Phospholipids and Fatty Acids Affect the Colonization of Urological Catheters by Proteus mirabilis
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. Bacterial Growth
2.3. Estimation of Cell-Surface Hydrophobicity (CSH)
2.4. Study on the Cell Adhesion Ability
2.5. Isolation of Cell Lipids
2.6. Preparation of Bacterial Fatty Acid Methyl Esters (FAME)
2.7. Qualitative Analysis of Fatty Acids by GC-MS Technique
2.8. Quantitation of Phospholipids by LC-MS/MS Method
2.9. Fluorescent Probe Investigations
2.10. Statistical Analyses
3. Results
3.1. Growth, Cell Hydrophobicity and Adhesion of the Pathogens to a Catheter Surface
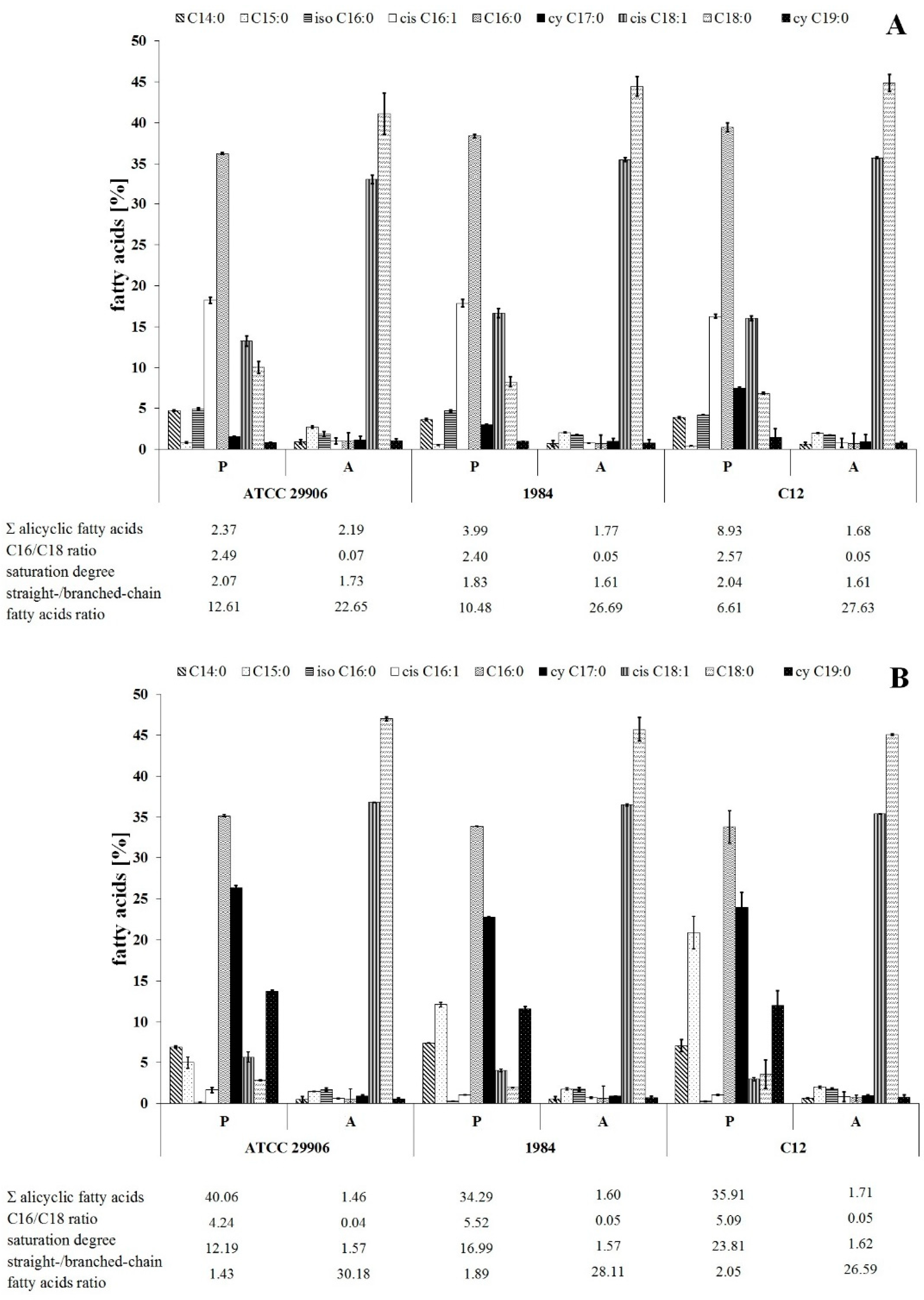
3.2. Fatty Acid Composition
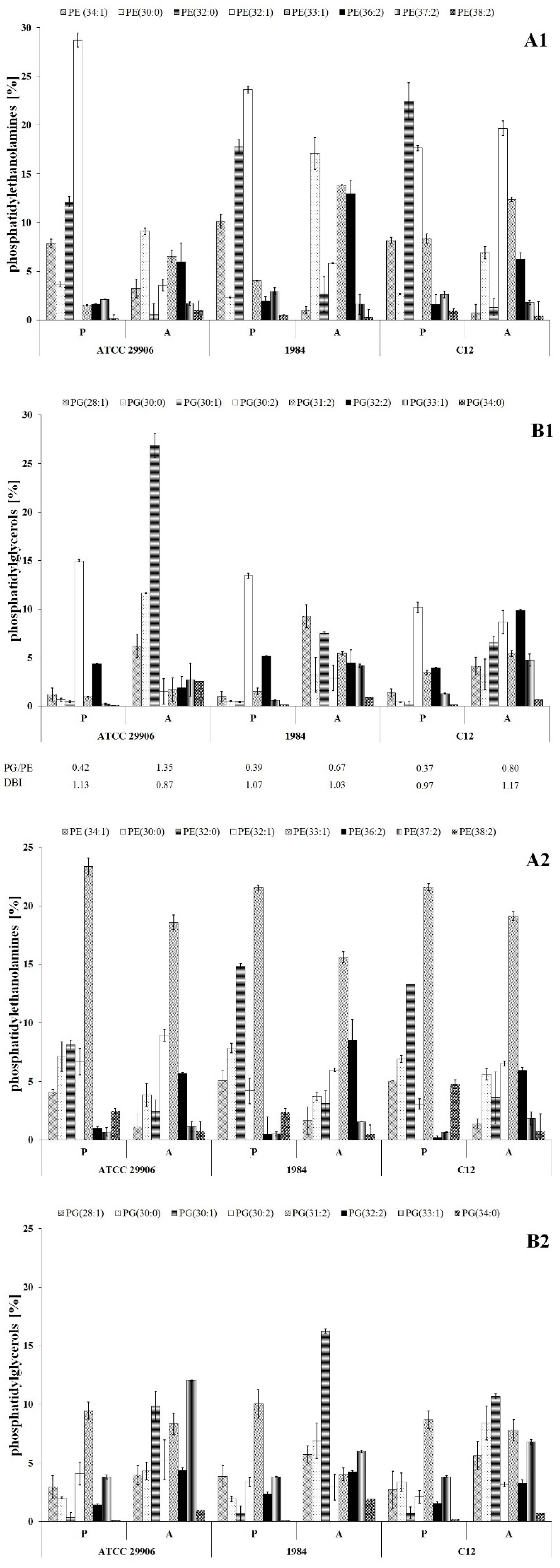
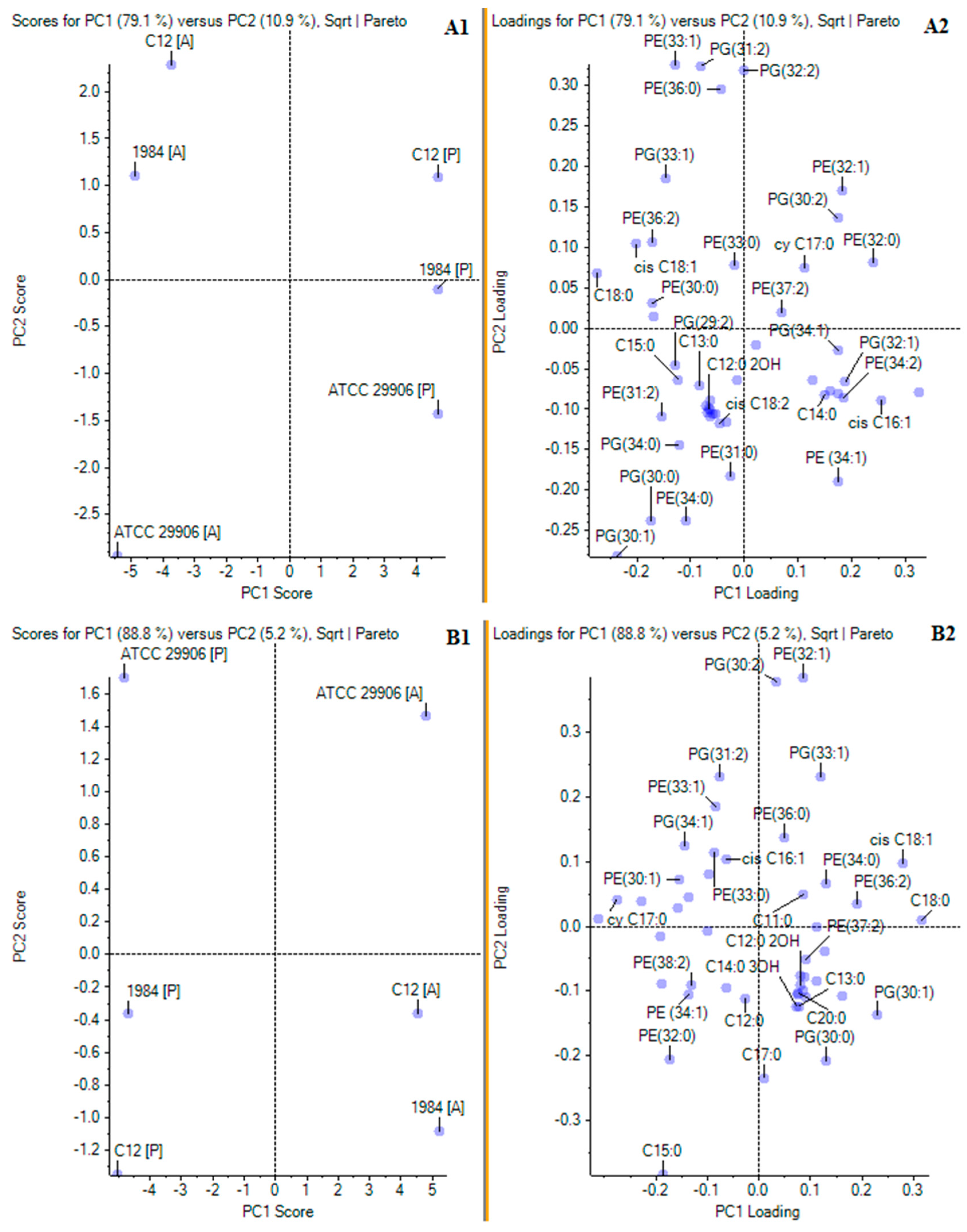
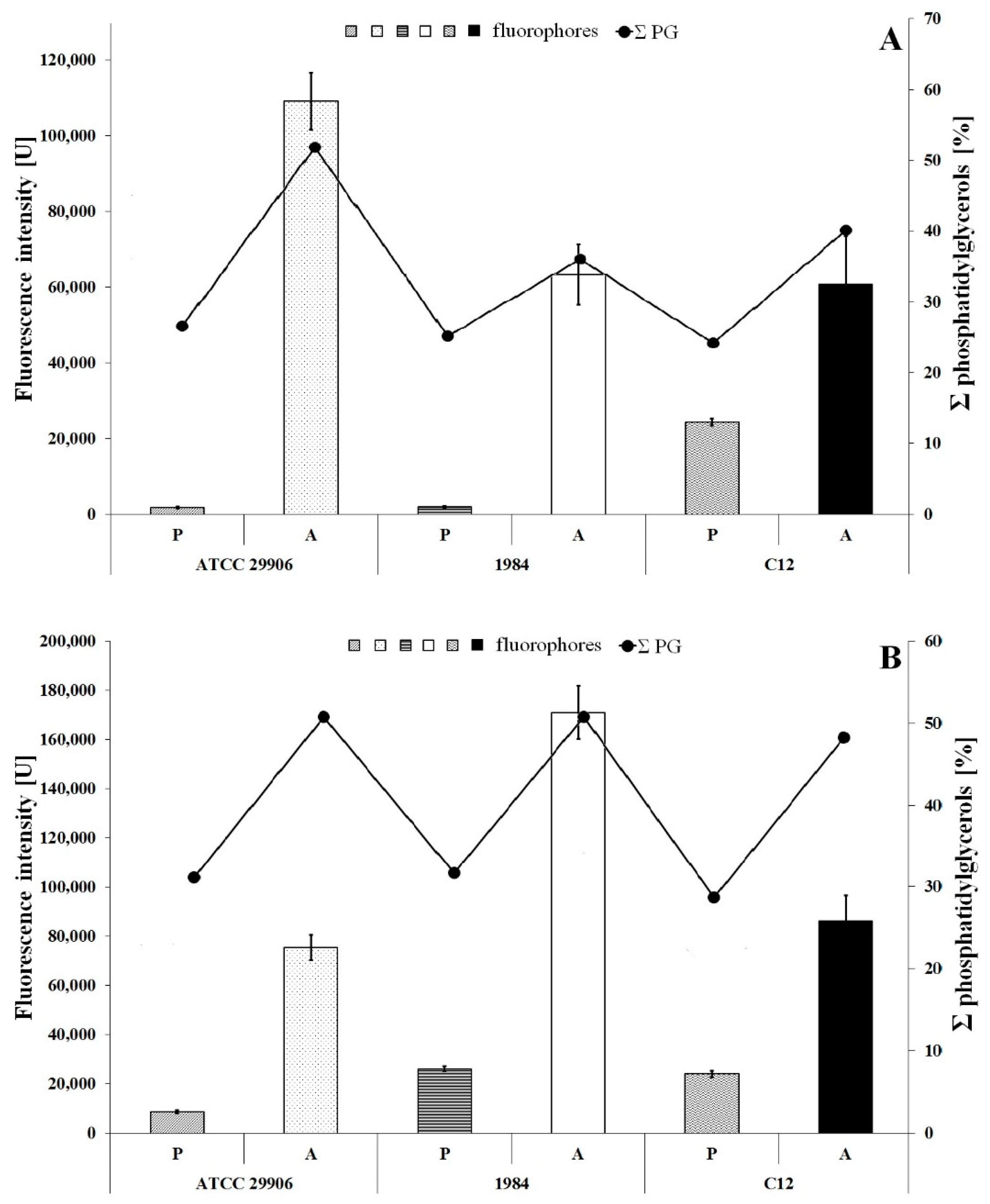
3.3. Phospholipid Profile
3.4. Inner Membrane Potential
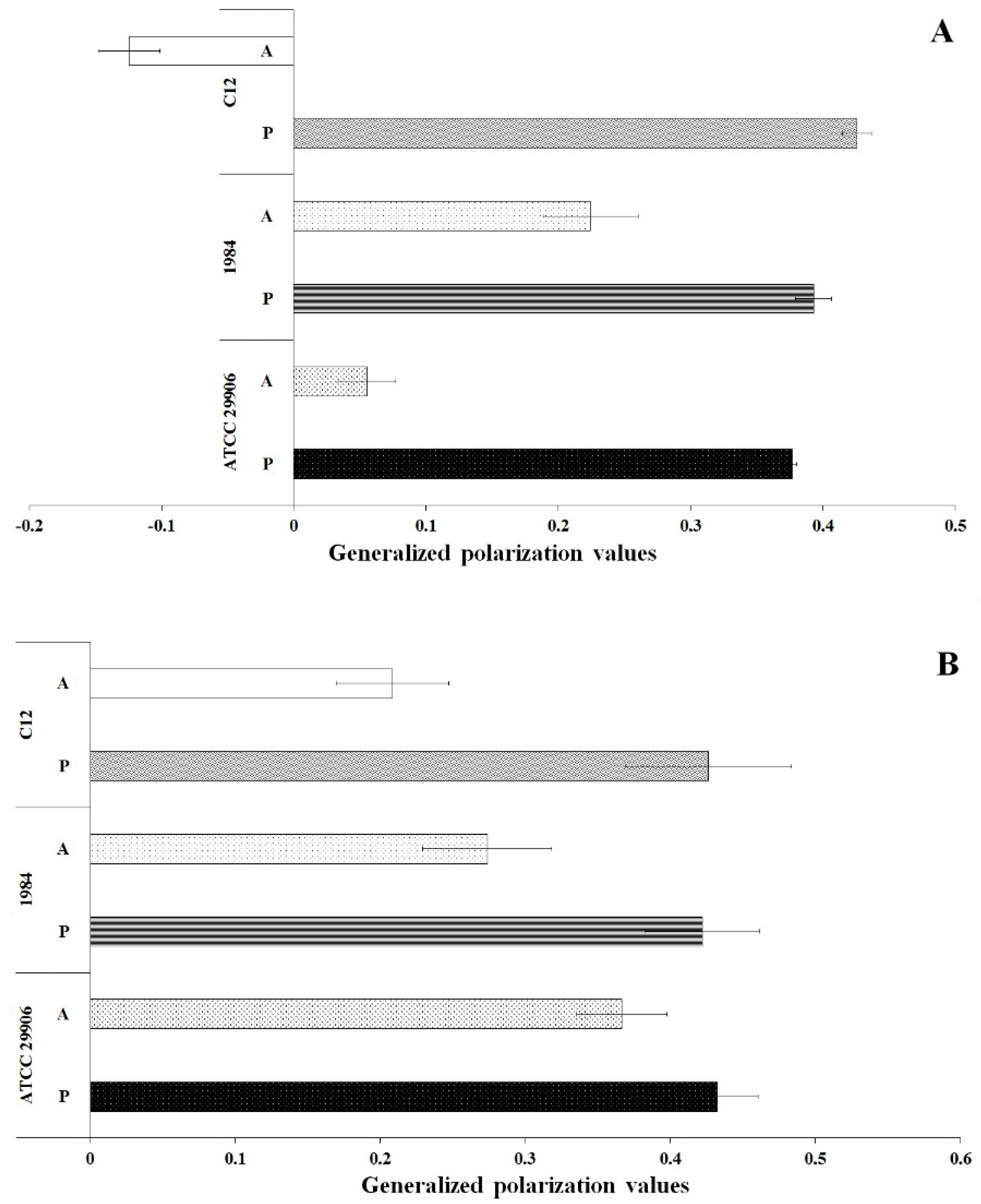
3.5. Fluidity of P. mirabilis Membranes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [CrossRef]
- Schaffer, J.N.; Pearson, M.M. Proteus mirabilis and urinary tract infections. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M. Pathogenesis of Proteus mirabilis infection. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Zabłotni, A.; Matusiak, D.; Arbatsky, N.P.; Moryl, M.; Maciejewska, A.; Kondakova, A.N.; Shashkov, A.S.; Ługowski, C.; Knirel, Y.A.; Różalski, A. Changes in the lipopolysaccharide of Proteus mirabilis 9B-m (O11a) clinical strain in response to planktonic or biofilm type of growth. Med. Microbiol. Immunol. 2018, 207, 129–139. [Google Scholar] [CrossRef]
- Kuan, L.; Schaffer, J.N.; Zouzias, C.D.; Pearson, M.M. Characterization of 17 chaperone-usher fimbriae encoded by Proteus mirabilis reveals strong conservation. J. Med. Microbiol. 2014, 63, 911–922. [Google Scholar] [CrossRef]
- Alamuri, P.; Löwer, M.; Hiss, J.A.; Himpsl, S.D.; Schneider, G.; Mobley, H.L.T. Adhesion, invasion, and agglutination mediated by two trimeric autotransporters in the human uropathogen Proteus mirabilis. Infect. Immun. 2010, 78, 4882–4894. [Google Scholar] [CrossRef]
- Pande, G. The role of membrane lipids in regulation of integrin functions. Curr. Opin. Cell. Biol. 2000, 12, 569–574. [Google Scholar] [CrossRef]
- Rowlett, V.W.; Mallampalli, V.K.P.S.; Karlstaedt, A.; Dowhan, W.; Taegtmeyer, H.; Margolin, W.; Vitrac, H. Impact of membrane phospholipid alterations in Escherichia coli on cellular function and bacterial stress adaptation. J. Bacteriol. 2017, 199, e00849-16. [Google Scholar] [CrossRef]
