Sirolimus Suppresses Phosphorylation of Cofilin and Reduces Interstitial Septal Thickness in Sporadic Lymphangioleiomyomatosis
Abstract
:1. Introduction
2. Results
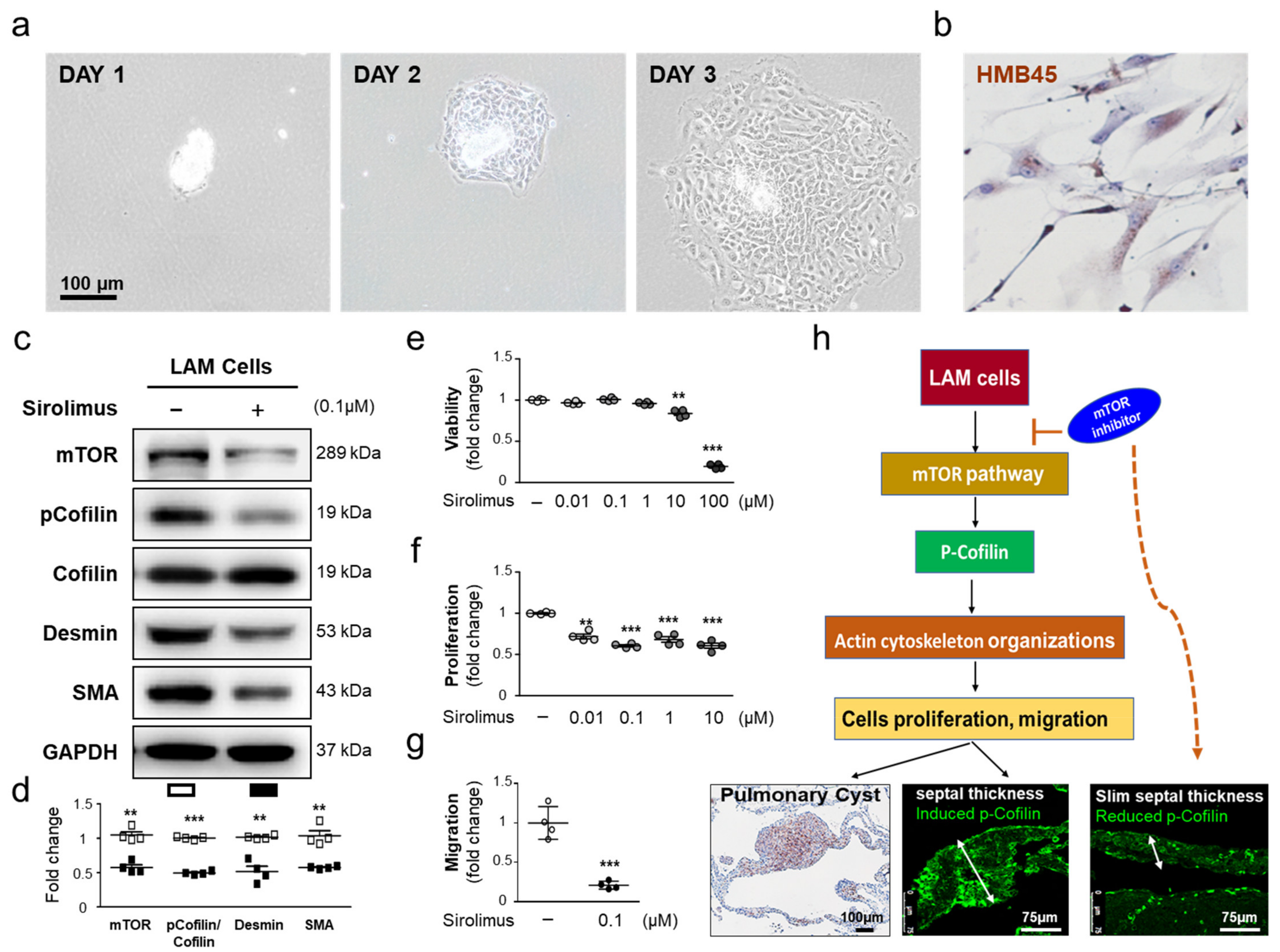
2.1. Pathological and Histological Analysis of LAM Patient Lung Tissues
2.2. Sirolimus Suppresses mTOR Expression in Lung Tissue of Patients with LAM
2.3. Interstitial Septal Thickness Is a Marker for the Efficacy of Sirolimus in LAM
2.4. The Effect of Sirolimus on p-Cofilin in the Interstitial LAM Septum
2.5. Sirolimus, an mTOR Inhibitor, Downregulates p-Cofilin Expression in LAM Cells
3. Discussion
4. Materials and Methods
4.1. Characteristics of Patient Lung Tissue
4.2. Immunohistochemical Analysis
4.3. Assessment of Cystic Area, and Interstitial Septal Thickness
4.4. Isolation and Culture of LAM Cells
4.5. Western Blot Analysis Characteristics of Patient Lung Tissue
4.6. Cell Viability and Proliferation Assay
4.7. Cell Migration Assay
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSLT | bilateral sequential lung transplantation |
| CT | computed tomography |
| ECMO | extracorporeal membrane oxygenation |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HRCT | high-resolution computed tomography |
| ILD | interstitial lung disease |
| LAM | lymphangioleiomyomatosis |
| LIMK | LIM kinase |
| LT | lung transplantation |
| mTOR | mammalian target of rapamycin |
| mTORC1 | the mammalian target of rapamycin complex 1 |
| PKA | protein kinase A |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| Rheb | Ras homolog enriched in brain |
| SEM | standard error of the mean |
| SLT | Single LT |
| SSh1 | Slingshot-1 |
| TESK1 | Testis-specific kinase 1 |
| TORSC | Taiwan Organ Registry and Sharing Center |
| TSC | tuberous sclerosis complex |
| TSC1 | Hamartin |
| TSC2 | tuberin |
| VEGF-D | vascular endothelial growth factor-D |
References
- Johnson, S.R. Lymphangioleiomyomatosis. Eur. Respir. J. 2006, 27, 1056–1065. [Google Scholar] [CrossRef] [Green Version]
- Ando, K.; Tobino, K.; Kurihara, M.; Kataoka, H.; Doi, T.; Hoshika, Y.; Takahashi, K.; Seyama, K. Quantitative CT analysis of small pulmonary vessels in lymphangioleiomyomatosis. Eur. J. Radiol. 2012, 81, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Meraj, R.; Wikenheiser-Brokamp, K.A.; Young, L.R.; McCormack, F.X. Lymphangioleiomyomatosis: New Concepts in Pathogenesis, Diagnosis, and Treatment. Semin. Respir. Crit. Care Med. 2012, 33, 486–497. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.; Li, Y.; Li, H.; Yang, P.; Xing, X. Solitary extrapulmonary lymphangioleiomyomatosis of the liver: A case report and literature review. Exp. Ther. Med. 2016, 12, 1499–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wienecke, R.; König, A.; DeClue, J.E. Identification of Tuberin, the Tuberous Sclerosis-2 Product. Tuberin Possesses Specific Rap1gap Activity. J. Biol. Chem. 1995, 270, 16409–16414. