl fumarate inhibits integrin α4 expression in multiple sclerosis models. Ann. Clin. Transl. Neurol. 2015, 2, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Soin, D.; Ito, K.; Dhib-Jalbut, S. Insight into the mechanism of action of dimethyl fumarate in multiple sclerosis. J. Mol. Med. 2019, 97, 463–472. [Google Scholar] [CrossRef]
- Kourakis, S.; Timpani, C.A.; de Haan, J.B.; Gueven, N.; Fischer, D.; Rybalka, E. Dimethyl Fumarate and Its Esters: A Drug with Broad Clinical Utility? Pharmaceuticals 2020, 13, 306. [Google Scholar] [CrossRef]
- Fleischer, V.; Friedrich, M.; Rezk, A.; Buhler, U.; Witsch, E.; Uphaus, T.; Bittner, S.; Groppa, S.; Tackenberg, B.; Bar-Or, A.; et al. Treatment response to dimethyl fumarate is characterized by disproportionate CD8+ T cell reduction in MS. Mult. Scler. 2018, 24, 632–641. [Google Scholar] [CrossRef]
- Ghadiri, M.; Rezk, A.; Li, R.; Evans, A.; Luessi, F.; Zipp, F.; Giacomini, P.S.; Antel, J.; Bar-Or, A. Dimethyl fumarate-induced lymphopenia in MS due to differential T-cell subset apoptosis. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e340. [Google Scholar] [CrossRef]
- Galloway, D.A.; Williams, J.B.; Moore, C.S. Effects of fumarates on inflammatory human astrocyte responses and oligodendrocyte differentiation. Ann. Clin. Transl. Neurol. 2017, 4, 381–391. [Google Scholar] [CrossRef]
- Wang, Q.; Chuikov, S.; Taitano, S.; Wu, Q.; Rastogi, A.; Tuck, S.J.; Corey, J.M.; Lundy, S.K.; Mao-Draayer, Y. Dimethyl fumarate protects neural stem/progenitor cells and neurons from oxidative damage through Nrf2-ERK1/2 MAPK pathway. Int. J. Mol. Sci. 2015, 16, 13885–13907. [Google Scholar] [CrossRef]
- Parodi, B.; Rossi, S.; Morando, S.; Cordano, C.; Bragoni, A.; Motta, C.; Usai, C.; Wipke, B.T.; Scannevin, R.H.; Mancardi, G.L.; et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta. Neuropathol. 2015, 130, 279–295. [Google Scholar] [CrossRef]
- Filippi, M.; Rocca, M.A.; Pagani, E.; De Stefano, N.; Jeffery, D.; Kappos, L.; Montalban, X.; Boyko, A.N.; Comi, G.; ALLEGRO Study Group. Placebo-controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J. Neurol. Neurosurg. Psychiatry 2014, 85, 851–858. [Google Scholar] [CrossRef]
- Thöne, J.; Linker, R.A. Laquinimod in the treatment of multiple sclerosis: A review of the data so far. Drug Des. Devel. Ther. 2016, 10, 1111–1118. [Google Scholar] [CrossRef]
