The Obscure Effect of Tribulus terrestris Saponins Plus Inulin on Liver Morphology, Liver Fatty Acids, Plasma Glucose, and Lipid Profile in SD Rats with and without Induced Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
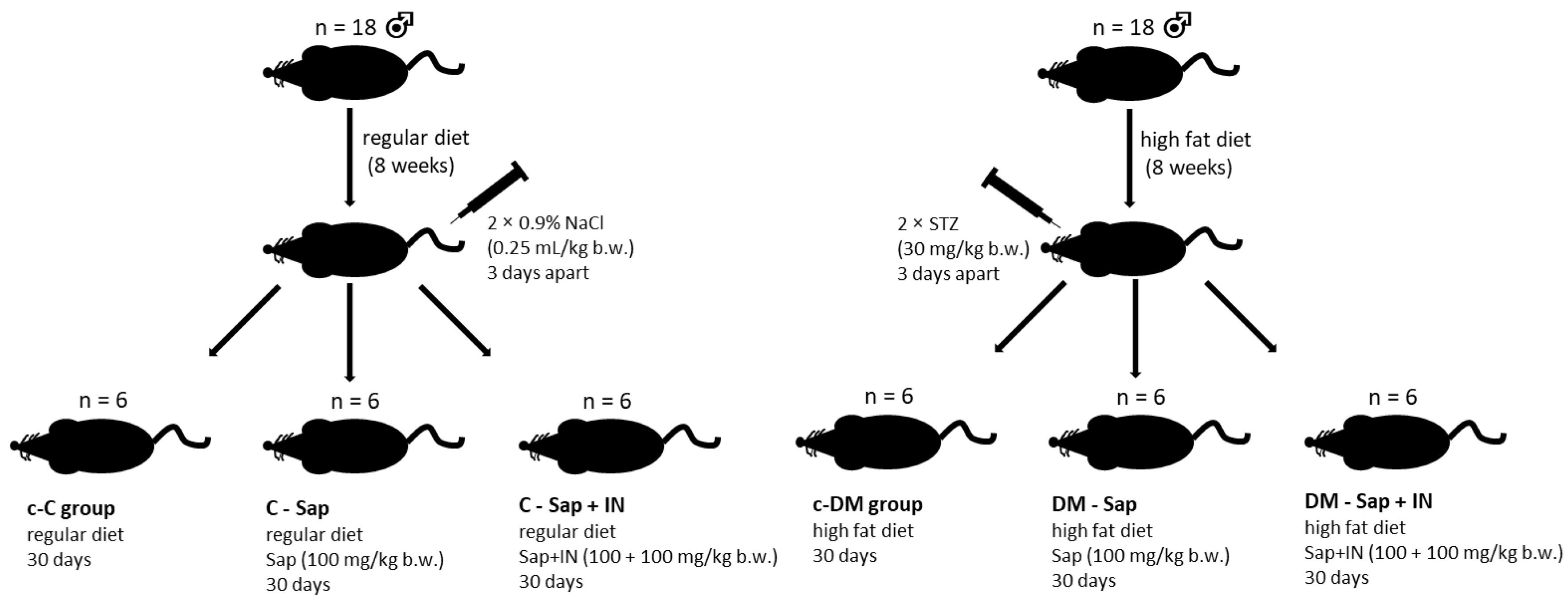
2.1. Animal Care and Maintenance
2.2. Histopathologic Evaluation
2.3. Isolation of Fatty Acids
2.4. Fatty Acid Methyl Esters Analysis
2.5. Fatty Acid Indices
2.6. Statistical Analysis
3. Results
3.1. Histological Evaluation of the Liver
3.2. The Baseline Plasma Lipid Profile, Glycated Hemoglobin, and Glucose
3.3. Fatty Acid Profile
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Firneisz, G. Non-alcoholic fatty liver disease and type 2 diabetes mellitus: The liver disease of our age? World J. Gastroenterol. 2014, 20, 9072–9089. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.D. Fatty liver: Role of inflammation and fatty acid nutrition. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 265–271. [Google Scholar] [CrossRef]
- Rafiq, N.; Bai, C.; Fang, Y.; Srishord, M.; McCullough, A.; Gramlich, T.; Younossi, Z.M. Long-Term Follow-Up of Patients with Nonalcoholic Fatty Liver. Clin. Gastroenterol. Hepatol. 2009, 7, 234–238. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [Green Version]
- El-Sayyad, H.I.; Al-Haggar, M.M.; El-Ghawet, H.A.; Bakr, I.H. Effect of maternal diabetes and hypercholesterolemia on fetal liver of albino Wistar rats. Nutrinets 2014, 30, 326–336. [Google Scholar] [CrossRef]
- Valenti, L.; Pelusi, S. Redefining fatty liver disease classification in 2020. Liver Int. 2020, 40, 1016–1017. [Google Scholar] [CrossRef] [PubMed]
- Toshikuni, N.; Tsutsumi, M.; Arisawa, T. Clinical differences between alcoholic liver disease and nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 8393–8406. [Google Scholar] [CrossRef]
- Pelusi, S.; Valenti, L. Hepatic fat as clinical outcome and therapeutic target for nonalcoholic fatty liver disease. Liver Int. 2018, 39, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romeo, S.; Sanyal, A.; Valenti, L. Leveraging Human Genetics to Identify Potential New Treatments for Fatty Liver Disease. Cell Metab. 2020, 31, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J. International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, C.; Cui, J.; Lu, J.; Yan, C.; Wei, X.; Zhao, X.; Li, N.; Li, S.; Xue, G.; et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019, 30, 675–688.e7. [Google Scholar] [CrossRef] [PubMed]
- Rosenstengel, S.; Stoeppeler, S.; Bahde, R.; Spiegel, H.-U.; Palmes, D. Type of Steatosis Influences Microcirculation and Fibrogenesis in Different Rat Strains. J. Investig. Surg. 2011, 24, 273–282. [Google Scholar] [CrossRef]
