Anti-Cancer and Electrochemical Properties of Thiogenistein—New Biologically Active Compound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Measurements
2.3. IR Measurements
2.4. Raman Spectroscopy Measurement
2.5. MS Spectrometry
2.6. Quantum Mechanical Modeling
2.7. In Vitro Study
3. Results and Discussion
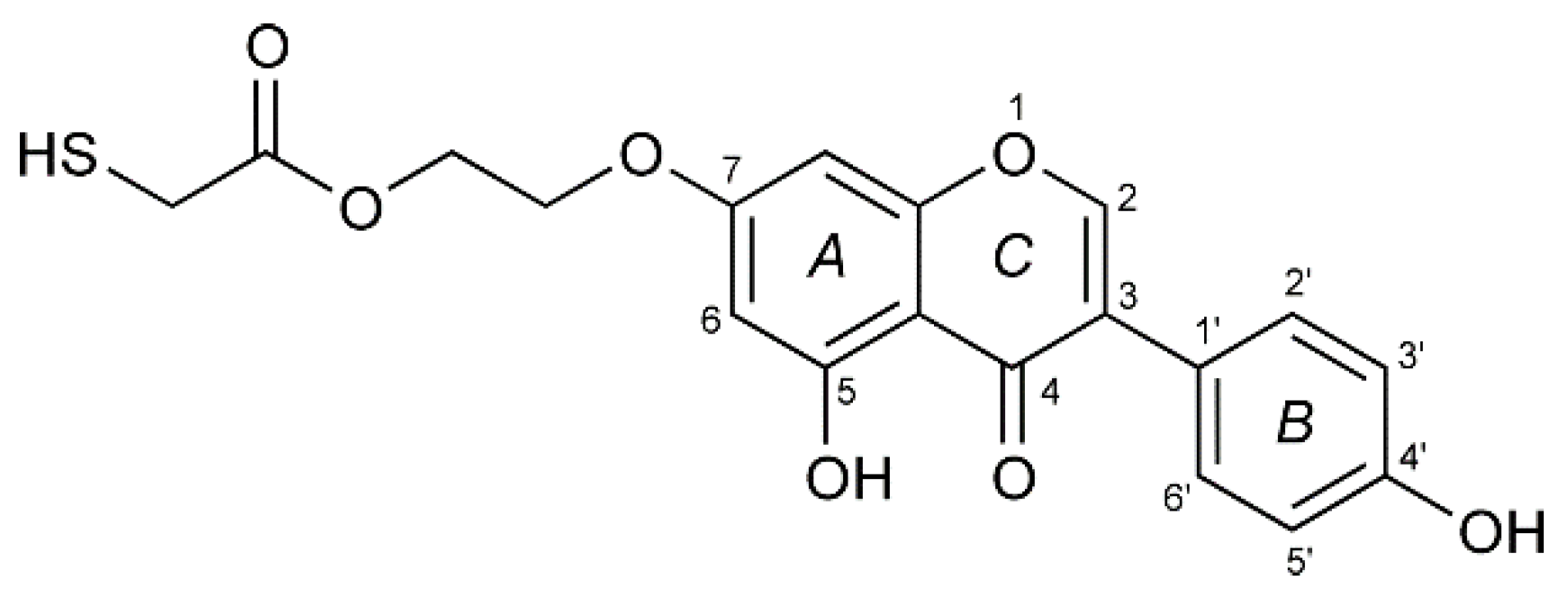