- Gandhi, A.; Shah, N.P. Effect of salt stress on morphology and membrane composition of Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum, and their adhesion to human intestinal epithelial-like Caco-2 cells. J. Dairy. Sci. 2016, 99, 2594–2605. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Waśko, A.; Paduch, R.; Skrzypek, T.; Sroka-Bartnicka, A. The effect of cell surface components on adhesion ability of Lactobacillus rhamnosus. Antonie Van Leeuwenhoek 2014, 106, 751–762. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell. Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef]
- Poortinga, A.T.; Bos, R.; Norde, W.; Busscher, H.J. Electric double layer interactions in bacterial adhesion to surfaces. Surf. Sci. Rep. 2002, 47, 197–301. [Google Scholar] [CrossRef]
- Prindle, A.; Liu, J.; Asally, M.; Ly, S.; Garcia-Ojalvo, J.; Süel, G.M. Ion channels enable electrical communication in bacterial communities. Nature 2015, 527, 59–63. [Google Scholar] [CrossRef]
- Lundberg, M.E.; Becker, E.C.; Choe, S. MstX and a putative potassium channel facilitate biofilm formation in Bacillus subtilis. PLoS ONE 2013, 30, e60993. [Google Scholar] [CrossRef]
- Rottem, S.; Markowitz, O.; Razin, S. Cerulenin-induced changes in the lipopolysaccharide content and phospholipid composition of Proteus mirabilis. Eur. J. Biochem. 1978, 85, 451–456. [Google Scholar] [CrossRef]
- Onaolapo, J.A.; Klemperer, R.M.M. The effect of environmental iron concentration on R-plasmid RPl-mediated changes in the lipopolysaccharide and fatty acid composition of Proteus mirabilis. FEMS Microbiol. Lett. 1998, 51, 141–144. [Google Scholar] [CrossRef]
- Rottem, S.; Markowitz, O.; Razin, S. Thermal regulation of the fatty acid composition of lipopolysaccharides and phospholipids of Proteus mirabilis. Eur. J. Biochem. 1978, 85, 445–450. [Google Scholar] [CrossRef]
- Sud, I.J.; Feingold, D.S. Mechanism of polymyxin B resistance in Proteus mirabilis. J. Bacteriol. 1970, 104, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, J.; Martin, H.H. Phospholipid and lipopolysaccharide in Proteus mirabilis and its stable protoplast L-form: Difference in content and fatty acid composition. Eur. J. Biochem. 1976, 67, 487–494. [Google Scholar] [CrossRef]