- Stöppeler, S.; Palmes, D.; Fehr, M.; Hölzen, J.P.; Zibert, A.; Siaj, R.; Schmidt, H.H.-J.; Spiegel, H.-U.; Bahde, R. Gender and strain-specific differences in the development of steatosis in rats. Lab. Anim. 2013, 47, 43–52. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A. Saponins; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Desai, S.D.; Desai, D.G.; Kaur, H. Saponin and their biological activities. Pharma Times 2009, 41, 13–16. [Google Scholar]
- Yoshiki, Y.; Kudou, S.; Okubo, K. Relationship between Chemical Structures and Biological Activities of Triterpenoid Saponins from Soybean. Biosci. Biotechnol. Biochem. 1998, 62, 2291–2299. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Li, C. Absorption, Disposition, and Pharmacokinetics of Saponins from Chinese Medicinal Herbs: What Do We Know and What Do We Need to Know More? Curr. Drug Metab. 2012, 13, 577–598. [Google Scholar] [CrossRef]
- Kostova, I.; Dinchev, D. Saponins in Tribulus terrestris—Chemistry and Bioactivity. Phytochem. Rev. 2005, 4, 111–137. [Google Scholar] [CrossRef]
- Wang, Z.-F.; Wang, B.-B.; Zhao, Y.; Wang, F.-X.; Sun, Y.; Guo, R.-J.; Song, X.-B.; Xin, H.-L.; Sun, X.-G. Furostanol and Spirostanol Saponins from Tribulus terrestris. Molecules 2016, 21, 429. [Google Scholar] [CrossRef] [Green Version]
- Dighe, S.U.; Jadhav, R.; Vikhe, D. Biological Potential of Tribulus terrestris. J. Drug Deliv. Ther. 2020, 10, 262–264. [Google Scholar] [CrossRef]
- Brown, K.; DeCoffe, D.; Molcan, E.; Gibson, D.L. Diet-Induced Dysbiosis of the Intestinal Microbiota and the Effects on Immunity and Disease. Nutrients 2012, 4, 1095. [Google Scholar] [CrossRef] [Green Version]
- McFarlane, G.T.; Cumming, J.H. The Colonic Flora, Fermentation and Large Bowel Digestive Function; Raven Press New York: New York, NY, USA, 1991. [Google Scholar]
- Bonsu, N.K.A.; Johnson, S. Effects of inulin fibre supplementation on serum glucose and lipid concentration in patients with type 2 diabetes. Int. J. Diabetes Metab. 2012, 21, 80–86. [Google Scholar]
- Parnell, J.A.; Reimer, R.A. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes 2012, 3, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Lv, X.-Y.; Li, J.; Xu, Z.-G.; Chen, L. The Characterization of High-Fat Diet and Multiple Low-Dose Streptozotocin Induced Type 2 Diabetes Rat Model. Exp. Diabetes Res. 2008, 2008, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Xu, Z.-J.; Fan, J.-G.; Ding, X.-D.; Qiao, L.; Wang, G.-L. Characterization of High-Fat, Diet-Induced, Non-alcoholic Steatohepatitis with Fibrosis in Rats. Dig. Dis. Sci. 2009, 55, 931–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misiakiewicz-Has, K.; Maciejewska, D.; Kolasa-Wołosiuk, A.; Pilutin, A.; Rzeszotek, S.; Wilk, A.; Szypulska-Koziarska, D.; Stachowska, E.; Łukomska, A.; Wiszniewska, B. Modulatory effect of inulin with soya isoflavones on plasma lipid profile and liver SCD-18 index in rats with induced type-2 diabetes mellitus. Histol. Histopathol. 