Effect of Neprilysin Inhibition for Ischemic Mitral Regurgitation after Myocardial Injury
Abstract
:1. Introduction
2. Results
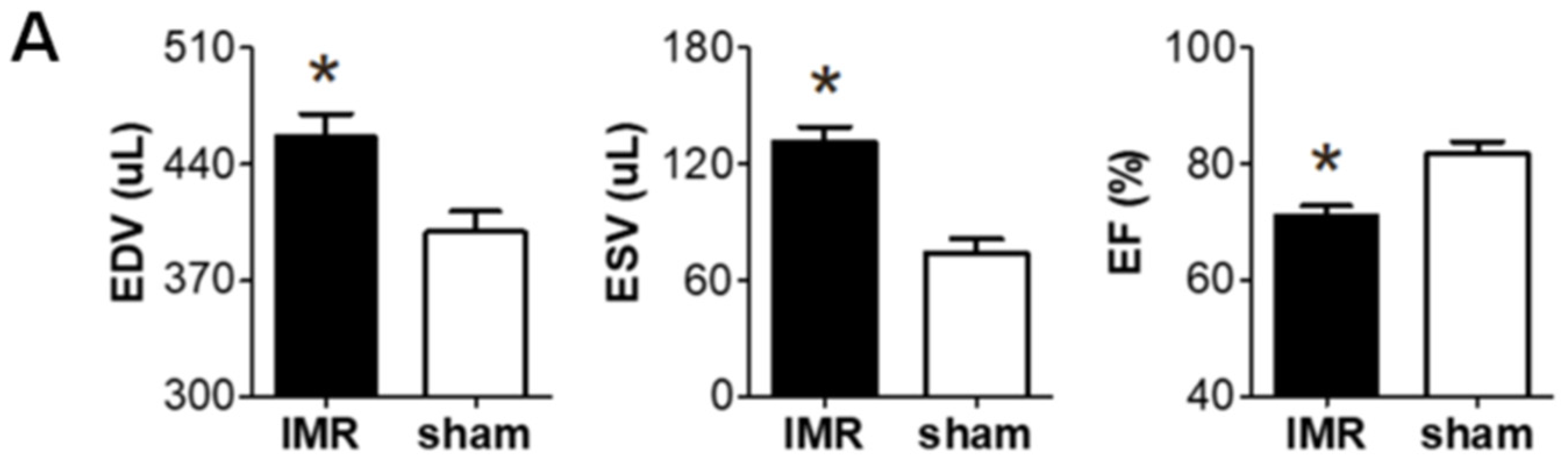
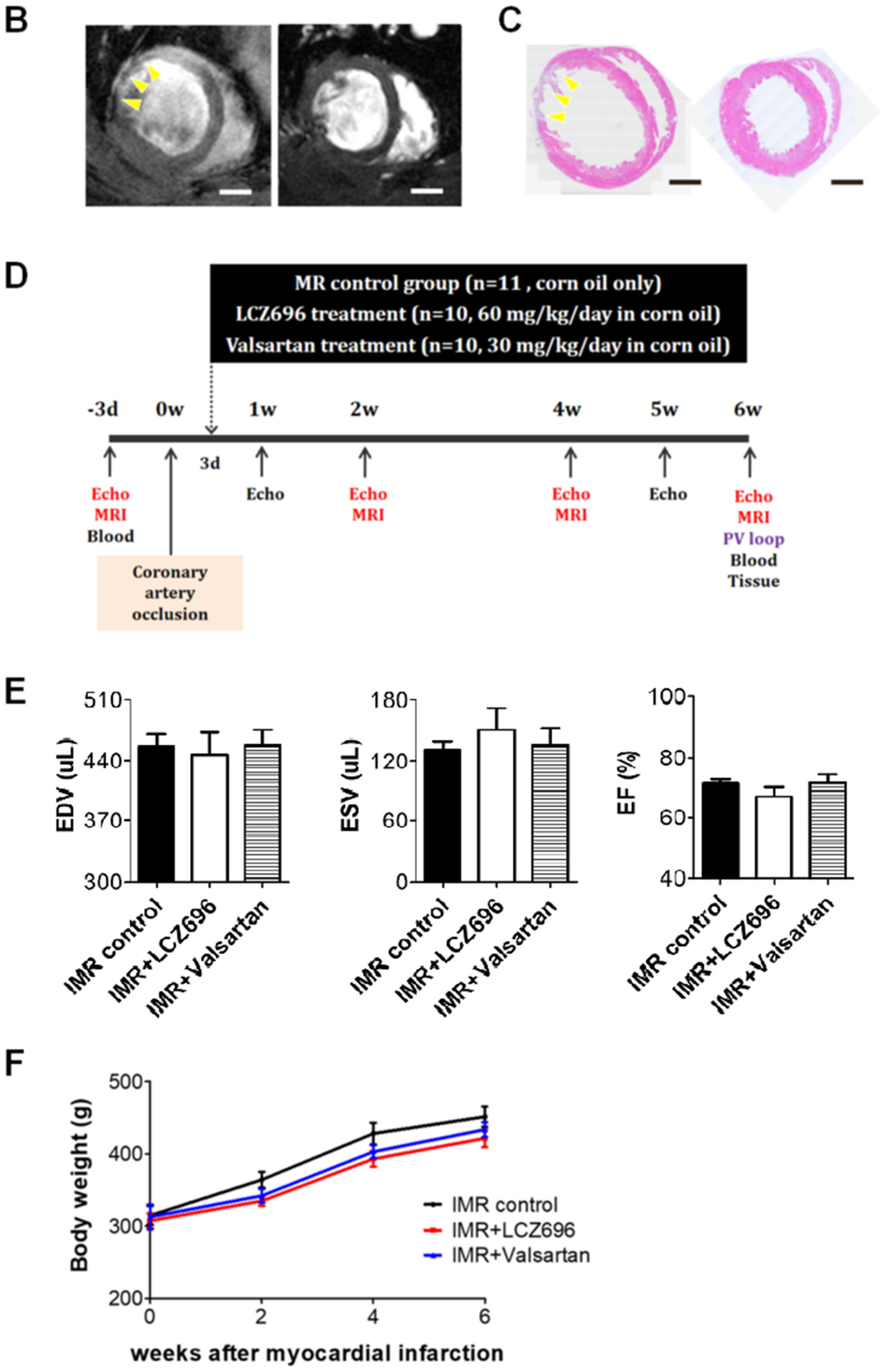
2.1. Left Ventricular Remodeling after Myocardial Infarction
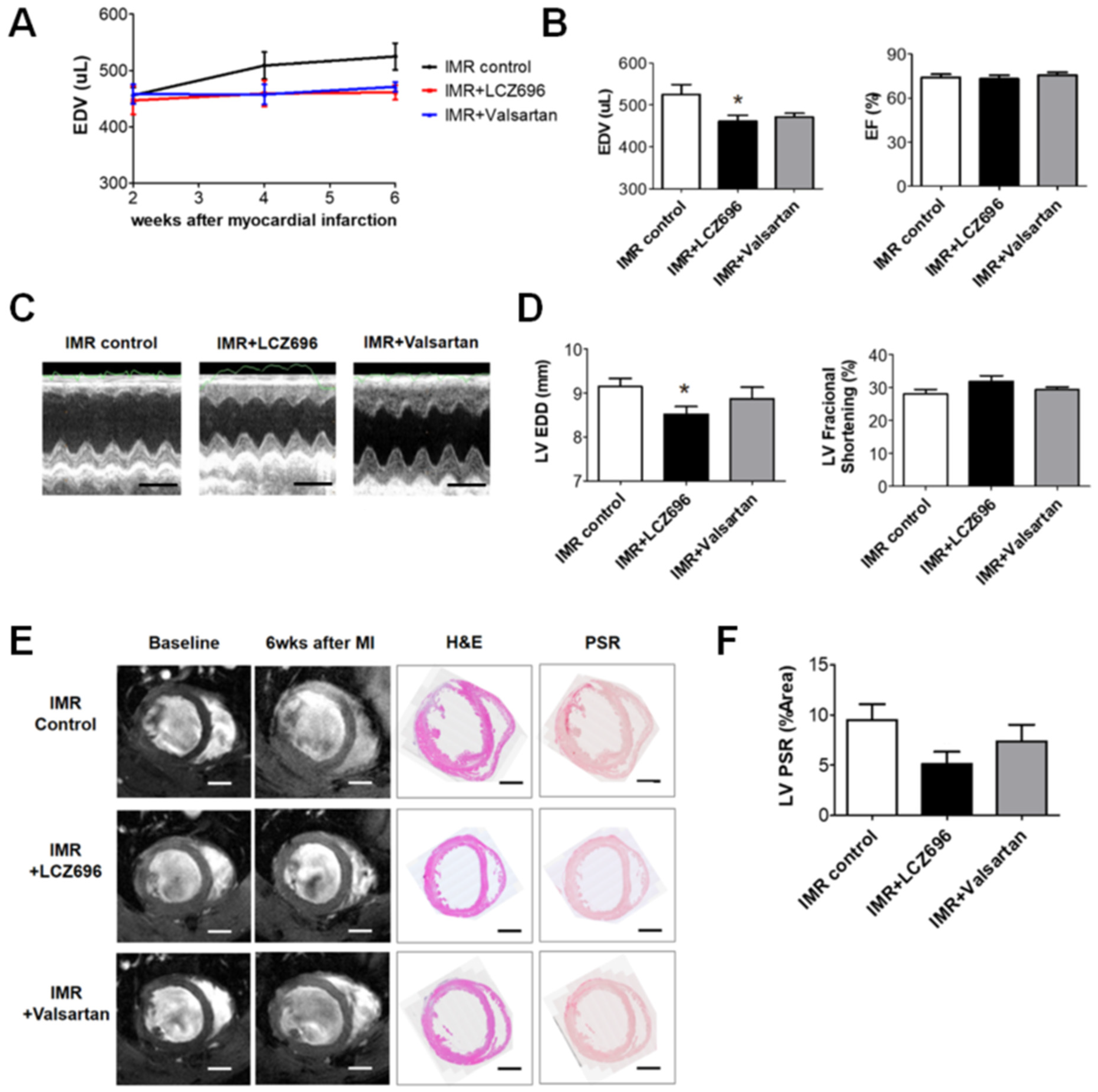
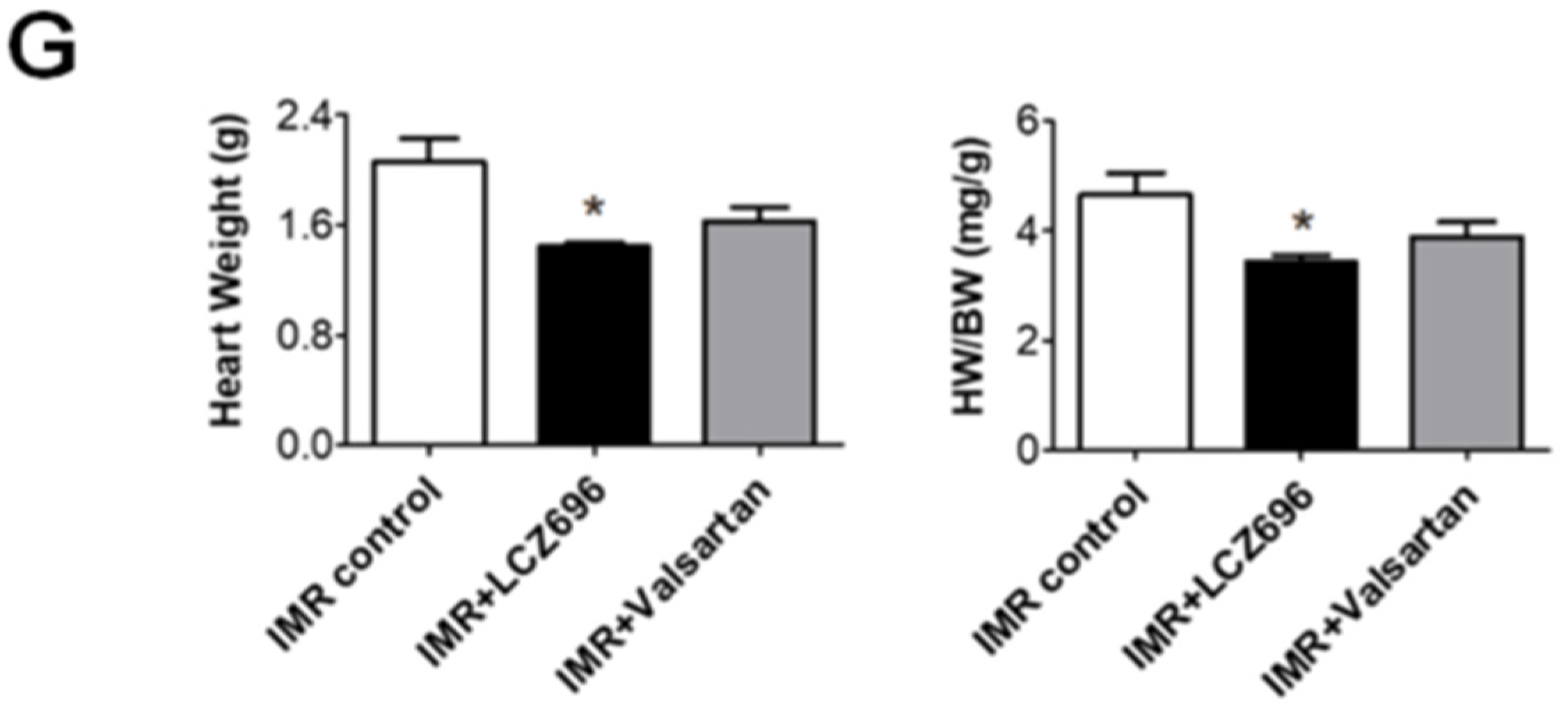
2.2. Neprilysin Inhibitor Facilitates Left Ventricular Reverse Remodeling
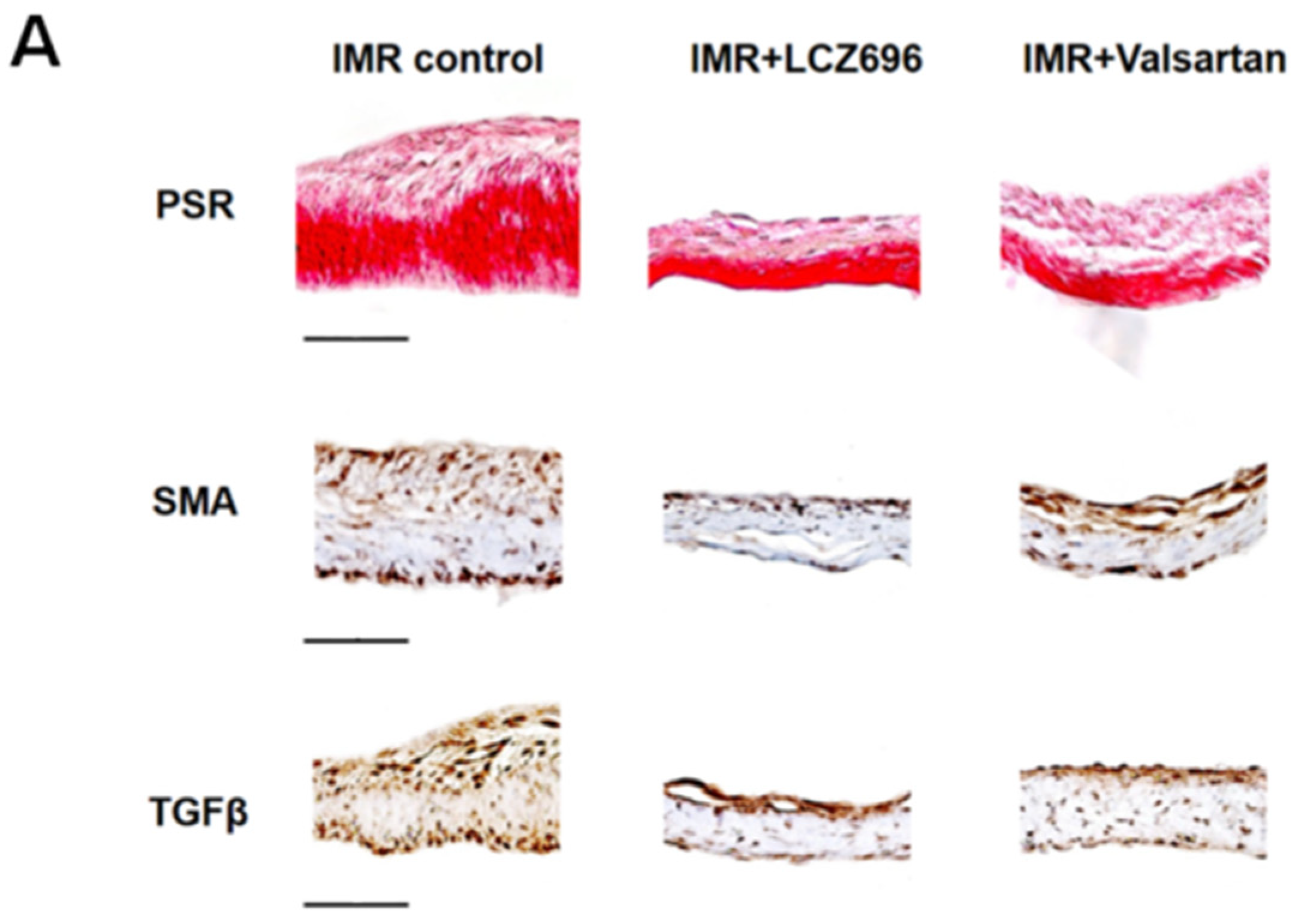
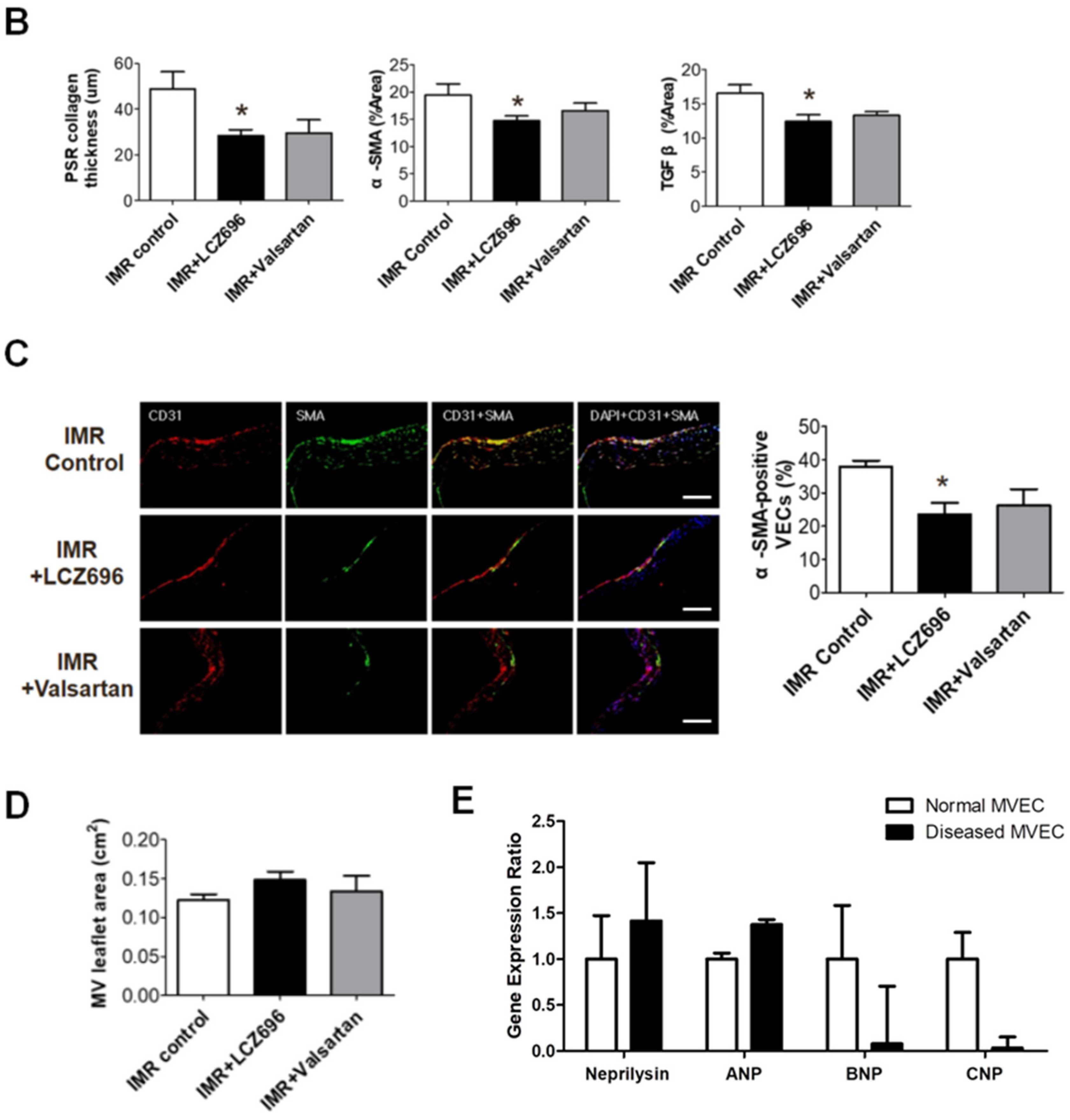
2.3. Neprilysin Inhibitor Suppresses Excessive Endothelial-to-Mesenchymal Transition which Mitigates Inadequate Leaflet Adaptation
2.4. The Effects of Neprilysin Inhibitor in Human Mitral Valve Endothelial Cells In Vitro Study
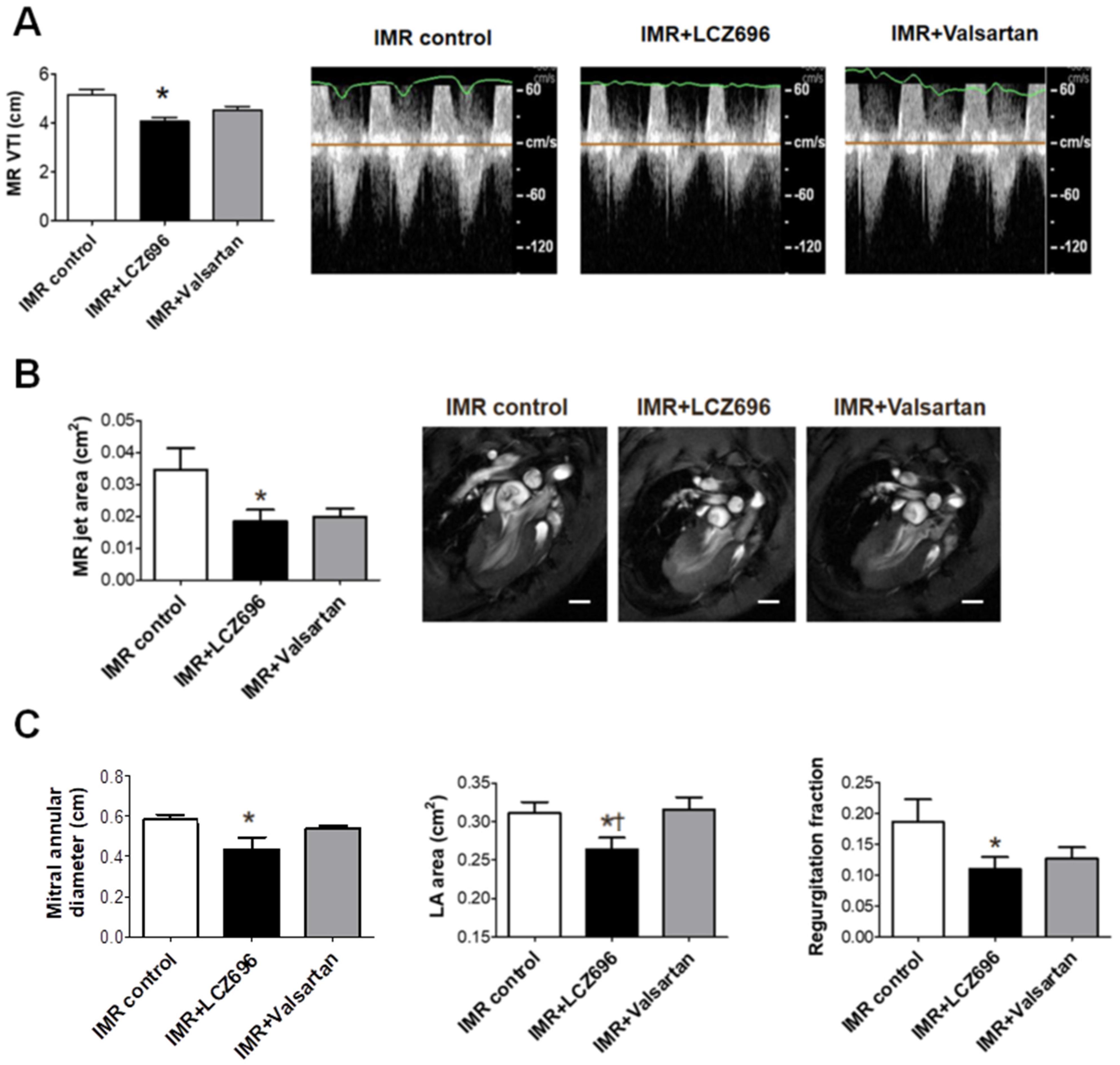
2.5. Neprilysin Inhibitor Attenuates Functional Mitral Regurgitation after Myocardial Infarction
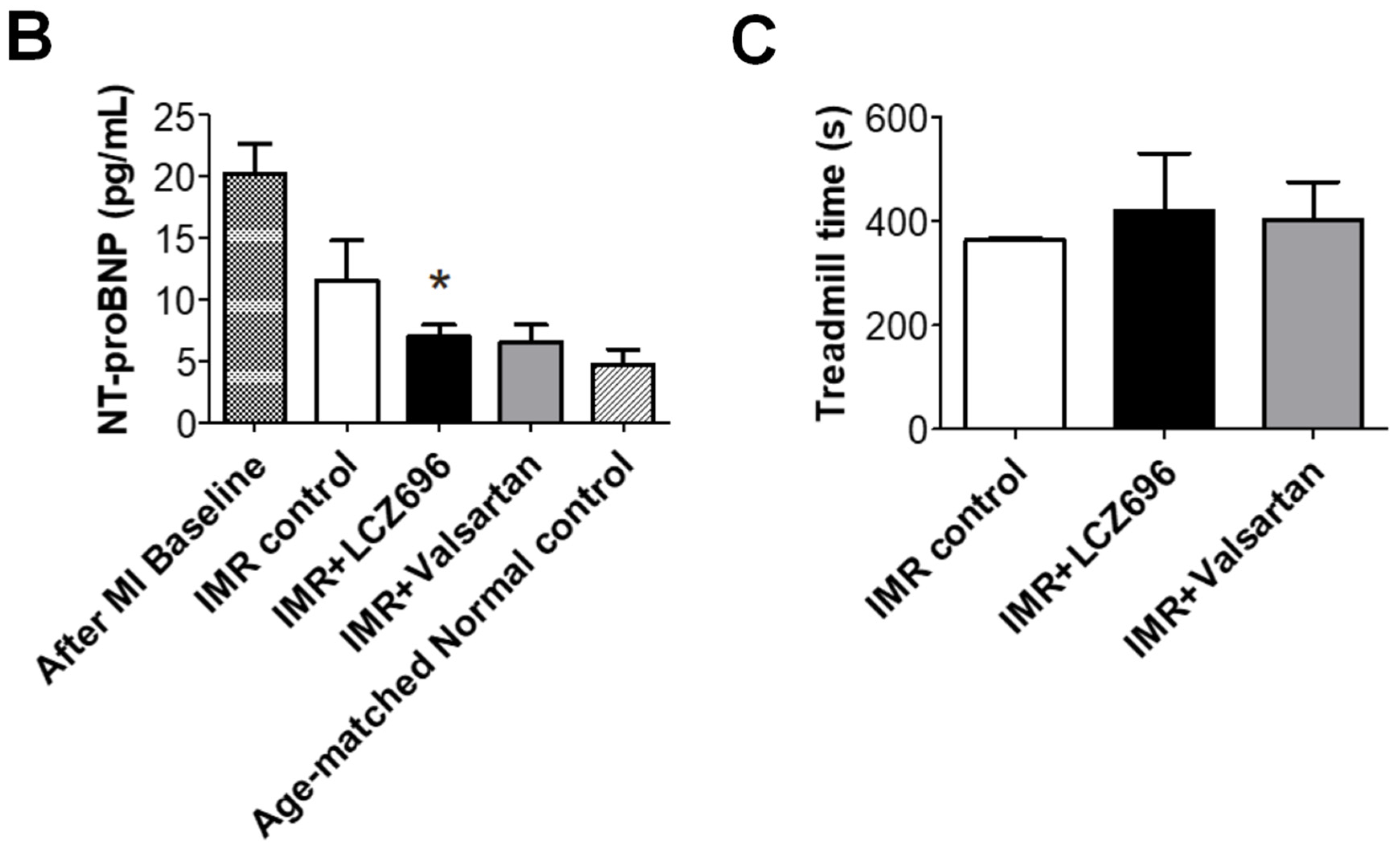
2.6. Functional Improvement in Pressure-Volume Loop Analysis and Treadmill Exercise Test
3. Discussion
4. Methods/Material
4.1. Experimental Animals
4.2. Myocardial Infarction for Creating Ischemic, Functional Mitral Regurgitation
4.3. Cardiac Magnetic Resonance Imaging
4.4. Echocardiography
4.5. Pressure-Volume Loop Analysis
4.6. Tissue Collection
4.7. Histology
4.8. Immunohistochemical Staining
4.9. Enzyme-Linked Immunosorbent Assay
4.10. Isolation of Human Mitral Valve Endothelial Cells and Cell Culture
4.11. In Vitro MVECs Study
4.12. Western Blotting