- Onaolapo, J.A.; Klemperer, R.M. Effect of R-plasmid RP1 on surface hydrophobicity of Proteus mirabilis. J. Gen. Microbiol. 1986, 132, 3303–3307. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Grela, E.; Ząbek, A.; Grabowiecka, A. Interferences in the optimization of the MTT assay for viability estimation of Proteus mirabilis. Avicenna J. Med. Biotechnol. 2015, 7, 159–167. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Stolarek, P.; Różalska, S.; Bernat, P. Lipidomic adaptations of the Metarhizium robertsii strain in response to the presence of butyltin compounds. Biochim. Biophys. Acta Biomembr. 2019, 1861, 316–326. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid. Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid. Res. 2010, 51, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Bernat, P.; Nykiel-Szymańska, J.; Stolarek, P.; Słaba, M.; Szewczyk, R.; Różalska, S. 2,4-dichlorophenoxyacetic acid-induced oxidative stress: Metabolome and membrane modifications in Umbelopsis isabellina, a herbicide degrader. PLoS ONE 2018, 13, e0199677. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Bremer, D.J.; Jeannotte, R.; Welti, R.; Yang, C. Membrane lipid composition and heat tolerance in cool-season turfgrasses, including a hybrid bluegrass. J. Am. Soc. Hortic. Sci. 2009, 134, 511–520. [Google Scholar] [CrossRef]
- Hofstetter, S.; Denter, C.; Winter, R.; McMullen, L.M.; Gänzle, M.G. Use of the fluorescent probe LAURDAN to label and measure inner membrane fluidity of endospores of Clostridium spp. J. Microbiol. Methods. 2012, 91, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Scavone, P.; Villar, S.; Umpiérrez, A.; Zunino, P. Role of Proteus mirabilis MR/P fimbriae and flagella in adhesion, cytotoxicity and genotoxicity induction in T24 and Vero cells. Pathog. Dis. 2015, 73, ftv017. [Google Scholar] [CrossRef] [PubMed]
- Zunino, P.; Sosa, V.; Allen, A.G.; Preston, A.; Schlapp, G.; Maskell, D.J. Proteus mirabilis fimbriae (PMF) are important for both bladder and kidney colonization in mice. Microbiology 2003, 149, 3231–3237. [Google Scholar] [CrossRef]
- Jiang, W.; Ubhayasekera, W.; Breed, M.C.; Norsworthy, A.N.; Serr, N.; Mobley, H.L.T.; Pearson, M.M.; Knight, S.D. MrpH, a new class of metal-binding adhesin, requires zinc to mediate biofilm formation. PLoS Pathog. 2020, 11, e1008707. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.P.D.; Pelayo, J.S.; Elias, W.P. Fimbriae of uropathogenic Proteus mirabilis. FEMS Immunol. Med. Microbiol. 2007, 51, 1–7. [Google Scholar] [CrossRef]