2019, 34, 18113. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Chong, M.F.-F.; Hodson, L.; Bickerton, A.S.; Roberts, R.; Neville, M.; Karpe, F.; Frayn, K.N.; Fielding, B.A. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am. J. Clin. Nutr. 2008, 87, 817–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of Δ6, Δ5, and Δ9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef] [PubMed]
- Drąg, J.; Goździalska, A.; Knapik-Czajka, M.; Gawędzka, A.; Gawlik, K.; Jaśkiewicz, J. Effect of high carbohydrate diet on elongase and desaturase activity and accompanying gene expression in rat’s liver. Genes Nutr. 2017, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Barnes, K.; Ball, L.; Desbrow, B.; Alsharairi, N.; Ahmed, F. Consumption and reasons for use of dietary supplements in an Australian university population. Nutrition 2016, 32, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Ercan, P.; El, S.N. Inhibitory effects of chickpea and Tribulus terrestris on lipase, α-amylase and α-glucosidase. Food Chem. 2016, 205, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Inui, Y.; Kawata, S.; Matsuzawa, Y.; Tokunaga, K.; Fujioka, S.; Tamura, S.; Kobatake, T.; Kenoxa, Y.; Odaka, H.; Matsuo, T.; et al. Inhibitory effect of a new α-glucosidase inhibitor on fatty liver in Zucker fatty rats. J. Hepatol. 1990, 10, 62–68. [Google Scholar] [CrossRef]
- Chiang, D.J.; Pritchard, M.T.; Nagy, L.E. Obesity, diabetes mellitus, and liver fibrosis. Am. J. Physiol. Liver Physiol. 2011, 300, G697–G702. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.H. Effect of Gross Saponins from Tribulus Terrestris on Hepatic Apoptosis in Mice’s Acute Hepatic Injury Induced by Tripterygium Glycosides; Heibei University Chinese Medicine: Heibei, China, 2009. [Google Scholar]
- Chi, X.-X.; Zhang, T.; Zhang, D.-J.; Yu, W.; Wang, Q.-Y.; Zhen, J.-L. Effects of isoflavones on lipid and apolipoprotein levels in patients with type 2 diabetes in Heilongjiang Province in China. J. Clin. Biochem. Nutr. 2016, 59, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Alles, M.S.; De Roos, N.M.; Bakx, J.C.; Van De Lisdonk, E.; Zock, P.L.; Hautvast, J.G. Consumption of fructooligosaccharides does not favorably affect blood glucose and serum lipid concentrations in patients with type 2 diabetes. Am. J. Clin. Nutr. 1999, 69, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venter, C.S.; Vorster, H.H.; Cummings, J.H. Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am. J. Gastroenterol. 1990, 85, 549–553. [Google Scholar] [PubMed]
- Yenge, G.D.; Lonkar, V.D.; Ranade, A.S.; Patodkar, V.R.; Mote, C.S.; Bhalerao, S.M.; Doiphode, A.Y. Dietary supplementation of combination of inulin and saponin on egg yolk lipid and serum biochemical profile in laying hens. Int. J. Sci. Environ. Technol. 2018, 7, 1711–1720. [Google Scholar]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Xiao, X.; Saha, P.; Olvera, R.A.; Lapek, J.D., Jr.; Zhang, L.; Wang, W.-B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e622. [Google Scholar] [CrossRef] [Green Version]