4.13. Real-Time qPCR
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Ethics Approval
References
- Pierard, L.A.; Carabello, B.A. Ischaemic mitral regurgitation: Pathophysiology, outcomes and the conundrum of treatment. Eur. Heart J. 2010, 31, 2996–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamas, G.A.; Mitchell, G.F.; Flaker, G.C.; Smit, S.C., Jr.; Gersh, B.J.; Basta, L.; Moyé, L.; Braunwald, E.; Pfeffer, M.A. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and ventricular enlargement investigators. Circulation 1997, 96, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; Enriquez-Sarano, M.; Nkomo, V.T.; Jacobsen, S.J.; Weston, S.A.; Meverden, R.A.; Roger, V.L. Heart failure and death after myocardial infarction in the community: The emerging role of mitral regurgitation. Circulation 2005, 111, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.; Dini, F.L.; Faggiano, P.; Cicoira, M.; Frattini, S.; Simioniuc, A.; Gullace, M.; Ghio, S.; Enriquez-Sarano, M.; Temporelli, P.L. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 1675–1680. [Google Scholar] [CrossRef]
- Levine, R.A.; Schwammenthal, E. Ischemic mitral regurgitation on the threshold of a solution: From paradoxes to unifying concepts. Circulation 2005, 112, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Asgar, A.W.; Mack, M.J.; Stone, G.W. Secondary mitral regurgitation in heart failure: Pathophysiology, prognosis, and therapeutic considerations. J. Am. Coll. Cardiol. 2015, 65, 1231–1248. [Google Scholar] [CrossRef] [Green Version]