- Scavone, P.; Iribarnegaray, V.; Caetano, A.L.; Schlapp, G.; Härtel, S.; Zunino, P. Fimbriae have distinguishable roles in Proteus mirabilis biofilm formation. Pathog. Dis. 2016, 74, ftw033. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Chien, H.F.; Huang, K.T.; Lin, W.Y.; Liaw, S.J. cAMP receptor protein regulates mouse colonization, motility, fimbria-mediated adhesion, and stress tolerance in uropathogenic Proteus mirabilis. Sci. Rep. 2017, 7, 7282. [Google Scholar] [CrossRef]
- Clapham, L.; McLean, R.J.C.; Nickel, J.C.; Downey, J.; Costerton, J.W. The influence of bacteria on struvite crystal habit and its importance in urinary stone formation. J. Cryst. Growth. 1990, 104, 475–484. [Google Scholar] [CrossRef]
- Jiang, S.S.; Liu, M.C.; Teng, L.J.; Wang, W.B.; Hsueh, P.R.; Liaw, S.J. Proteus mirabilis pmrI, an RppA-regulated gene necessary for polymyxin B resistance, biofilm formation, and urothelial cell invasion. Antimicrob. Agents Chemother. 2010, 54, 1564–1571. [Google Scholar] [CrossRef]
- Oder, M.; Kompare, B.; Bohince, K.; Torkar, K.G. The impact of material surface roughness and temperature on the adhesion of Legionella pneumophila to contact surfaces. Int. J. Environ. Health Res. 2015, 25, 469–479. [Google Scholar] [CrossRef]
- May, H.C.; Yu, J.J.; Shrihari, S.; Seshu, J.; Klose, K.E.; Cap, A.P.; Chambers, J.P.; Guentzel, M.N.; Arulanandam, B.P. Thioredoxin modulates cell surface hydrophobicity in Acinetobacter baumannii. Front. Microbiol. 2019, 10, 2849. [Google Scholar] [CrossRef]
- Stancu, M.M. Response mechanisms in Serratia marcescens IBBPo15 during organic solvents exposure. Curr. Microbiol. 2016, 73, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, F.; Lagha, R.; Said, K.; Kallel, H.; Gharbi, J. Detection of cell surface hydrophobicity, biofilm and fimbirae genes in Salmonella isolated from tunisian clinical and poultry meat. Iran. J. Public Health 2014, 43, 423–431. [Google Scholar] [PubMed]
- Jadhav, S.; Hussain, A.; Devi, S.; Kumar, A.; Parveen, S.; Gandham, N.; Wieler, L.H.; Ewers, C.; Ahmed, N. Virulence characteristics and genetic affinities of multiple drug resistant uropathogenic Escherichia coli from a semi urban locality in India. PLoS ONE 2011, 6, e18063. [Google Scholar] [CrossRef]
- Peerbooms, P.G.; Verweij, A.M.; MacLaren, D.M. Uropathogenic properties of Proteus mirabilis and Proteus vulgaris. J. Med. Microbiol. 1985, 19, 55–60. [Google Scholar] [CrossRef]
- Mitsuyama, J.; Itoh, Y.; Takahata, M.; Okamoto, S.; Yasuda, T. In vitro antibacterial activities of tosufloxacin against and uptake of tosufloxacin by outer membrane mutants of Escherichia coli, Proteus mirabilis, and Salmonella typhimurium. Antimicrob. Agents Chemother. 1992, 36, 2030–2036. [Google Scholar] [CrossRef][Green Version]