- Madeline Bross, M.; Hackett, M.; Bernitsas, E. Approved and Emerging Disease Modifying Therapies on Neurodegeneration in Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 4312. [Google Scholar] [CrossRef] [PubMed]
- Lühder, F.; Kebir, H.; Odoardi, F.; Litke, T.; Sonneck, M.; Alvarez, J.I.; Winchenbach, J.; Eckert, N.; Hayardeny, L.; Sorani, E.; et al. Laquinimod enhances central nervous system barrier functions. Neurobiol. Dis. 2017, 102, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; Jeffery, D.; Kappos, L.; Montalban, X.; Boyko, A.; Rocca, M.A.; Filippi, M. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N. Engl. J. Med. 2012, 366, 1000–1009. [Google Scholar] [CrossRef]
- Steinman, L.; Zamvil, S.S. Virtutes and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005, 26, 565–571. [Google Scholar] [CrossRef]
- Giovannoni, G.; Knappertz, V.; Steinerman, J.R.; Tansy, A.P.; Li, T.; Krieher, S.; Uccelli, A.; Uitdehaad, B.M.J.; Montalban, X.; Hartung, H.P.; et al. A randomized, placebo-controlled, phase 2 trial of laquinimod in primary progressive multiple sclerosis. Neurology 2020, 95, e1027–e1040. [Google Scholar] [CrossRef]
- Comi, G.; Vollmer, T.; Montalban, X.; Ziemssen, T.; Boyko, A.; Vermersch, P.; Rachmilewitz, T.; Sasson, N.; Gorfine, T.; Knappertz, V.; et al. Baseline characteristics of patients enrolled in CONCERTO-A study of 0.6 and 1.2 mg/day oral laquinimod for relapsing-remitting multiple sclerosis. Neurology 2015, 84, P7.216. [Google Scholar]
- Faissner, S.; Gold, R. Oral Therapies for Multiple Sclerosis. Cold Spring Harb. Perspect Med. 2019, 9, a032011. [Google Scholar] [CrossRef]
- Wegner, C.; Stadelmann, C.; Pförtner, R.; Raymond, E.; Feigelson, S.; Alon, R.; Timan, B.; Hayardeny, L.; Brück, W. Laquinimod interferes with migratory capacity of T cells and reduces IL-17 levels, inflammatory demyelination and acute axonal damage in mice with experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2010, 227, 133–143. [Google Scholar] [CrossRef]
- Jolivel, V.; Luessi, F.; Masri, J.; Kraus, H.P.; Hubo, M.; Poisa-Beiro, L.; Klebow, S.; Paterka, M.; Yogev, N.; Tumani, H.; et al. Modulation of dendritic cell properties by laquinimod as a mechanism for modulating multiple sclerosis. Brain 2013, 136, 1048–1066. [Google Scholar] [CrossRef]
- Thöne, J.; Ellrichmann, G.; Seubert, S.; Peruga, I.; Lee, D.H.; Conrad, R.; Hayardeny, L.; Comi, G.; Wiese, S.; Linker, R.A.; et al. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am. J. Pathol. 2012, 180, 267–274. [Google Scholar] [CrossRef]