- Agricola, E.; Ielasi, A.; Oppizzi, M.; Faggiano, P.; Ferri, L.; Calabrese, A.; Vizzardi, E.; Alfieri, O.; Margonato, A. Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur. J. Heart Fail. 2009, 11, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Grigioni, F.; Detaint, D.; Avierinos, J.F.; Scott, C.; Tajik, J.; Enriquez-Sarano, M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 260–267. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, e57–e185. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R.; et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Doughty, R.N.; Whalley, G.A.; Walsh, H.A.; Gamble, G.D.; Lopez-Sendon, J.; Sharpe, N.; Investigators, C.E.S. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: The capricorn echo substudy. Circulation 2004, 109, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.D.; Skali, H.; Anavekar, N.S.; Bourgoun, M.; Barvik, S.; Ghali, J.K.; Warnica, J.W.; Khrakovskaya, M.; Arnold, J.M.O.; Schwartz, Y.; et al. Changes in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarction. Circulation 2005, 111, 3411–3419. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.A.; Hagége, A.A.; Judge, D.P.; Padala, M.; Dal-Bianco, J.P.; Aikawa, E.; Beaudoin, J.; Bischoff, J.; Bouatia-Naji, N.; Bruneval, P.; et al. Mitral valve disease—Morphology and mechanisms. Nat. Rev. Cardiol. 2015, 12, 689–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lui, K.O.; Zhou, B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 2018, 15, 445–456. [Google Scholar] [CrossRef]