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Rodríguez, G.; Steagall, W.K.; Samsel, L.; Dagur, P.K.; McCoy, J.P.; Tunc, I.; Pirooznia, M.; Wang, J.-A.; Darling, T.N.; Moss, J. Circulating Lymphangioleiomyomatosis Tumor Cells With Loss of Heterozygosity in the TSC2 Gene Show Increased Aldehyde Dehydrogenase Activity. Chest 2019, 156, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Van Slegtenhorst, M.; de Hoogt, R.; Hermans, C.; Nellist, M.; Janssen, B.; Verhoef, S.; Lindhout, D.; Ouweland, A.V.D.; Halley, D.; Young, J.; et al. Identification of the Tuberous Sclerosis Gene TSC1 on Chromosome 9q34. Science 1997, 277, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Rosner, M.; Hanneder, M.; Siegel, N.; Valli, A.; Hengstschläger, M. The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat. Res. Mutat. Res. 2008, 658, 234–246. [Google Scholar] [CrossRef]
- Huang, J.; Manning, B.D. The TSC1–TSC2 complex: A molecular switchboard controlling cell growth. Biochem. J. 2008, 412, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Mazhab-Jafari, M.T.; Marshall, C.B.; Ishiyama, N.; Ho, J.; Di Palma, V.; Stambolic, V.; Ikura, M. An Autoinhibited Noncanonical Mechanism of GTP Hydrolysis by Rheb Maintains mTORC1 Homeostasis. Structure 2012, 20, 1528–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarbassov, D.D.; Ali, S.M.; Kim, D.-H.; Guertin, D.A.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Rictor, a Novel Binding Partner of mTOR, Defines a Rapamycin-Insensitive and Raptor-Independent Pathway that Regulates the Cytoskeleton. Curr. Biol. 2004, 14, 1296–1302. [Google Scholar] [CrossRef] [Green Version]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Rüegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- McCormack, F.X.; Inoue, Y.; Moss, J.; Singer, L.G.; Strange, C.; Nakata, K.; Barker, A.F.; Chapman, J.T.; Brantly, M.L.; Stocks, J.M.; et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 2011, 364, 1595–1606. [Google Scholar] [CrossRef]
- Yao, J.; Taveira-DaSilva, A.M.; Jones, A.M.; Julien-Williams, P.; Stylianou, M.; Moss, J. Sustained Effects of Sirolimus on Lung Function and Cystic Lung Lesions in Lymphangioleiomyomatosis. Am. J. Respir. Crit. Care Med. 2014, 190, 1273–1282. [Google Scholar] [CrossRef] [Green Version]
- Taveira-DaSilva, A.M.; Jones, A.M.; Julien-Williams, P.; Stylianou, M.; Moss, J. Long-Term Effect of Sirolimus on Serum Vascular Endothelial Growth Factor D Levels in Patients With Lymphangioleiomyomatosis. Chest 2018, 153, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wu, X.; Xu, W.; Tian, X.; Yang, Y.; Wang, S.-T.; Liu, S.; Xu, X.; Xu, K.-F. Long-term efficacy and safety of sirolimus therapy in patients with lymphangioleiomyomatosis. Orphanet. J. Rare Dis. 2019, 14, 206. [Google Scholar] [CrossRef]