- Flemer, B.; Gaci, N.; Borrel, G.; Sanderson, I.; Chaudhary, P.P.; Tottey, W.; O’Toole, P.W.; Brugère, J.-F. Fecal microbiota variation across the lifespan of the healthy laboratory rat. Gut Microbes 2017, 8, 428–439. [Google Scholar] [CrossRef]
- Green, C.D.; Ozguden-Akkoc, C.G.; Wang, Y.; Jump, D.B.; Olson, L.K. Role of fatty acid elongases in determination of de novo synthesized monounsaturated fatty acid species. J. Lipid Res. 2010, 51, 1871–1877. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Xu, C.; Xu, L.; Yu, C.; Miao, M.; Li, Y. Independent association of HbA1c and nonalcoholic fatty liver disease in an elderly Chinese population. BMC Gastroenterol. 2013, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Kato, T.; Atsumi, A.; Yamamoto, T.; Inoue, N.; Ishikawa, M.; Okada, S.; Ishigaki, N.; et al. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat. Med. 2007, 13, 1193–1202. [Google Scholar] [CrossRef]
- Li, Z.Z.; Berk, M.; McIntyre, T.M.; Feldstein, A.E. Hepatic Lipid Partitioning and Liver Damage in Nonalcoholic Fatty Liver Disease: Role of Stearoylcoa desaturase. J. Biol. Chem. 2009, 284, 5637–5644. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cao, Y.; Fu, Y.; Guo, G.; Zhang, X. Liver fatty acid composition in mice with or without nonalcoholic fatty liver disease. Lipids Health Dis. 2011, 10, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, A.M.; Chagas, T.R.; Nunes, E.A. Fatty liver and n-3 fatty acids ingestion: New mechanisms and perspectives from pre-clinical animal models. Liver Pancreat. Sci. 2016, 1, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Bargut, T.; Frantz, E.D.C.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. Effects of a Diet Rich in n-3 Polyunsaturated Fatty Acids on Hepatic Lipogenesis and Beta-Oxidation in Mice. Lipids 2014, 49, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Taltavull, N.; Muñoz-Cortés, M.; Lluís, L.; Jové, M.; Fortuño, À.; Molinar-Toribio, E.; Torres, J.L.; Pazos, M.; Medina, I.; Nogués, M.R. Eicosapentaenoic acid/docosahexaenoic acid 1:1 ratio improves histological alterations in obese rats with metabolic syndrome. Lipids Health Dis. 2014, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.A.; Marvyn, P.M.; Henao, J.J.A.; Bradley, R.M.; Stark, K.; Duncan, R.E. Fasting enriches liver triacylglycerol with n-3 polyunsaturated fatty acids: Implications for understanding the adipose–liver axis in serum docosahexaenoic acid regulation. Genes Nutr. 2015, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatner, D.F.; Majumdar, S.K.; Kumashiro, N.; Petersen, M.C.; Rahimi, Y.; Gattu, A.K.; Bears, M.; Camporez, J.