- Debonnaire, P.; Al Amri, I.; Leong, D.P.; Joyce, E.; Katsanos, S.; Kamperidis, V.; Schalij, M.J.; Bax, J.J.; Marsan, N.A.; Delgado, V. Leaflet remodelling in functional mitral valve regurgitation: Characteristics, determinants, and relation to regurgitation severity. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 290–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connell, P.S.; Azimuddin, A.F.; Kim, S.E.; Ramirez, F.; Jackson, M.S.; Little, S.H.; Grande-Allen, K.J. Regurgitation hemodynamics alone cause mitral valve remodeling characteristic of clinical disease states in vitro. Ann. Biomed. Eng. 2016, 44, 954–967. [Google Scholar] [CrossRef] [Green Version]
- Beaudoin, J.; Dal-Bianco, J.P.; Aikawa, E.; Bischoff, J.; Guerrero, J.L.; Sullivan, S.; Bartko, P.E.; Handschumacher, M.D.; Kim, D.H.; Wylie-Sears, J.; et al. Mitral leaflet changes following myocardial infarction: Clinical evidence for maladaptive valvular remodeling. Circ. Cardiovasc. Imaging 2017, 10, e006512. [Google Scholar] [CrossRef] [Green Version]
- Chaput, M.; Handschumacher, M.D.; Tournoux, F.; Hua, L.; Guerrero, J.L.; Vlahakes, G.J.; Levine, R.A. Mitral leaflet adaptation to ventricular remodeling: Occurrence and adequacy in patients with functional mitral regurgitation. Circulation 2008, 118, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Chaput, M.; Handschumacher, M.D.; Guerrero, J.L.; Holmvang, G.; Dal-Bianco, J.P.; Sullivan, S.; Vlahakes, G.J.; Hung, J.; Levine, R.A. Mitral leaflet adaptation to ventricular remodeling: Prospective changes in a model of ischemic mitral regurgitation. Circulation 2009, 120, S99–S103. [Google Scholar] [CrossRef] [Green Version]
- Kunzelman, K.S.; Quick, D.W.; Cochran, R.P. Altered collagen concentration in mitral valve leaflets: Biochemical and finite element analysis. Ann. Thorac. Surg. 1998, 66, S198–S205. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter mitral-valve repair in patients with heart failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Park, S.J.; Shin, S.H.; Hong, G.R.; Lee, S.; Kim, M.S.; Yun, S.C.; Song, M.J.; Park, S.W.; King, J.J. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation 2019, 139, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Noe, A.; Chandra, P.; Al-Fayoumi, S.; Ligueros-Saylan, M.; Sarangapani, R.; Maahs, S.; Ksander, G.; Gigel, D.F.; Jeng, Y.F.; et al. Pharmacokinetics and pharmacodynamics of lcz696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (arni). J. Clin. Pharmacol. 2010, 50, 401–414. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.R.; Shi, C.V.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubers, S.A.; Brown, N.J. Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation 2016, 133, 1115–1124. [Google Scholar] [CrossRef]
- Sacks, C.A.; Jarcho, J.A.; Curfman, G.D. Paradigm shifts in heart-failure therapy—A timeline. N. Engl. J. Med. 2014, 371, 989–991. [Google Scholar] [CrossRef]