- Brück, W.; Pförtner, R.; Pham, T.; Zhang, J.; Hayardeny, L.; Piryatinsky, V.; Hanisch, U.K.; Regen, T.; van Rossum, D.; Brakelmann, L.; et al. Reduced astrocytic NF-κB activation by laquinimod protects from cuprizone-induced demyelination. Acta. Neuropathol. 2012, 124, 411–424. [Google Scholar] [CrossRef]
- Zou, L.P.; Abbas, N.; Volkmann, I.; Nennesmo, I.; Levi, M.; Wahren, B.; Winblad, B.; Hedlund, G.; Zhu, J. Suppression of experimental autoimmune neuritis by ABR-215062 is associated with altered Th1/Th2 balance and inhibited migration of inflammatory cells into the peripheral nerve tissue. Neuropharmacology 2002, 42, 731–739. [Google Scholar] [CrossRef]
- Gregson, A.; Thompson, K.; Tsirka, S.E.; Selwood, D.L. Emerging small-molecule treatments for multiple sclerosis: Focus on B cells. F1000Research 2019, 8, F1000. [Google Scholar] [CrossRef]
- Van Doorn, R.; Nijland, P.G.; Dekker, N.; Witte, M.E.; Lopes-Pinheiro, M.A.; van het Hof, B.; Kooij, G.; Reijerkerk, A.; Dijkstra, C.; van van der Walk, P.; et al. Fingolimod attenuates ceramide-induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes. Acta. Neuropathol. 2012, 124, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Cyster, J.G.; Hla, T. FTY720: Sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am. J. Transplant. 2004, 4, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Mehling, M.; Brinkmann, V.; Antel, J.; Bar-Or, A.; Goebels, N.; Vedrine, C.; Kristofic, C.; Kuhle, J.; Lindberg, R.L.; Kappos, L. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 2008, 71, 1261–1267. [Google Scholar] [CrossRef]
- Kimura, A.; Ohmori, T.; Ohkawa, R.; Madoiwa, S.; Mimuro, J.; Murakami, T.; Kobayashi, E.; Hoshino, Y.; Yatomi, Y.; Sakata, Y. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem. Cells 2007, 25, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Estrada-Hernandez, T.; Paik, J.H.; Wu, M.T.; Venkataraman, K.; Brinkmann, V.; Claffey, K.; Hla, T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 2003, 278, 47281–47290. [Google Scholar] [CrossRef]
- Chun, J.; Hartung, H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.E.; Jung, C.G.; Kim, H.J.; Kennedy, T.E.; Soliven, B.; Antel, J.P. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann. Neurol. 2008, 63, 61–71. [Google Scholar] [CrossRef]