-P.G.; Cline, G.W.; Jurczak, M.J.; et al. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc. Natl. Acad. Sci. USA 2015, 112, 1143–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flannery, C.; Dufour, S.; Rabøl, R.; Shulman, G.I.; Petersen, K.F. Skeletal Muscle Insulin Resistance Promotes Increased Hepatic De Novo Lipogenesis, Hyperlipidemia, and Hepatic Steatosis in the Elderly. Diabetes 2012, 61, 2711–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, J.E.; Ramos–Roman, M.A.; Browning, J.D.; Parks, E.J. Increased De Novo Lipogenesis Is a Distinct Characteristic of Individuals with Nonalcoholic Fatty Liver Disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Roumans, K.H.M.; Lindeboom, L.; Veeraiah, P.; Remie, C.M.E.; Phielix, E.; Havekes, B.; Bruls, Y.; Brouwers, M.C.G.J.; Ståhlman, M.; Alssema, M.; et al. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nat. Commun. 2020, 11, 1891. [Google Scholar] [CrossRef]
- Paton, C.M.; Ntambi, J.M. Loss of stearoyl-CoA desaturase activity leads to free cholesterol synthesis through increased Xbp-1 splicing. Am. J. Physiol. Metab. 2010, 299, E1066–E1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef] [Green Version]
- Pinnamaneni, S.K.; Southgate, R.J.; Febbraio, M.A.; Watt, M.J. Stearoyl CoA desaturase 1 is elevated in obesity but protects against fatty acid-induced skeletal muscle insulin resistance in vitro. Diabetologia 2006, 49, 3027–3037. [Google Scholar] [CrossRef] [Green Version]


| Group | N (n) | Histological Grades of Steatosis Number of Evaluated Histological Fields (Percentage of Grade of Steatosis) | Fibrosis Stage (N) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 4 | ||
| c-C | 6 (60) | 60 (100) | 0 (0) | 0 (0) | 0 (0) | 6 | 0 | 0 | 0 | 0 |
| C-Sap | 6 (60) | 60 (100) | 0 (0) | 0 (0) | 0 (0) | 6 | 0 | 0 | 0 | 0 |
| C-Sap + IN | 6 (60) | 6 (10) b | 20 (33.3) c | 28 (46.7) c | 6 (10) b | 4 | 2 | 0 | 0 | 0 |
| c-DM | 6 (60) | 0 (0) | 3 (5) | 44 (73.3) | 13 (21.7) | 1 | 4 | 1 | 0 | 0 |
| DM-Sap | 6 (60) | 42 (70) * | 13 (21.7) a | 5 (8.3) * | 0 (0) * | 5 | 1 | 0 | 0 | 0 |
| DM-Sap + IN | 6 (60) | 53 (88.3) * | 7 (11.7) | 0 (0) * | 0 (0) * | 5 | 1 | 0 | 0 | 0 |
| Parameters | Control Groups | Diabetes Groups | ||||
|---|---|---|---|---|---|---|
| c-C | C-Sap | C-Sap + IN | c-DM | DM-Sap | DM-Sap + IN | |
| HbA1c/HbF (%)-IFCC | 7.97 ± 0.55 | 8.15 ± 1.44 | 8.87 ± 1.46 | 8.38 ± 1.44 | 9 ± 1.92 | 10.08 ± 1.66 |
| Glucose (mg/dL) | 69.33 ± 4.93 | 69.33 ± 5.42 | 76 ± 5.48 b | 184.5 ± 46.48 * | 252 ± 135.55 | 274.5 ± 64.78 ∆ |
| Triglycerides (mg/dL) | 39.83 ± 23.61 | 38.0 ± 13.18 | 48.33 ± 14.31 | 67.0 ± 35.39 | 40.0 ± 21.4 | 42.33 ± 17.99 |
| Cholesterol (mg/dL) | 59.0 ± 8.07 | 64.83 ± 8.08 | 61.83 ± 8.61 | 51.5 ± 9.07 | 46.83 ± 14.08 | 55.5 ± 19.89 |