- Von Lueder, T.G.; Wang, B.H.; Kompa, A.R.; Huang, L.; Webb, R.; Jordaan, P.; Ater, D.; Krum, H. Angiotensin receptor neprilysin inhibitor lcz696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ. Heart Fail. 2015, 8, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Kato, J. Natriuretic peptides and neprilysin inhibition in hypertension and hypertensive organ damage. Peptides 2020, 132, 170352. [Google Scholar] [CrossRef]
- Castleberry, A.W.; Williams, J.B.; Daneshmand, M.A.; Honeycutt, E.; Shaw, L.K.; Samad, Z.; Lopes, R.D.; Alexander, J.H.; Mathew, J.P.; Velazquez, E.J.; et al. Surgical revascularization is associated with maximal survival in patients with ischemic mitral regurgitation: A 20-year experience. Circulation 2014, 129, 2547–2556. [Google Scholar] [CrossRef] [Green Version]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.; Fonarow, C.C.; Givertz, M.M.; et al. 2016 acc/aha/hfsa focused update on new pharmacological therapy for heart failure: An update of the 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of america. Circulation 2016, 134, e282–e293. [Google Scholar]
- Ruilope, L.M.; Dukat, A.; Bohm, M.; Lacourciere, Y.; Gong, J. Lefkowitz MP. Blood-pressure reduction with lcz696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010, 375, 1255–1266. [Google Scholar] [CrossRef]