- Barske, C.; Osinde, M.; Mir, A.K.; Schwab, M.; Thallmair, M.; Klein, C.; Dev, K.K. FTY720 (fingolimod) enhances the number of progenitor and mature oligodendrocytes. Mult. Scler. 2007, 13, S148. [Google Scholar]
- Smith, P.A.; Schmid, C.; Zurbruegg, S.; Jivkov, M.; Doelemeyer, A.; Theil, D.; Dubost, V.; Beckmann, N. Fingolimod inhibits brain atrophy and promotes brain-derived neurotrophic factor in an animal model of multiple sclerosis. J. Neuroimmunol. 2018, 318, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, S.D.; Nicole, O.; Peavy, R.D.; Montoya, L.M.; Lee, C.J.; Murphy, T.J.; Traynelis, S.F.; Hepler, J.R. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol. Pharmacol. 2003, 64, 1199–1209. [Google Scholar] [CrossRef]
- Miron, V.E.; Schubart, A.; Antel, J.P. Central nervous system-directed effects of FTY720 (fingolimod). J. Neurol. Sci. 2008, 274, 13–17. [Google Scholar] [CrossRef]
- Noda, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 2013, 256, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Giovannoni, G.; Baker, D. Fingolimod modulates microglial activation to augment markers of remyelination. J. Neuroinflammation 2011, 8, 76. [Google Scholar] [CrossRef]
- Chaudhry, B.; Cohen, J.A.; Conway, D.S. Sphingosine 1-Phosphate Receptor Modulators for the Treatment of Multiple Sclerosis. Neurotherapeutics 2017, 14, 859–887. [Google Scholar] [CrossRef]
- Groves, A.; Kihara, Y.; Chun, J. Fingolimod: Direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J. Neurol. Sci. 2013, 328, 9–18. [Google Scholar] [CrossRef]
- Kappos, L.; Fox, R.J.; Burcklen, M.; Freedman, M.S.; Havrdová, E.K.; Hennessy, B.; Hohlfeld, R.; Lublin, F.; Montalban, X.; Pozzilli, C.; et al. Ponesimod Compared With Teriflunomide in Patients With Relapsing Multiple Sclerosis in the Active-Comparator Phase 3 OPTIMUM Study. A Randomized Clinical Trial. JAMA Neurol. 2021, 78, 558–567. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, X.; Zhang, L.; Shen, J. Neurovascular Unit: A critical role in ischemic stroke. CNS Neurosci. Ther. 2021, 27, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Del Zoppo, G.J. The neurovascular unit in the setting of stroke. J. Intern. Med. 2010, 267, 156–171. [Google Scholar] [CrossRef]
- Park, S.-J.; Im, D.-S. Sphingosine 1-Phosphate Receptor Modulators and Drug Discovery. Biomol. Ther. 2017, 25, 80–90. [Google Scholar] [CrossRef]