- Czerwonka, G.; Guzy, A.; Kałuża, K.; Grosicka, M.; Dańczuk, M.; Lechowicz, Ł.; Gmiter, D.; Kowalczyk, P.; Kaca, W. The role of Proteus mirabilis cell wall features in biofilm formation. Arch. Microbiol. 2016, 198, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Bartodziejska, B.; Błaszczyk, A.; Wykrota, M.; Kwil, I.; Babicka, D.; Różalski, A. Investigation of hydrophobicity of Proteus vulgaris strains and ability of Proteus vulgaris and Proteus penneri strains to penetrate bladder membrane HCV T-29 cells (Article in Polish). Med. Doświad. Mikrobiol. 2002, 54, 335–344. [Google Scholar]
- Drumm, B.; Neumann, A.W.; Policova, Z.; Sherman, P.M. Bacterial cell surface hydrophobicity properties in the mediation of in vitro adhesion by the rabbit enteric pathogen Escherichia coli strain RDEC-1. J. Clin. Invest. 1989, 84, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Vanhaecke, E.; Remon, J.P.; Moors, M.; Raes, F.; De Rudder, D.; Van Peteghem, A. Kinetics of Pseudomonas aeruginosa adhesion to 304 and 316-L stainless steel: Role of cell surface hydrophobicity. Appl. Environ. Microbiol. 1990, 56, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, T.; Sperling, S.; Seifert, J.; von Bergen, M.; Steiniger, F.; Wick, L.Y.; Heipieper, H.J. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl. Environ. Microbiol. 2012, 78, 6217–6224. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.J.; Flores-Mireles, A.L. Urinary catheter coating modifications: The race against catheter-associated infections. Coatings 2020, 10, 23. [Google Scholar] [CrossRef]
- van Merode, A.E.J.; van der Mei, H.C.; Busscher, H.J.; Krom, B.P. Influence of culture heterogeneity in cell surface charge on adhesion and biofilm formation by Enterococcus faecalis. J. Bacteriol. 2006, 188, 2421–2426. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Turner, M.S.; Dykes, G.A. Influence of cell surface hydrophobicity on attachment of Campylobacter to abiotic surfaces. Food Microbiol. 2011, 28, 942–950. [Google Scholar] [CrossRef]
- Gilbert, P.; Evans, D.J.; Evans, E.; Duguid, I.G.; Brown, M.R.W. Surface characteristics and adhesion of Escherichia coli and Staphylococcus epidermidis. J. Appl. Microbiol. 1991, 71, 72–77. [Google Scholar] [CrossRef]