| HDL (mg/dL) | 19.5 ± 1.97 | 18.5 ± 2.17 | 17.67 ± 2.25 | 16.83 ± 1.6 * | 17.5 ± 7.26 | 18.67 ± 4.93 |
| LDL (mg/dL) | 9.0 ± 3.35 | 10.33 ± 2.73 | 9.0 ± 2.76 | 6.67 ± 2.94 | 7.5 ± 1.87 | 7.67 ± 3.44 |
| Rate | ||||||
| TC/HDL | 2.04 ± 1.24 | 2.12 ± 0.87 | 2.78 ± 0.87 | 3.98 ± 2.02 b | 2.75 ± 1.81 | 2.59 ± 1.66 |
| Fatty Acids (%) | Control Groups | Diabetes Groups | ||||
|---|---|---|---|---|---|---|
| c-C | C-Sap | C-Sap + IN | c-DM | DM-Sap | DM-Sap + IN | |
| C12:0 Lauric Acid | 2.08 ± 0.34 | 2.03 ± 0.31 | 1.63 ± 0.12 * | 2.58 ± 0.58 | 3.22 ± 1.17 b | 3.34 ± 0.39 Δ |
| C14:0 Myristic | 0.52 ± 0.25 | 0.50 ± 0.14 | 0.55 ± 0.19 | 0.52 ± 0.23 | 0.68 ± 0.03 | 0.75 ± 0.14 Δ |
| C14:1 Myristoleic | 0.36 ± 0.07 | 0.28 ± 0.03 * | 0.33 ± 0.06 | 0.32 ± 0.02 | 0.35 ± 0.04 | 0.33 ± 0.07 |
| C15:0 Pentadecanoic | 0.21 ± 0.09 | 0.34 ± 0.12 | 0.46 ± 0.25 | 0.12 ± 0.02 | 0.4 ± 0.96 | 0.12 ± 0.02 |
| C16:0 Palmitic | 22.82 ± 0.73 | 23.24 ± 0.79 | 23.51 ± 0.81 | 21.29 ± 1.33 * | 20.71 ± 5.94 | 21.68 ± 0.53 |
| C16:1n7 Palmitoleic | 0.41 ± 0.1 | 0.36 ± 0.08 | 0.53 ± 0.12 | 0.28 ± 0.13 | 0.29 ± 0.12 | 0.24 ± 0.08 |
| C17:0 Heptadecanoic | 0.67 ± 0.08 | 0.66 ± 0.08 | 0.55 ± 0.07 * | 0.36 ± 0.02 * | 0.44 ± 0.03 Δ | 0.46 ± 0.05 Δ |
| C18:0 Stearic | 18.14 ± 2.2 | 21.08 ± 0.5 * | 17.98 ± 4.17 | 22.13 ± 44.44 | 22.98 ± 10.33 | 22.49 ± 1.09 |
| C18:1n9 Oleic | 11.57 ± 2.31 | 9.03 ± 0.54 * | 10.96 ± 2.42 | 15.88 ± 38.57 | 14.16 ± 20.81 | 13.92 ± 2.24 |
| C18:1n-7 Vaccenic | 4.1 ± 0.21 | 3.38 ± 0.28 * | 3.55 ± 0.13 * | 2.47 ± 0.88 | 2.27 ± 0.46 | 2.28 ± 0.32 |
| 18:2n-6 Linoleic | 18.81 ± 0.92 | 17.77 ± 0.94 | 20.22 ± 2.58 | 13.66 ± 1.1 * | 12.51 ± 5.42 | 12.31 ± 0.95 b |
| 18:3n-6 γ-Linoleic | 0.19 ± 0.05 | 0.23 ± 0.07 | 0.18 ± 0.07 | 0.13 ± 0.03 | 0.14 ± 0.01 | 0.13 ± 0.04 |
| 18:3n-3 Linolenic | 0.77 ± 0.13 | 0.63 ± 0.1 | 0.87 ± 0.21 | 0.27 ± 0.1 * | 0.19 ± 0.04 | 0.16 ± 0.02 b |
| C20:0 Arachidic | 0.31 ± 0.07 | 0.26 ± 0.03 | 0.24 ± 0.03 | 0.2 ± 0.01 | 0.21 ± 0.01 | 0.18 ± 0.03 |
| C20:1 11-Eicosenoic | 0.33 ± 0.16 | 0.23 ± 0.13 | 0.4 ± 0.31 | 0.08 ± 0.01 | 0.1 ± 0.01 | 0.09 ± 0.03 |
| C20:2n6 Eicosadienoic | 0.57 ± 0.11 | 0.57 ± 0.1 | 0.4 ± 0.11* | 0.33 ± 0.06 | 0.28 ± 0.02 | 0.28 ± 0.09 |
| C20:3n6 Eicosatrienoic | 0.59 ± 0.07 | 0.61 ± 0.12 | 0.51 ± 0.18 | 0.75 ± 0.1 | 0.73 ± 0.25 | 0.78 ± 0.13 |
| C20:4n6 Arachidonic | 10.83 ± 1.33 | 12.69 ± 1.88 | 11.03 ± 1.59 | 11.64 ± 39.83 | 14.33 ± 14.45 | 13.8 ± 1.52 |
| C20:5n3 Eicosapentaenoic | 0.43 ± 0.12 | 0.34 ± 0.06 | 0.49 ± 0.12 | 0.22 ± 0.03 | 0.22 ± 0.03 | 0.19 ± 0.04 |
| C22:4n6 Docosatetraenoic | 0.63 ± 0.18 | 0.47 ± 0.11 | 0.52 ± 0.12 | 0.55 ± 0.04 | 0.47 ± 0.12 | 0.43 ± 0.06 |
| C22:5n3 Docosapentaenoic | 1.21 ± 0.47 | 1,0 ± 0.36 | 1.13 ± 0.35 | 1.04 ± 1.3 | 0.63 ± 0.1 | 0.77 ± 0.16 |
| C22:6n3 Docosahexaenoic | 4.44 ± 0.69 | 4.31 ± 0.81 | 3.98 ± 0.61 | 5.17 ± 5.69 | 4.49 ± 2.86 | 5.3 ± 1.08 |