- Aloizou, A.-M.; Siokas, V.; Pateraki, G.; Liampas, I.; Bakirtzis, C.; Tsouris, Z.; Lazopoulos, G.; Calina, D.; Docea, A.O.; Tsatsakis, A.; et al. Thinking Outside the Ischemia Box: Advancements in the Use of Multiple Sclerosis Drugs in Ischemic Stroke. J. Clin. Med. 2021, 10, 630. [Google Scholar] [CrossRef]
- Elkins, J.; Veltkamp, R.; Montaner, J.; Johnston, S.C.; Singhal, A.B.; Becker, K.; Lansberg, M.G.; Tang, W.; Chang, I.; Muralidharan, K.; et al. Safety and Efficacy of Na-talizumab in Patients with Acute Ischaemic Stroke (ACTION): A Randomised, Place-bo-Controlled, Double-Blind Phase 2 Trial. Lancet Neurol. 2017, 16, 217–226. [Google Scholar] [CrossRef]
- Elkind, M.S.V.; Veltkamp, R.; Montaner, J.; Johnston, S.C.; Singhal, A.B.; Becker, K.; Lansberg, M.G.; Tang, W.; Kasliwal, R.; Elkins, J. Natalizumab in Acute Ischemic Stroke (ACTION II): A Randomized, Placebo-Controlled Trial. Neurology 2020, 95, e1091–e1104. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, N.; Ren, L.; Yan, Y.; Sun, N.; Li, Y.-J.; Han, W.; Xue, R.; Liu, Q.; Hao, J.; et al. Impact of an Immune Modulator Fingolimod on Acute Ischemic Stroke. Proc. Natl. Acad. Sci. USA 2014, 111, 18315–18320. [Google Scholar] [CrossRef]
- Zhu, Z.; Fu, Y.; Tian, D.; Sun, N.; Han, W.; Chang, G.; Dong, Y.; Xu, X.; Liu, Q.; Huang, D.; et al. Combination of the Immune Modulator Fingolimod With Alteplase in Acute Ischemic Stroke: A Pilot Trial. Circulation 2015, 132, 1104–1112. [Google Scholar] [CrossRef]
- Tian, D.; Shi, K.; Zhu, Z.; Yao, J.; Yang, X.; Su, L.; Zhang, S.; Zhang, M.; Gon-zales, R.J.; Liu, Q.; et al. Fingolimod Enhances the Efficacy of Delayed Alteplase Ad-ministration in Acute Ischemic Stroke by Promoting Anterograde Reperfusion and Retrograde Collateral Flow. Ann. Neurol. 2018, 84, 717–728. [Google Scholar] [CrossRef]
- Liantao, Z.; Jing, Z.; Lingling, L.; Hua, L. Efficacy of Fingolimod Combined with Alteplase in Acute Ischemic Stroke and Rehabilitation Nursing. Pak. J. Pharm. Sci. 2019, 32, 413–419. [Google Scholar]
- Rejdak, K.; Stelmasiak, Z.; Grieb, P. Cladribine induces long lasting oligoclonal bands disappearance in relapsing multiple sclerosis patients: 10-year observational study. Mult. Scler. Relat. Disord. 2019, 27, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, K.; Coyle, P.K.; Sylvester, E.; Galazka, A.; Dangond, F.; Grosso, M.; Leist, T.P. The Development of Cladribine Tablets for the Treatment of Multiple Sclerosis: A Comprehensive Review. Drugs 2020, 80, 1901–1928. [Google Scholar] [CrossRef]