- Ando, K.; Okada, Y.; Akiba, M.; Kondo, T.; Kawamura, T.; Okumura, M.; Chen, F.; Date, H.; Shiraishi, T.; Iwasaki, A.; et al. Lung Transplantation for Lymphangioleiomyomatosis in Japan. PLoS ONE 2016, 11, e0146749. [Google Scholar] [CrossRef]
- Wang, W.; Mouneimne, G.; Sidani, M.; Wyckoff, J.; Chen, X.; Makris, A.; Goswami, S.; Bresnick, A.R.; Condeelis, J.S. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J. Cell Biol. 2006, 173, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Huang, Y.; Zhao, J.; Wu, L.; Qi, Q.; Liu, Y.; Li, G.; Li, J.; Liu, H.; Wu, H. Cofilin: A Promising Protein Implicated in Cancer Metastasis and Apoptosis. Front. Cell Dev. Biol. 2021, 9, 599065. [Google Scholar] [CrossRef]
- Li, J.; Yang, R.; Yang, H.; Chen, S.; Wang, L.; Li, M.; Yang, S.; Feng, Z.; Bi, J. NCAM regulates the proliferation, apoptosis, autophagy, EMT, and migration of human melanoma cells via the Src/Akt/mTOR/cofilin signaling pathway. J. Cell. Biochem. 2020, 121, 1192–1204. [Google Scholar] [CrossRef]
- Ohashi, K.; Fujiwara, S.; Watanabe, T.; Kondo, H.; Kiuchi, T.; Sato, M.; Mizuno, K. LIM Kinase Has a Dual Role in Regulating Lamellipodium Extension by Decelerating the Rate of Actin Retrograde Flow and the Rate of Actin Polymerization. J. Biol. Chem. 2011, 286, 36340–36351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estenne, M.; De Francquen, P.; Wellens, F.; Leclerc, J.-L.; Vanderhoeft, P.; Yernault, J.-C.; Primo, G. Combined heart-and-lung transplantation for lymphangioleiomyomatosis. Lancet 1984, 323, 275. [Google Scholar] [CrossRef]
- Tanaka, H.; Imada, A.; Morikawa, T.; Shibusa, T.; Satoh, M.; Sekine, K.; Abe, S. Diagnosis of pulmonary lymphangioleiomyomatosis by HMB45 in surgically treated spontaneous pneumothorax. Eur. Respir. J. 1995, 8, 1879–1882. [Google Scholar] [CrossRef] [Green Version]
- Ballou, L.M.; Lin, R.Z. Rapamycin and mTOR kinase inhibitors. J. Chem. Biol. 2008, 1, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, K.G.; Fingar, D.C. Mammalian Target of Rapamycin (mTOR): Conducting the Cellular Signaling Symphony. J. Biol. Chem. 2010, 285, 14071–14077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Finlay, G.A.; Kotloff, R.M.; Strange, C.; Wilson, K.C.; Young, L.R.; Taveira-DaSilva, A.M.; Johnson, S.; Cottin, V.; Sahn, S.A.; et al. Lymphangioleiomyomatosis Diagnosis and Management: High-Resolution Chest Computed Tomography, Transbronchial Lung Biopsy, and Pleural Disease Management. An Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2017, 196, 1337–1348. [Google Scholar] [CrossRef]
- Johnson, S.R.; Cordier, J.F.; Lazor, R.; Cottin, V.; Costabel, U.; Harari, S.; Reynaud-Gaubert, M.; Boehler, A.; Brauner, M.; Popper, H.; et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur. Respir. J. 2010, 35, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northrup, H.; Krueger, D.A. Tuberous sclerosis complex diagnostic criteria update: Recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 2013, 49, 243–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henske, E.P.; McCormack, F.X. Lymphangioleiomyomatosis—a wolf in sheep’s clothing. J. Clin. Investig. 2012, 122, 3807–3816. [Google Scholar] [CrossRef] [Green Version]