- Manna, S.; Ghanty, C.; Baindara, P.; Barik, T.K.; Mandal, S.M. Electrochemical communication in biofilm of bacterial community. J. Basic Microbiol. 2020, 60, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, J.M.; Asally, M. Microbiologist’s guide to membrane potential dynamics. Trends Microbiol. 2020, 28, 304–314. [Google Scholar] [CrossRef]
- Siciliano, J.C.; Toutant, M.; Derkinderen, P.; Sasaki, T.; Girault, J.A. Differential regulation of proline-rich tyrosine kinase 2/cell adhesion kinase beta (PYK2/CAKbeta) and pp125(FAK) by glutamate and depolarization in rat hippocampus. J. Biol. Chem. 1996, 271, 28942–28946. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Zeng, X.A.; Liu, Z.W.; Brennan, C.S. Variations in cellular membrane fatty acid composition of Escherichia coli in resistance to pulsed electric fields induced by eugenol. J. Food Process. Preserv. 2018, 42, e13740. [Google Scholar] [CrossRef]
- Nesbitt, J.A., III; Lennarz, W.J. Comparison of lipids and lipopolysaccharide from bacillary and L forms of Proteus P18. J. Bacteriol. 1965, 89, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, Y.; Fang, Y.; Huang, Z.; Kan, B.; Wang, D. Proteus alimentorum sp. nov. isolated from pork and lobster in Ma’anshan city, China. Int. J. Syst. Evol. Microbiol. 2018, 68, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.C.; da Fonseca, M.M. Preventing biofilm formation: Promoting cell separation with terpenes. FEMS Microbiol. Ecol. 2007, 61, 406–413. [Google Scholar] [CrossRef]
- Moorman, M.A.; Thelemann, C.A.; Zhou, S.; Pestka, J.J.; Linz, J.E.; Ryser, E.T. Altered hydrophobicity and membrane composition in stress-adapted Listeria innocua. J. Food Prot. 2008, 71, 182–185. [Google Scholar] [CrossRef]
- Lagha, R.; Ben Abdallah, F.; Ellafi, A.; Bekir, K.; Bakhrouf, A. Biofilm formation, cell surface hydrophobicity, and fatty acids analysis of starved Salmonella enterica serovar Typhimurium in seawater. Foodborne. Pathog. Dis. 2012, 9, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek, E.; Sałek, K.; Guzik, U.; Dudzińska-Bajorek, B. Cell surface properties and fatty acids composition of Stenotrophomonas maltophilia under the influence of hydrophobic compounds and surfactants. N. Biotechnol. 2013, 30, 173–182. [Google Scholar] [CrossRef]
- Nishida, T.; Hori, R.; Morita, N.; Okuyama, H. Membrane eicosapentaenoic acid is involved in the hydrophobicity of bacterial cells and affects the entry of hydrophilic and hydrophobic compounds. FEMS Microbiol. Lett. 2010, 306, 91–96. [Google Scholar] [CrossRef]
- Hori, R.; Nishida, T.; Okuyama, H. Hydrophilic and hydrophobic compounds antithetically affect the growth of eicosapentaenoic acid-synthesizing Escherichia coli recombinants. Open. Microbiol. J. 2011, 5, 114–118. [Google Scholar] [CrossRef]