- Leist, T.P.; Weissert, R. Cladribine: Mode of Action and Implications for Treatment of Multiple Sclerosis. Clin. Neuropharm. 2011, 34, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.Ø.; Hyrlov, K.H.; Elkjaer, M.R.; Weber, A.B.; Pedersen, A.E.; Svenningsen, A.F.; Illes, Z. Cladribine modifies functional properties of microglia. Clin. Exp. Immunol. 2020, 201, 328–340. [Google Scholar] [CrossRef]
- Mitosek-Szewczyk, K.; Stelmasiak, Z.; Bartosik-Psujek, H.; Benliak, E. Impact of cladribine on soluble adhesion molecules in multiple sclerosis. Acta. Neurol. Scand. 2010, 122, 409–413. [Google Scholar] [CrossRef]
- Smal, C.; Vertommen, D.; Bertrand, L.; Ntamashimikiro, S.; Rider, M.H.; Van Den Neste, E.; Bontemps, F. Identification of in vivo phosphorylation sites on human deoxycytidine kinase. Role of Ser-74 in the control of enzyme activity. J. Biol. Chem. 2006, 281, 4887–4893. [Google Scholar] [CrossRef]
- Beutler, E.; Sipe, J.; Romine, J.; McMillan, R.; Zyroff, J.; Koziol, J. Treatment of multiple sclerosis and other autoimmune diseases with cladribine. Semin. Hematol. 1996, 33, 45–52. [Google Scholar]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Sørensen, P.S.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef]
- Milligan, N.M.; Newcombe, R.; Compston, D.A.S. A double-blind controlled trial of high-dose methylprednisolone in patients with multiple-sclerosis.1. Clinical effects. J. Neurol. Neurosurg. Psychiatry 1987, 50, 511–516. [Google Scholar] [CrossRef]
- Sloka, J.S.; Stefanelli, M. The mechanism of action of methylprednisolone in the treatment of multiple sclerosis. Mult. Scler. 2005, 11, 425–432. [Google Scholar] [CrossRef]
- Wüst, S.; van den, B.J.; Tischner, D.; Kleiman, A.; Tuckermann, J.P.; Gold, R.; Lühder, F.; Reichardt, H.M. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. Immunol. J. 2008, 180, 8434–8443. [Google Scholar] [CrossRef] [PubMed]
- Leussink, V.I.; Jung, S.; Merschdorf, U.; Toyka, K.V.; Gold, R. Highdose methylprednisolone therapy in multiple sclerosis induces apoptosis in peripheral blood leukocytes. Arch. Neurol. 2001, 58, 91–97. [Google Scholar] [CrossRef]
- Martinez-Caceres, E.M.; Barrau, M.A.; Brieva, L.; Espejo, C.; Barbera, N.; Montalban, X. Treatment with methylprednisolone in relapses of multiple sclerosis patients: Immunological evidence of immediate and short-term but not long-lasting effects. Clin. Ecp. Immunol. 2002, 127, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Gelati, M.; Corsini, E.; Dufour, A.; Massa, G.; Giombini, S.; Solero, C.L.; Salmaggi, A. High-dose methylprednisolone reduces cytokine-induced adhesion molecules on human brain endothelium. Can. J. Neurol. Sci. 2000, 27, 241–244. [Google Scholar] [CrossRef]