- Huber, K.M.; Klann, E.; Costa-Mattioli, M.; Zukin, R.S. Dysregulation of Mammalian Target of Rapamycin Signaling in Mouse Models of Autism. J. Neurosci. 2015, 35, 13836–13842. [Google Scholar] [CrossRef]
- Ghosh, M.; Song, X.; Mouneimne, G.; Sidani, M.; Lawrence, D.S.; Condeelis, J.S. Cofilin Promotes Actin Polymerization and Defines the Direction of Cell Motility. Science 2004, 304, 743–746. [Google Scholar] [CrossRef] [Green Version]
- Prass, M.; Jacobson, K.; Mogilner, A.; Radmacher, M. Direct measurement of the lamellipodial protrusive force in a migrating cell. J. Cell Biol. 2006, 174, 767–772. [Google Scholar] [CrossRef]
- Collazo, J.; Zhu, B.; Larkin, S.; Martin, S.K.; Pu, H.; Horbinski, C.; Koochekpour, S.; Kyprianou, N. Cofilin drives cell-invasive and metastatic responses to TGF-beta in prostate cancer. Cancer Res. 2014, 74, 2362–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krymskaya, V. Smooth Muscle like Cells in Pulmonary Lymphangioleiomyomatosis. Proc. Am. Thorac. Soc. 2008, 5, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steagall, W.K.; Pacheco-Rodriguez, G.; Glasgow, C.G.; Ikeda, Y.; Lin, J.-P.; Zheng, G.; Moss, J. Osteoprotegerin Contributes to the Metastatic Potential of Cells with a Dysfunctional TSC2 Tumor-Suppressor Gene. Am. J. Pathol. 2013, 183, 938–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steagall, W.K.; Pacheco-Rodriguez, G.; Darling, T.N.; Torre, O.; Harari, S.; Moss, J. The Lymphangioleiomyomatosis Lung Cell and Its Human Cell Models. Am. J. Respir. Cell Mol. Biol. 2018, 58, 678–683. [Google Scholar] [CrossRef]
- Julian, L.M.; Delaney, S.P.; Wang, Y.; Goldberg, A.A.; Doré, C.; Yockell-Lelièvre, J.; Tam, R.; Giannikou, K.; McMurray, F.; Shoichet, M.S.; et al. Human Pluripotent Stem Cell–Derived TSC2-Haploinsufficient Smooth Muscle Cells Recapitulate Features of Lymphangioleiomyomatosis. Cancer Res. 2017, 77, 5491–5502. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Yu, J.J.; Perl, A.K.; Wikenheiser-Brokamp, K.A.; Riccetti, M.; Zhang, E.Y.; Sudha, P.; Adam, M.; Potter, A.; Kopras, E.J.; et al. Single Cell Transcriptomic Analysis Identifies a Unique Pulmonary Lymphangioleiomyomatosis Cell. Am. J. Respir. Crit. Care Med. 2020, 202, 1373–1387. [Google Scholar] [CrossRef]
- Karbowniczek, M.; Astrinidis, A.; Balsara, B.R.; Testa, J.R.; Lium, J.H.; Colby, T.V.; McCormack, F.X.; Henske, E.P. Recurrent lymphangiomyomatosis after transplantation: Genetic analyses reveal a metastatic mechanism. Am. J. Respir. Crit. Care Med. 2003, 167, 976–982. [Google Scholar] [CrossRef]