- Solomon, S.D. Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor lcz696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Lancet 2012, 380, 1387–1395. [Google Scholar]
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K. Investigators P-H. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N. Engl. J. Med. 2019, 380, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Lee, S.; Kim, S.M.; Lee, E.J.; Lee, S.R.; Kim, D.H.; Jang, J.Y.; Kang, S.W.; Lee, K.U.; Chang, E.U.; et al. Dipeptidyl peptidase-4 induces aortic valve calcification by inhibiting insulin-like growth factor-1 signaling in valvular interstitial cells. Circulation 2017, 135, 1935–1950. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Flanagan, T.C.; Lu, C.C.; French, A.T.; Argyle, D.J. Corcoran BM. Culture and characterisation of canine mitral valve interstitial and endothelial cells. Vet. J. 2015, 204, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Gould, R.A.; Butcher, J.T. Isolation of valvular endothelial cells. JoVE (J. Vis. Exp.) 2010, 46, e2158. [Google Scholar] [CrossRef] [Green Version]


| Variables | IMR Control | IMR + LCZ696 | IMR + Valsartan | p Value |
|---|---|---|---|---|
| SBP, mmHg | 120.6 ± 3.0 | 118.7 ± 3.4 | 118.9 ± 4.0 | 0.925 |
| DBP, mmHg | 67.7 ± 3.9 | 66.2 ± 3.6 | 63.3 ± 3.0 | 0.559 |
| MAP, mmHg | 85.4 ± 2.8 | 83.7 ± 2.2 | 81.8 ± 2.8 | 0.483 |
| HR, bpm | 352 ± 23 | 336 ± 20 | 363 ± 16 | 0.152 |
| LVESP, mmHg | 106.6 ± 7.2 | 118.9 ± 4.4 | 102.7 ± 7.0 | 0.182 |
| LVEDP, mmHg | 7.7 ± 0.9 | 7.9 ± 1.0 | 11.2 ± 2.3 | 0.697 |