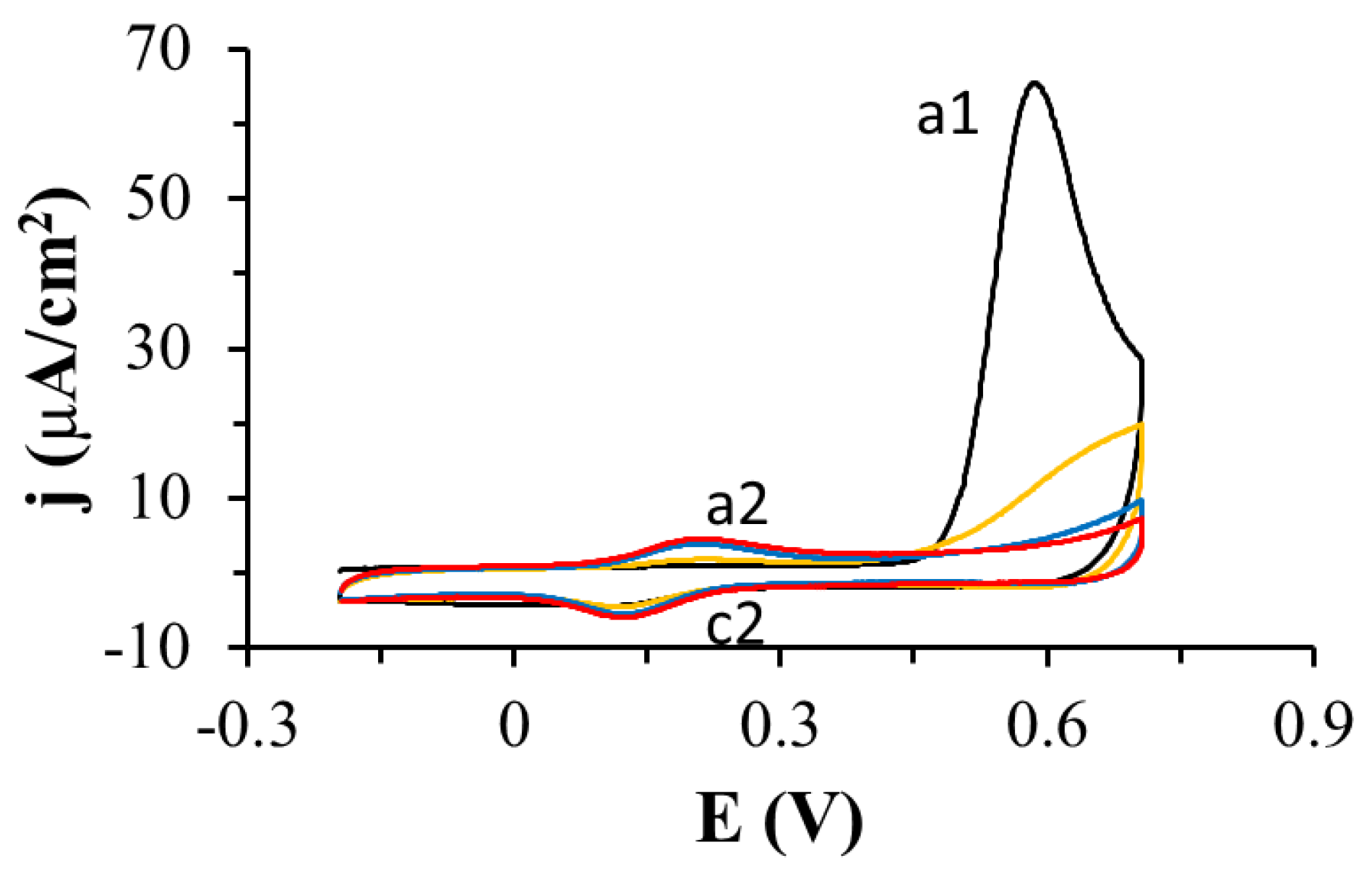
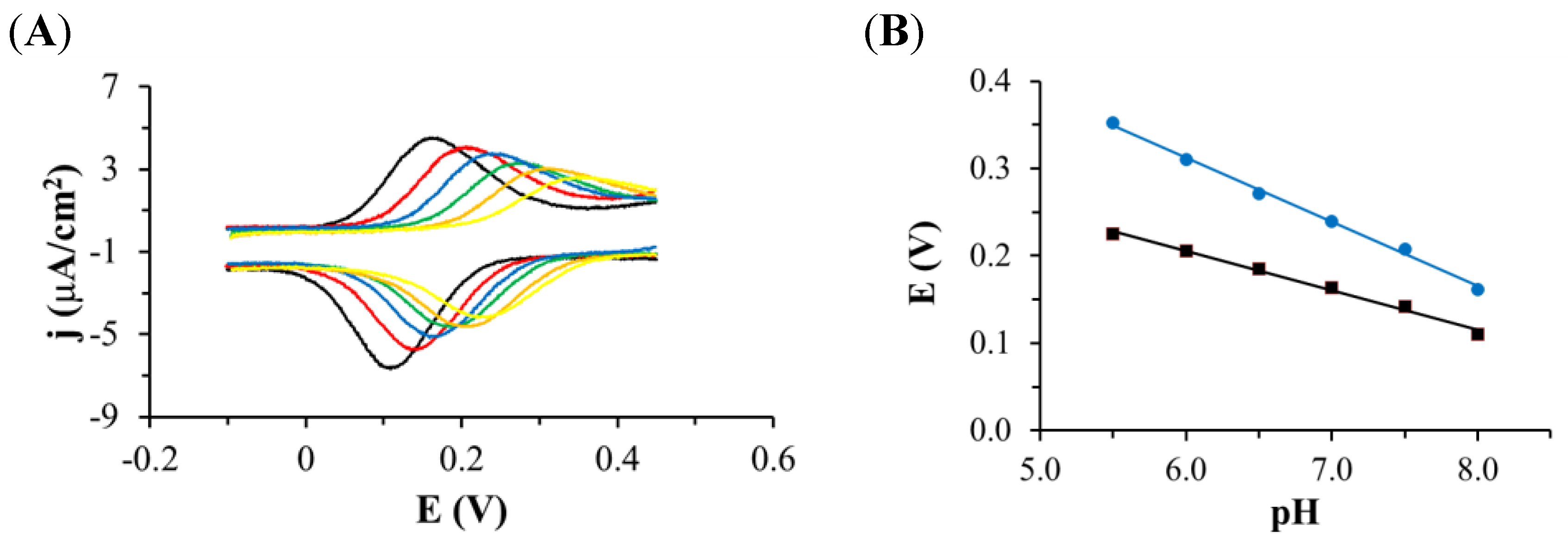