- Bittmann, I.; Rolf, B.; Amann, G.; Löhrs, U. Recurrence of lymphangioleiomyomatosis after single lung transplantation: New insights into pathogenesis. Hum. Pathol. 2003, 34, 95–98. [Google Scholar] [CrossRef]
- Wang, W.; Eddy, R.J.; Condeelis, J. The cofilin pathway in breast cancer invasion and metastasis. Nat. Rev. Cancer 2007, 7, 429–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; Pacheco-Rodriguez, G.; Haughey, M.; Samsel, L.; Xu, S.; Wu, H.-P.; McCoy, J.P.; Stylianou, M.; Darling, T.; Moss, J. Sirolimus decreases circulating lymphangioleiomyomatosis cells in patients with lymphangioleiomyomatosis. Chest 2014, 145, 108–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-J.; Hsu, H.-C.; Ho, W.-J.; Chang, G.-J.; Pang, J.-H.S.; Chen, W.-J.; Huang, C.-C.; Lai, Y.-J. Cathepsin S promotes the development of pulmonary arterial hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L1–L13. [Google Scholar] [CrossRef] [PubMed]




| LAM | LAM/Sirolimus | |
|---|---|---|
| Subjects n | 7 | 6 |
| Male/Female | 0/7 | 0/6 |
| Recipient age at listing (years) | 48.43 (38.21–55.75) | 45.17 (37.01–53.33) |
| Recipient age at transplant (years) | 48.43 (41.11–55.75) | 46.50 (37.95–55.05) |
| FEV1 (% predicted) | 29.83 (7.49–52.17) | 21.70 (14.73–28.67) |
| FEV1/FVC | 40.66 (21.69–59.62) | 33.85 (29.62–38.08) |
| DLco (ml/min/mmHg) | 5.14 (4.50–5.77) | 3.88 (1.47–6.29) |
| mPAP (mmHg) | 22.43 (16.16–28.70) | 28.40 (21.10–35.70) |
| PVR (Wood Unit) | 2.31 (1.17–3.45) | 3.22 (2.01–4.43) |
| CO (L/min/m2) | 4.24 (3.74–4.74) | 4.72 (3.81–5.63) |
| 6MWD (m) | 280.8 (137.02–424.58) | 291 (171.75–410.25) |
| Septum (μm) | 502.91 (457.63–548.19) | 278.83 (212.5–345.15) *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-L.; Chen, P.-R.; Lai, Y.-J.; Hsu, H.-H. Sirolimus Suppresses Phosphorylation of Cofilin and Reduces Interstitial Septal Thickness in Sporadic Lymphangioleiomyomatosis. Int. J. Mol. Sci. 2021, 22, 8564. https://doi.org/10.3390/ijms22168564
Huang Y-L, Chen P-R, Lai Y-J, Hsu H-H. Sirolimus Suppresses Phosphorylation of Cofilin and Reduces Interstitial Septal Thickness in Sporadic Lymphangioleiomyomatosis. International Journal of Molecular Sciences. 2021; 22(16):8564. https://doi.org/10.3390/ijms22168564
Chicago/Turabian StyleHuang, Yen-Lin, Po-Ru Chen, Ying-Ju Lai, and Hsao-Hsun Hsu. 2021. "Sirolimus Suppresses Phosphorylation of Cofilin and Reduces Interstitial Septal Thickness in Sporadic Lymphangioleiomyomatosis" International Journal of Molecular Sciences 22, no. 16: 8564. https://doi.org/10.3390/ijms22168564
APA StyleHuang, Y.-L., Chen, P.-R., Lai, Y.-J., & Hsu, H.-H. (2021). Sirolimus Suppresses Phosphorylation of Cofilin and Reduces Interstitial Septal Thickness in Sporadic Lymphangioleiomyomatosis. International Journal of Molecular Sciences, 22(16), 8564. https://doi.org/10.3390/ijms22168564