- Xu, J.; Kim, G.M.; Ahmed, S.H.; Xu, J.; Yan, P.; Xu, X.M.; Hsu, C.Y. Glucocorticoid receptor-mediated suppression of activator protein-1 activation and matrix metalloproteinase expression after spinal cord injury. J. Neurosci. 2001, 21, 92–97. [Google Scholar] [CrossRef]
- Förster, C.; Silwedel, C.; Golenhofen, N.; Burek, M.; Kietz, S.; Mankertz, J.; Drenckhahn, D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 2005, 565, 475–486. [Google Scholar] [CrossRef]
- Burek, M.; Förster, C.Y. Cloning and characterization of the murine claudin-5 promoter. Mol. Cell Endocrinol. 2009, 298, 19–24. [Google Scholar] [CrossRef]
- Salvador, E.; Shityakov, S.; Förster, C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014, 355, 597–605. [Google Scholar] [CrossRef]
- Saade, C.; Bou-Fakhredin, R.; Yousem, D.M.; Asmar, K.; Naffaa, L.; El-Merhi, F. Gadolinium and Multiple Sclerosis: Vessels, Barriers of the Brain, and Glymphatics. AJNR Am. J. Neuroradio. 2018, 39, 2168–2176. [Google Scholar] [CrossRef]
- Prinz, C.; Starke, L.; Millward, L.; Fillmer, A.; Delgado, P.R.; Waiczies, H.; Pohlmann, A.; Rothe, M.; Nazare, M.; Paul, F.; et al. In vivo detection of teriflunomide-derived fluorine signal during neuroinflammation using fluorine MR spectroscopy. Theranostics 2021, 11, 2490–2504. [Google Scholar] [CrossRef]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef]
- Pardridge, W.P. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Demetzos, C. Promising Nanotechnology Approaches in Treatment of Autoimmune Diseases of Central Nervous System. Brain Sci. 2020, 10, 338. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Gonzalez-Carter, D.; Liu, X.; Tockary, T.A.; Dirisala, A.; Toh, K.; Anraku, Y.; Kataoka, K. Targeting nanoparticles to the brain by exploiting the blood–brain barrier impermeability to selectively label the brain endothelium. Proc. Natl. Acad. Sci. USA 2020, 117, 19141–19150. [Google Scholar] [CrossRef]