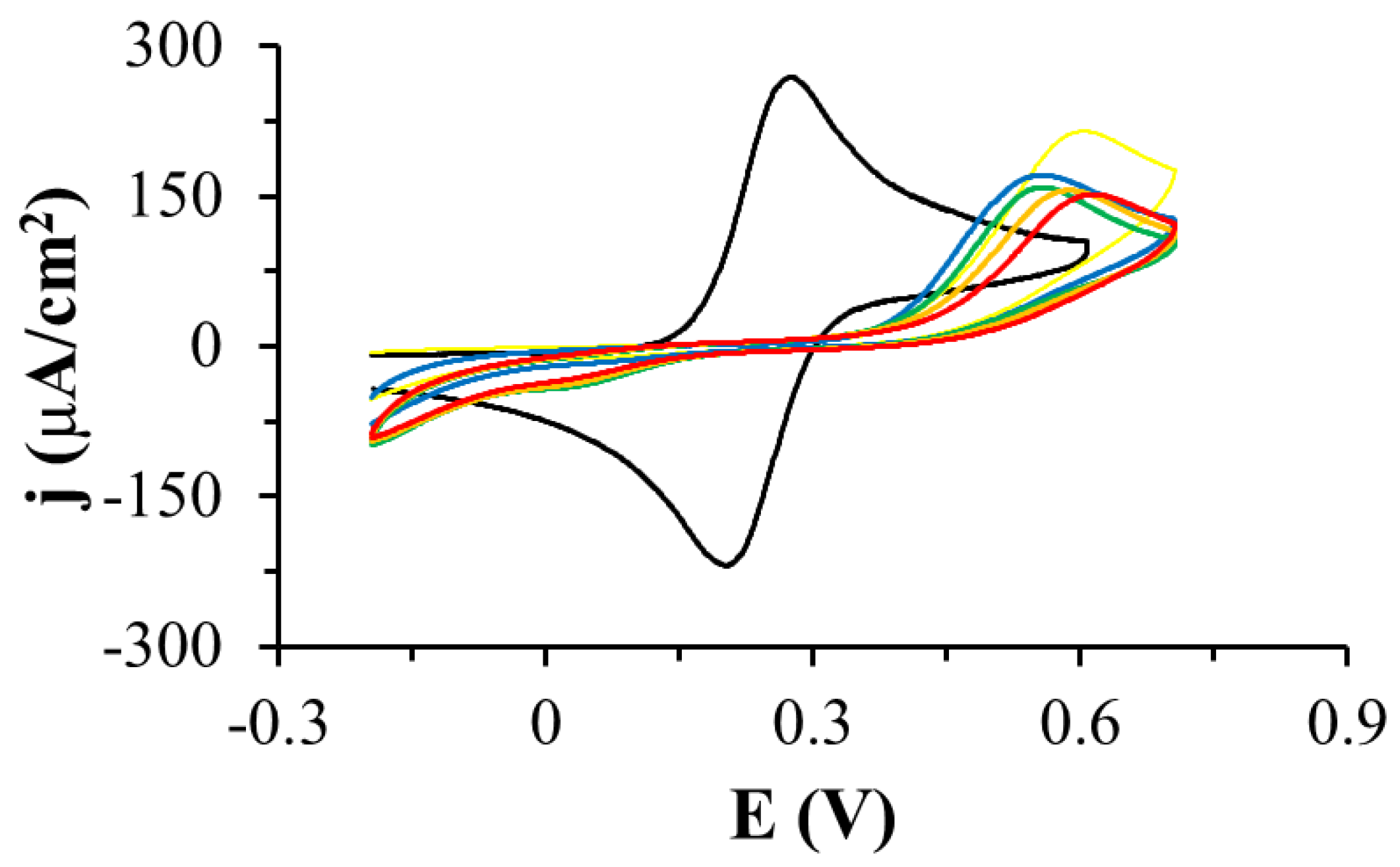
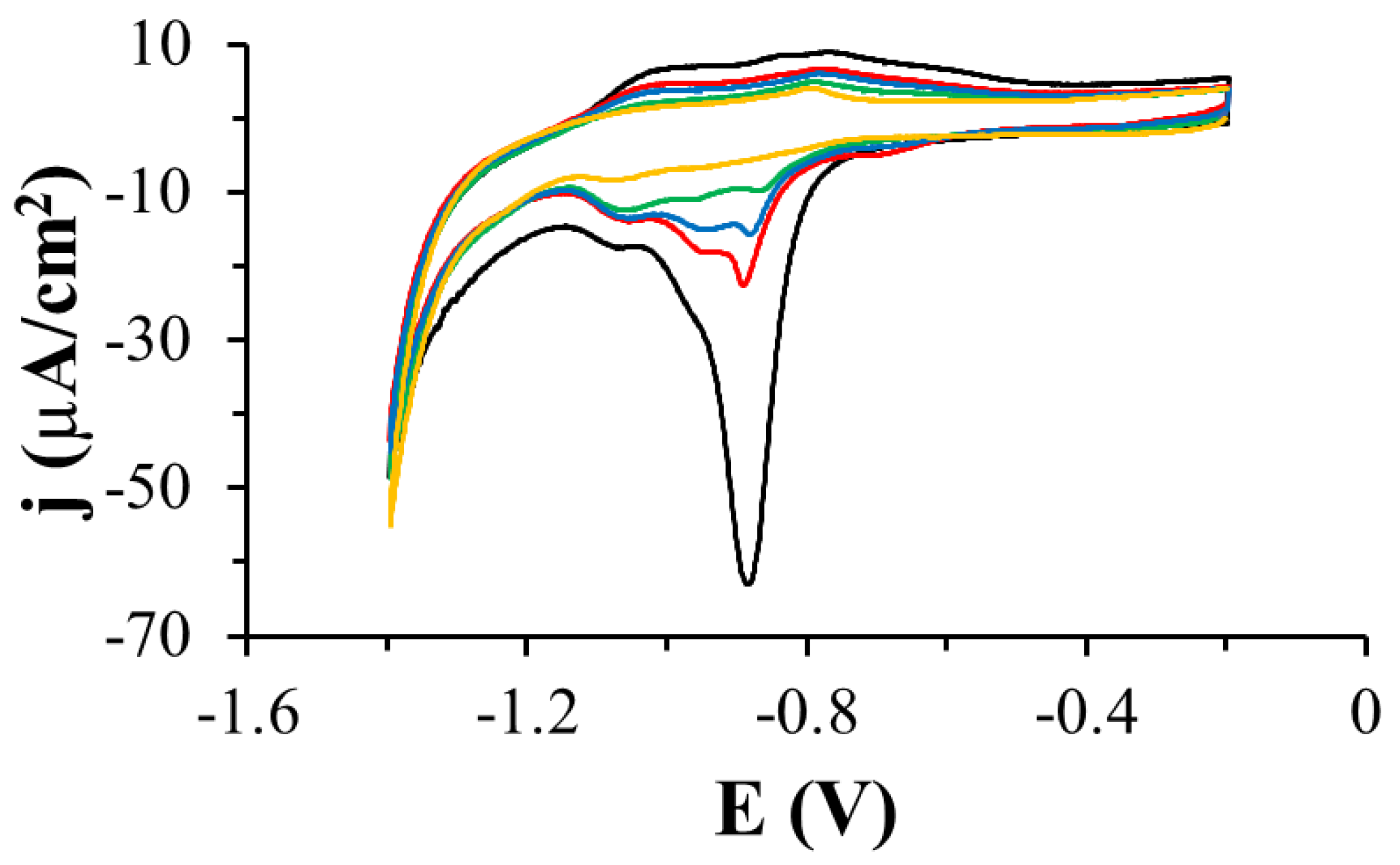
3.1. Electrochemistry