- Bentley-Hewitt, K.; Narbad, A.; Majsak-Newman, G. Lactobacilli survival and adhesion to colonic epithelial cell lines is dependent on long chain fatty acid exposure. Eur. J. Lipid. Sci. Technol. 2017, 119, 1700062. [Google Scholar] [CrossRef]
- Mrozik, A.; Piotrkowska-Seget, Z.; Łabużek, S. Cytoplasmatic bacterial membrane responses to environmental perturbations. Pol. J. Environ. 2004, 13, 478–494. [Google Scholar]
- Skotland, T.; Sandvig, K. The role of PS 18:0/18:1 in membrane function. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Gianotti, A.; Serrazanetti, D.; Kamdem, S.S.; Guerzoni, M.E. Involvement of cell fatty acid composition and lipid metabolism in adhesion mechanism of Listeria monocytogenes. Int. J. Food. Microbiol. 2008, 123, 9–17. [Google Scholar] [CrossRef]
- Iimura, Y.; Hara, S.; Otsuka, K. Fatty acids as hydrophobic substance on cell surface of film strain of Saccharomyces. Agric. Biol. Chem. 1980, 44, 1223–1229. [Google Scholar] [CrossRef]
- Nakayama, R.; Kumagal, H.; Tochikura, T. gamma-Glutamyltranspeptidase from Proteus mirabilis: Localization and activation by phospholipids. J. Bacteriol. 1984, 160, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Rottem, S.; Hasin, M.; Razin, S. The outer membrane of Proteus mirabilis II. The extractable lipid fraction and electron-paramagnetic resonance analysis of the outer and cytoplasmic membranes. Biochim. Biophys. Acta. Biomembr. 1975, 375, 395–405. [Google Scholar] [CrossRef]
- Ming, X.; Daeschel, M.A. Correlation of cellular phospholipid content with nisin resistance of Listeria monocytogenes scott A. J. Food. Prot. 1995, 58, 416–420. [Google Scholar] [CrossRef]
- Paul, S.; Chaudhuri, K.; Chatterjee, A.N.; Das, J. Presence of exposed phospholipids in the outer membrane of Vibrio cholerae. J. Gen. Microbiol. 1992, 138, 755–761. [Google Scholar] [CrossRef]
- Hart, M.E.; Champlin, F.R. Susceptibility to hydrophobic molecules and phospholipid composition in Pasteurella multocida and Actinobacillus lignieresii. Antimicrob. Agents. Chemother. 1988, 32, 1354–1359. [Google Scholar] [CrossRef]
- Yu, C.; Li, M.; Sun, Y.; Wang, X.; Chen, Y. Phosphatidylethanolamine deficiency impairs Escherichia coli adhesion by downregulating lipopolysaccharide synthesis, which is reversible by high galactose/lactose cultivation. Cell. Commun. Adhes. 2017, 23, 1–10. [Google Scholar] [CrossRef]