| Parameter | ||||||
| ∑-SFA | 44.74 ± 3.05 | 48.12 ± 0.56 * | 44.92 ± 4.15 | 47.2 ± 2.45 | 48.64 ± 1.44 | 49.01 ± 0.93 |
| ∑-PUFA | 38.4 ± 0.75 | 38.61 ± 0.66 | 39.31 ± 1.65 | 33.76 ± 2.26 * | 34.19 ± 2.06 | 34.13 ± 1.9 |
| ∑-MUFA | 16.78 ± 2.83 | 13.27 ± 0.54 * | 15.77 ± 2.66 | 19.04 ± 3.25 * | 17.16 ± 2.48 | 16.86 ± 2.78 |
| PUFA/SFA | 0.86 ± 0.07 | 0.8 ± 0.02 c | 0.88 ± 0.12 | 0.72 ± 0.06 * | 0.7 ± 0.05 | 0.7 ± 0.03 |
| ∑-n-6 | 31.62 ± 0.87 | 32.34 ± 1.68 | 32.85 ± 1.42 | 27.06 ± 1.77 * | 28.47 ± 1.74 | 27.71 ± 1.35 |
| ∑-n-3 | 6.86 ± 0.97 | 6.27 ± 1.2 | 6.46 ± 0.56 | 6.7 ± 0.83 | 5.72 ± 0.86 | 6.42 ± 1.15 |
| n-6/n-3 | 4.71 ± 0.47 | 5.36 ± 1.27 | 5.11 ± 0.44 | 4.08 ± 0.47 | 5.06 ± 0.76 Δ | 4.44 ± 0.89 |
| DNL index | 1.22 ± 0.08 | 1.31 ± 0.08 | 1.17 ± 0.12 | 1.56 ± 0.07 * | 1.66 ± 0.14 | 1.77 ± 0.16 |
| SCD16 index | 0.02 ± 0.005 | 0.02 ± 0.004 | 0.02 ± 0.005 | 0.01 ± 0.007 | 0.01 ± 0.007 | 0.01 ± 0.004 |
| SCD18 index | 0.66 ± 0.23 | 0.43 ± 0.04 * | 0.68 ± 0.37 | 0.75 ± 0.26 | 0.62 ± 0.13 | 0.62 ± 0.14 |
| ∆6D index | 0.01 ± 0.003 | 0.01 ± 0.004 | 0.009 ± 0.003 | 0.01 ± 0.002 | 0.01 ± 0.004 | 0.01 ± 0.004 |
| Elovl-5 index | 3.38 ± 0.89 | 2.96 ± 1.14 | 3.35 ± 2.07 | 5.71 ± 1.73 * | 5.03 ± 0.95 | 7.44 ± 4.92 |
| Elovl-6 index | 0.79 ± 0.09 | 0.91 ± 0.05 * | 0.77 ± 0.22 | 1.05 ± 0.18 * | 1.11 ± 0.12 | 1.04 ± 0.08 |
| Elongation FA index | 1.59 ± 0.48 | 1.3 ± 0.05 | 1.24 ± 0.14 | 1.79 ± 0.1 | 1.8 ± 0.1 | 1.68 ±0.06 Δ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misiakiewicz-Has, K.; Maciejewska-Markiewicz, D.; Rzeszotek, S.; Pilutin, A.; Kolasa, A.; Szumilas, P.; Stachowska, E.; Wiszniewska, B. The Obscure Effect of Tribulus terrestris Saponins Plus Inulin on Liver Morphology, Liver Fatty Acids, Plasma Glucose, and Lipid Profile in SD Rats with and without Induced Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 8680. https://doi.org/10.3390/ijms22168680
Misiakiewicz-Has K, Maciejewska-Markiewicz D, Rzeszotek S, Pilutin A, Kolasa A, Szumilas P, Stachowska E, Wiszniewska B. The Obscure Effect of Tribulus terrestris Saponins Plus Inulin on Liver Morphology, Liver Fatty Acids, Plasma Glucose, and Lipid Profile in SD Rats with and without Induced Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2021; 22(16):8680. https://doi.org/10.3390/ijms22168680
Chicago/Turabian StyleMisiakiewicz-Has, Kamila, Dominika Maciejewska-Markiewicz, Sylwia Rzeszotek, Anna Pilutin, Agnieszka Kolasa, Paweł Szumilas, Ewa Stachowska, and Barbara Wiszniewska. 2021. "The Obscure Effect of Tribulus terrestris Saponins Plus Inulin on Liver Morphology, Liver Fatty Acids, Plasma Glucose, and Lipid Profile in SD Rats with and without Induced Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 22, no. 16: 8680. https://doi.org/10.3390/ijms22168680