- Balasa, A.; Șerban, G.; Chinezu, R.; Hurghiș, C.; Tămaș, F.; Manu, D. The Involvement of Exosomes in Glioblastoma Development, Diagnosis, Prognosis, and Treatment. Brain Sci. 2020, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, U.; Putz, U.; Low, L.H.; Silke, J.; Tan, S.S.; Howitt, J. Engineered Exosomes as Vehicles for Biologically Active Proteins. Mol. Ther. 2017, 25, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef]
- Kappos, L.; Bar-Or, A.; Cree, B.A.; Fox, R.J.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnold, D.L.; Arnould, S.; Scherz, T.; et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 2018, 391, 1263–1273. [Google Scholar] [CrossRef]

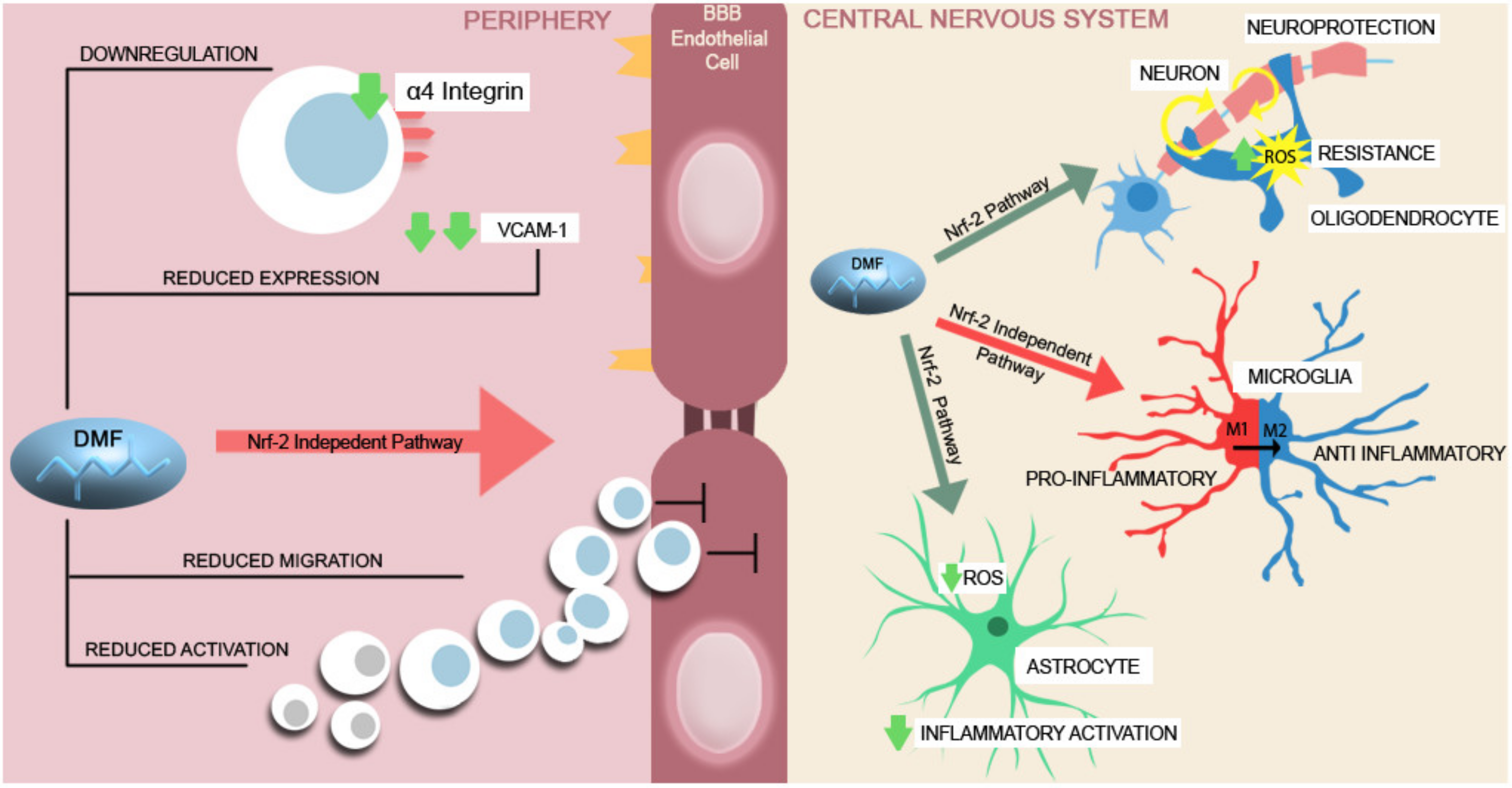
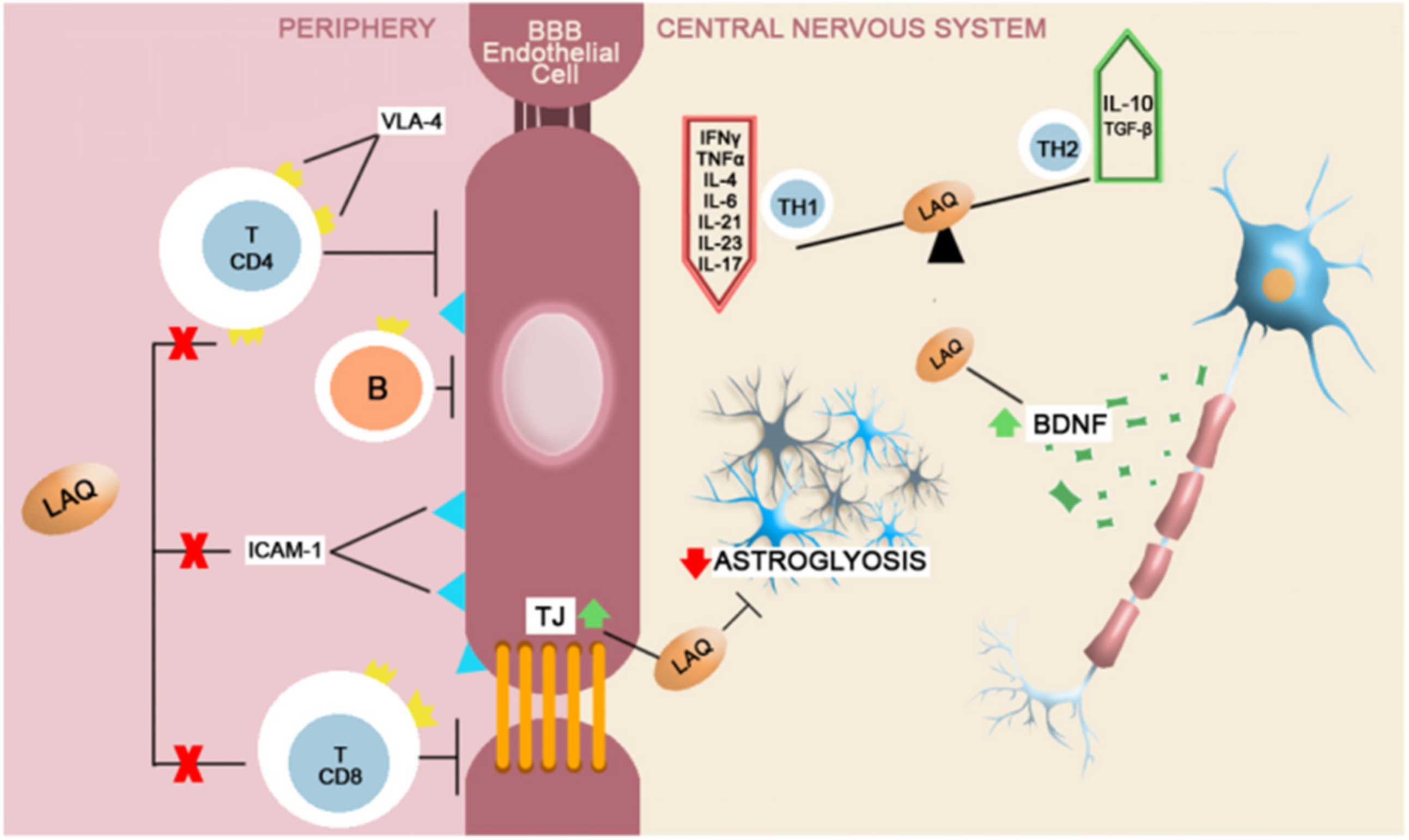
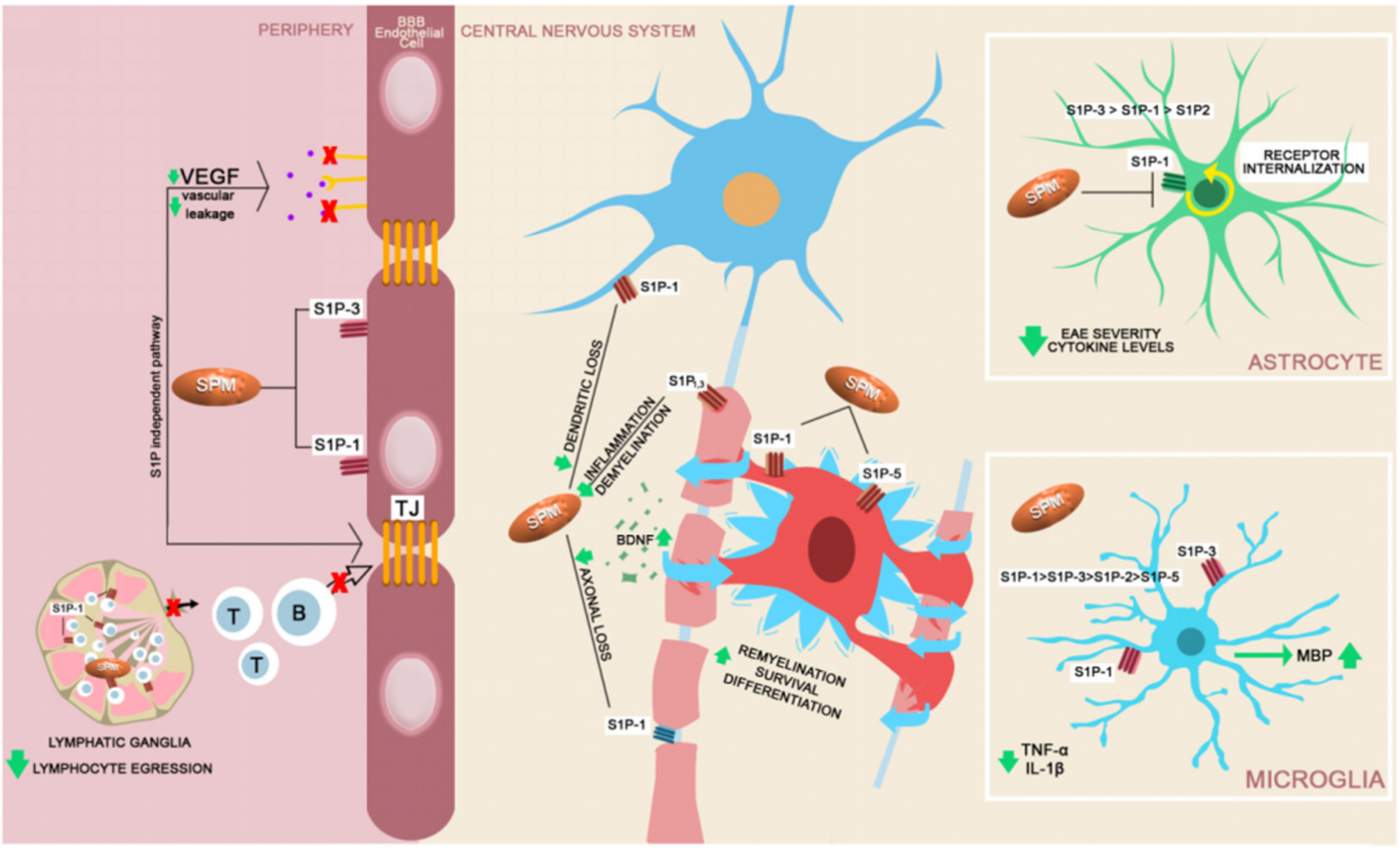

| Agent | Effects on the blood–brain barrier |
|---|---|
| Dimethyl fumarate | ↓ expression of adhesion molecules (VCAM-1 1, ICAM-1 2) [84] |
| Laquinimod | ↓ expression of adhesion molecule ICAM-1 [102] ↓ expression of MMP-9 [103] |
| Fingolimod | ↓ S1P1/S1P3 4 expression on the surface of the endothelial cells [110,111] ↓ VEGF 5 and ↓ vascular permeability [112] |
| Cladribine | ↓ICAM-1, E-selectin ↓ MMP-2, -9 [136,139] |
| Natalizumab | Blocks the interaction between the α-4 integrin (lymphocyte surface) and VCAM-1 (endothelial surface) [73] |
| Interferon-beta | Ensures structural BBB stability in murine models [69,70,71] |
| Cortisone | ↓ expression of adhesion molecules (VCAM-1, ICAM-1, E selectin) [144,148] ↓ MMP-1 and -9 [149] ↑ up-regulation of TJ (occludine, claudin) [150,151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasa, R.; Barcutean, L.; Mosora, O.; Manu, D. Reviewing the Significance of Blood–Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int. J. Mol. Sci. 2021, 22, 8370. https://doi.org/10.3390/ijms22168370
Balasa R, Barcutean L, Mosora O, Manu D. Reviewing the Significance of Blood–Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. International Journal of Molecular Sciences. 2021; 22(16):8370. https://doi.org/10.3390/ijms22168370
Chicago/Turabian StyleBalasa, Rodica, Laura Barcutean, Oana Mosora, and Doina Manu. 2021. "Reviewing the Significance of Blood–Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment" International Journal of Molecular Sciences 22, no. 16: 8370. https://doi.org/10.3390/ijms22168370
APA StyleBalasa, R., Barcutean, L., Mosora, O., & Manu, D. (2021). Reviewing the Significance of Blood–Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. International Journal of Molecular Sciences, 22(16), 8370. https://doi.org/10.3390/ijms22168370