| −dP/dt, mmHg/s | 6940 ± 509 | 8660 ± 388 *† | 5946 ± 853 | 0.019 |
| +dP/dt, mmHg/s | 7370 ± 504 | 8623 ± 273 † | 6799 ± 444 | 0.014 |
| ESPVR, mmHg/µL | 0.471 ± 0.22 | 0.621 ± 0.31 | 0.798 ± 0.50 | 0.142 |
| EDPVR, mmHg/µL | 0.126 ± 0.10 | 0.126 ± 0.07 | 0.279 ± 0.22 | 0.163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Hwang, H.-S.; Song, N.; Kang, G.-H.; Choi, K.-H.; Ji, E.; Song, J.-M.; Kang, D.-H. Effect of Neprilysin Inhibition for Ischemic Mitral Regurgitation after Myocardial Injury. Int. J. Mol. Sci. 2021, 22, 8598. https://doi.org/10.3390/ijms22168598
Lee S, Hwang H-S, Song N, Kang G-H, Choi K-H, Ji E, Song J-M, Kang D-H. Effect of Neprilysin Inhibition for Ischemic Mitral Regurgitation after Myocardial Injury. International Journal of Molecular Sciences. 2021; 22(16):8598. https://doi.org/10.3390/ijms22168598
Chicago/Turabian StyleLee, Sahmin, Hyo-Sook Hwang, Naaleum Song, Geun-Hyung Kang, Kyoung-Hee Choi, Eunhye Ji, Jong-Min Song, and Duk-Hyun Kang. 2021. "Effect of Neprilysin Inhibition for Ischemic Mitral Regurgitation after Myocardial Injury" International Journal of Molecular Sciences 22, no. 16: 8598. https://doi.org/10.3390/ijms22168598
APA StyleLee, S., Hwang, H. -S., Song, N., Kang, G. -H., Choi, K. -H., Ji, E., Song, J. -M., & Kang, D. -H. (2021). Effect of Neprilysin Inhibition for Ischemic Mitral Regurgitation after Myocardial Injury. International Journal of Molecular Sciences, 22(16), 8598. https://doi.org/10.3390/ijms22168598