- Benamara, H.; Rihouey, C.; Jouenne, T.; Alexandre, S. Impact of the biofilm mode of growth on the inner membrane phospholipid composition and lipid domains in Pseudomonas aeruginosa. Biochim. Biophys. Acta 2011, 1808, 98–105. [Google Scholar] [CrossRef]
- Casares, D.; Escriba, P.V.; Rossello, C.A. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef]
- Wan, N.; Wang, H.; Ng, C.K.; Mukherjee, M.; Ren, D.; Cao, B.; Tang, Y.J. Bacterial metabolism during biofilm growth investigated by 13C tracing. Front. Microbiol. 2018, 9, 2657. [Google Scholar] [CrossRef]
- Alim, D.; Sircaik, S.; Panwar, S.L. The significance of lipids to biofilm formation in Candida albicans: An emerging perspective. J. Fungi 2018, 4, 140. [Google Scholar] [CrossRef]
- Pisithkul, T.; Schroeder, J.W.; Trujillo, E.A.; Yeesin, P.; Stevenson, D.M.; Chaiamarit, T.; Coon, J.J.; Wang, J.D.; Amador-Noguez, D. Metabolic remodeling during biofilm development of Bacillus subtilis. mBio 2019, 10, e00623-19. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.A.; Tricerri, M.A.; Gratton, E. Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 7314–7319. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Matsumoto, S.; Kasai, T.; Yoshizawa, E.; Okamoto, S.; Yoshikawa, H.Y.; Taniguchi, H.; Takebe, T. Defining lineage-specific membrane fluidity signatures that regulate adhesion kinetics. Stem. Cell Rep. 2018, 11, 852–860. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolarek, P.; Bernat, P.; Szczerbiec, D.; Różalski, A. Phospholipids and Fatty Acids Affect the Colonization of Urological Catheters by Proteus mirabilis. Int. J. Mol. Sci. 2021, 22, 8452. https://doi.org/10.3390/ijms22168452
Stolarek P, Bernat P, Szczerbiec D, Różalski A. Phospholipids and Fatty Acids Affect the Colonization of Urological Catheters by Proteus mirabilis. International Journal of Molecular Sciences. 2021; 22(16):8452. https://doi.org/10.3390/ijms22168452
Chicago/Turabian StyleStolarek, Paulina, Przemysław Bernat, Dominika Szczerbiec, and Antoni Różalski. 2021. "Phospholipids and Fatty Acids Affect the Colonization of Urological Catheters by Proteus mirabilis" International Journal of Molecular Sciences 22, no. 16: 8452. https://doi.org/10.3390/ijms22168452
APA StyleStolarek, P., Bernat, P., Szczerbiec, D., & Różalski, A. (2021). Phospholipids and Fatty Acids Affect the Colonization of Urological Catheters by Proteus mirabilis. International Journal of Molecular Sciences, 22(16), 8452. https://doi.org/10.3390/ijms22168452