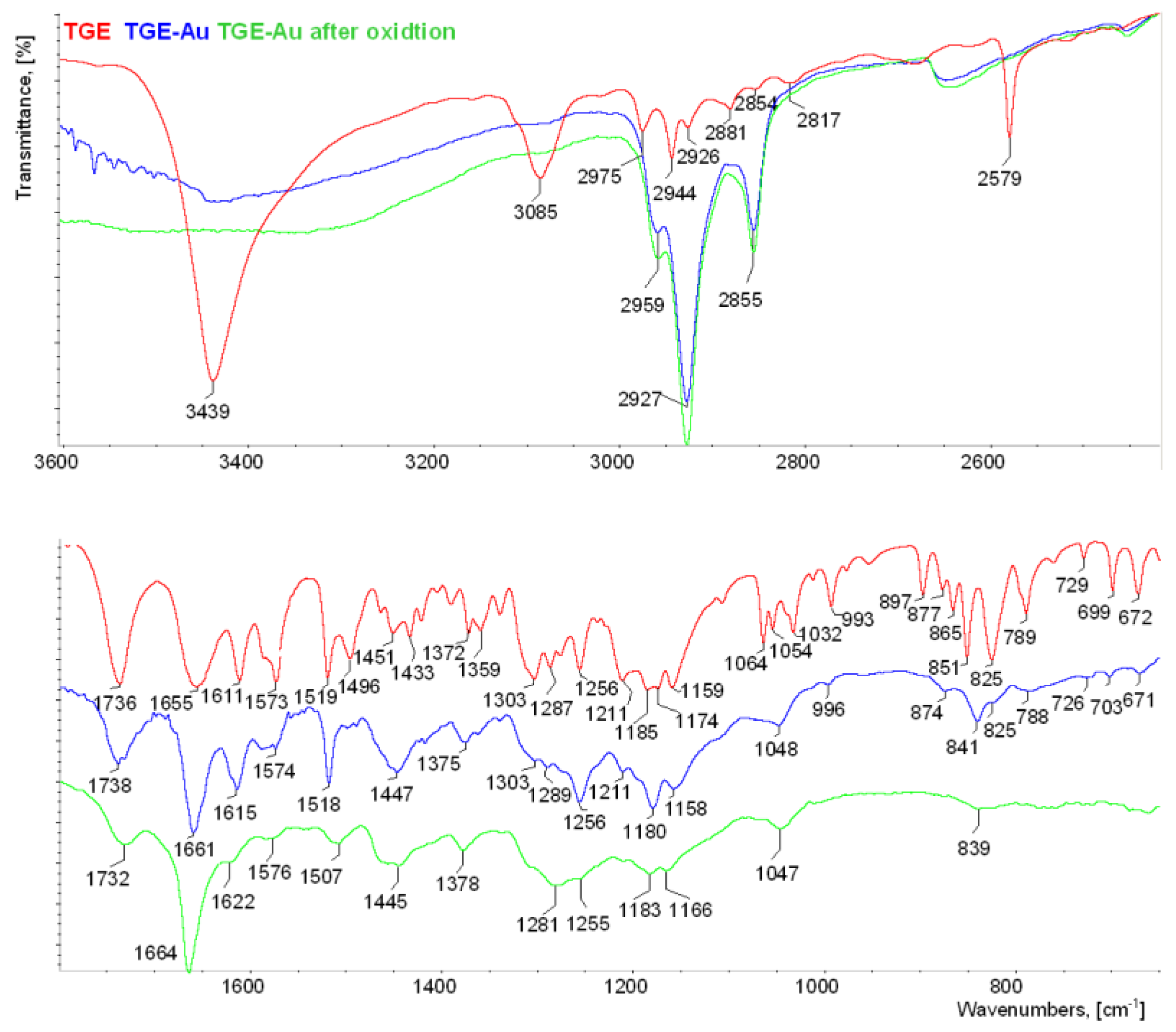
3.2. IR Spectroscopy
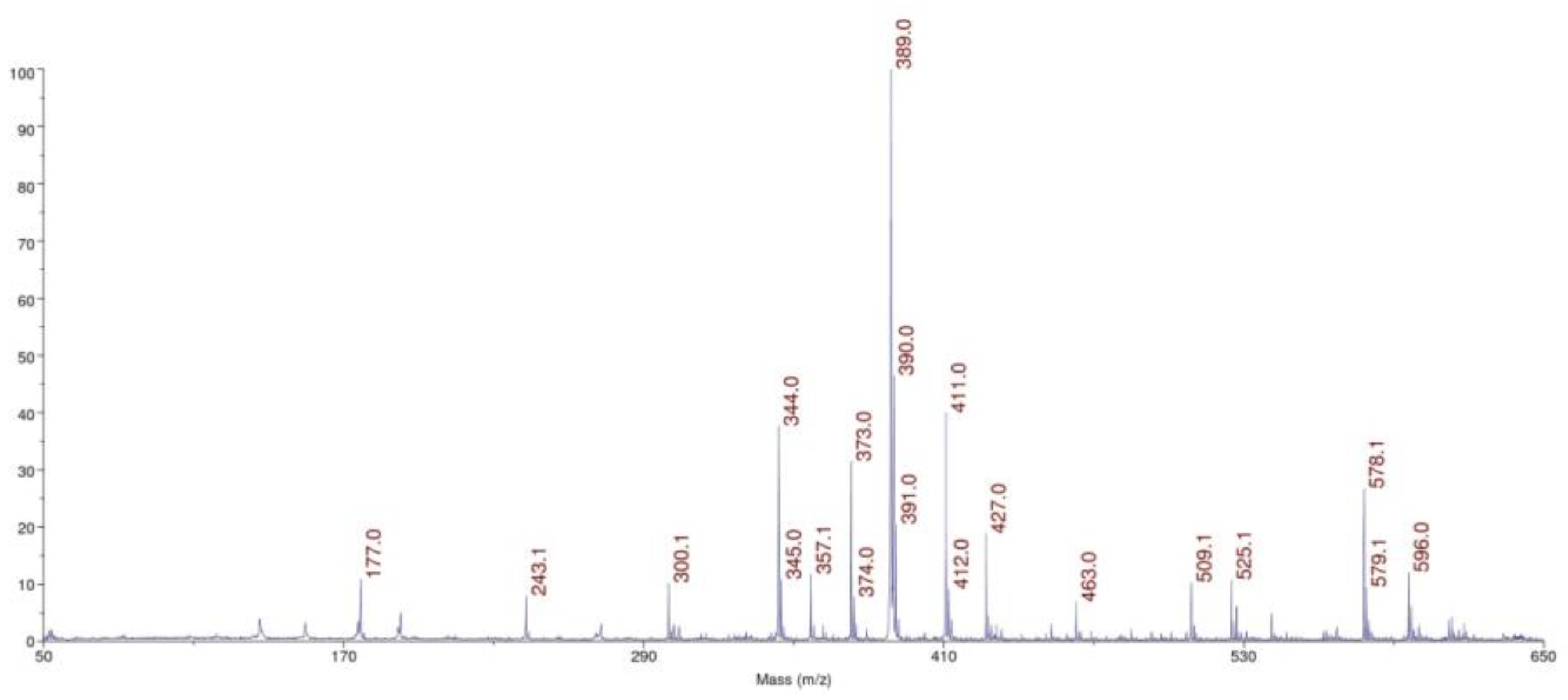
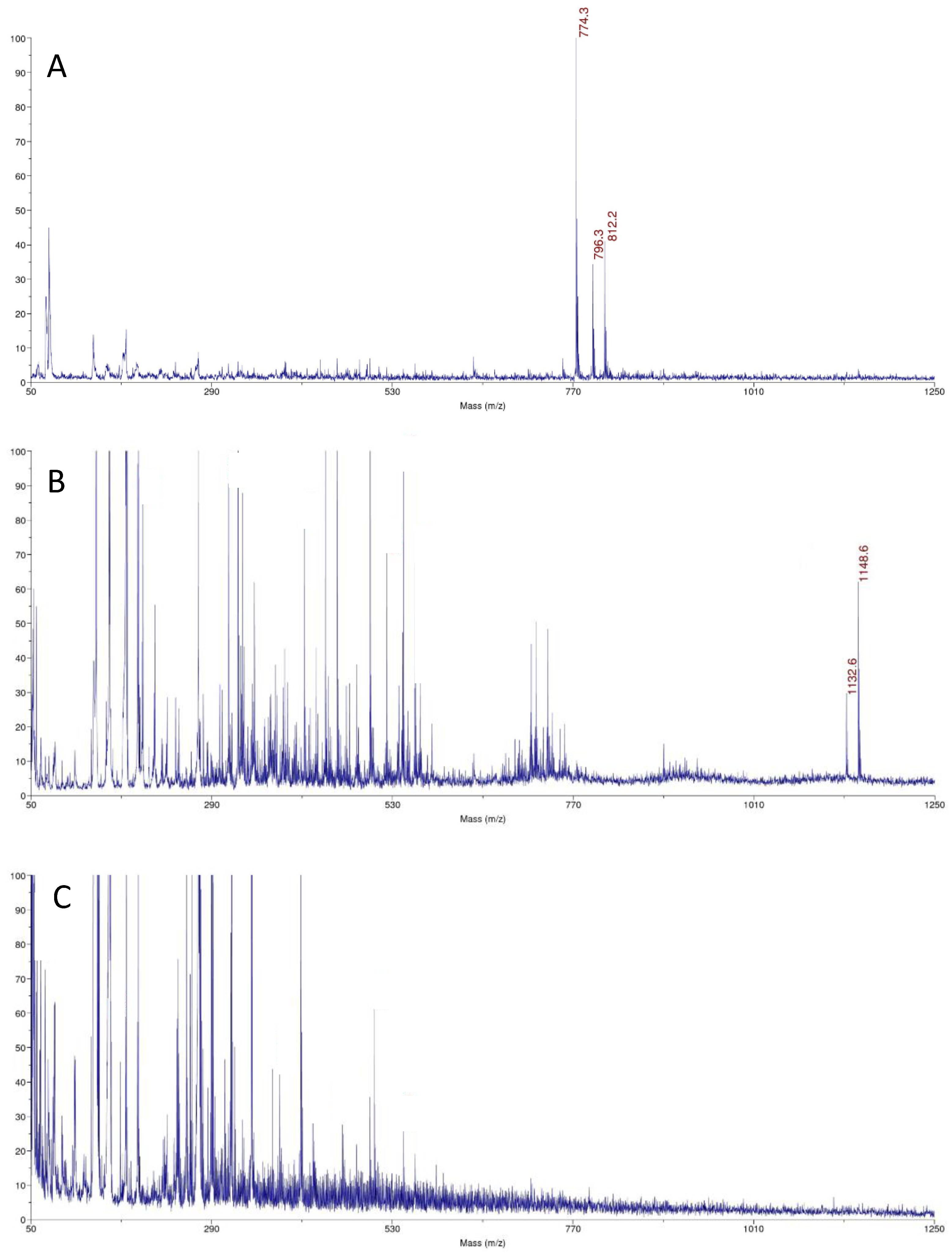
3.3. MALDI-TOF MS
3.4. Molecular Modeling and the Quantum Mechanical Density Functional Calculations
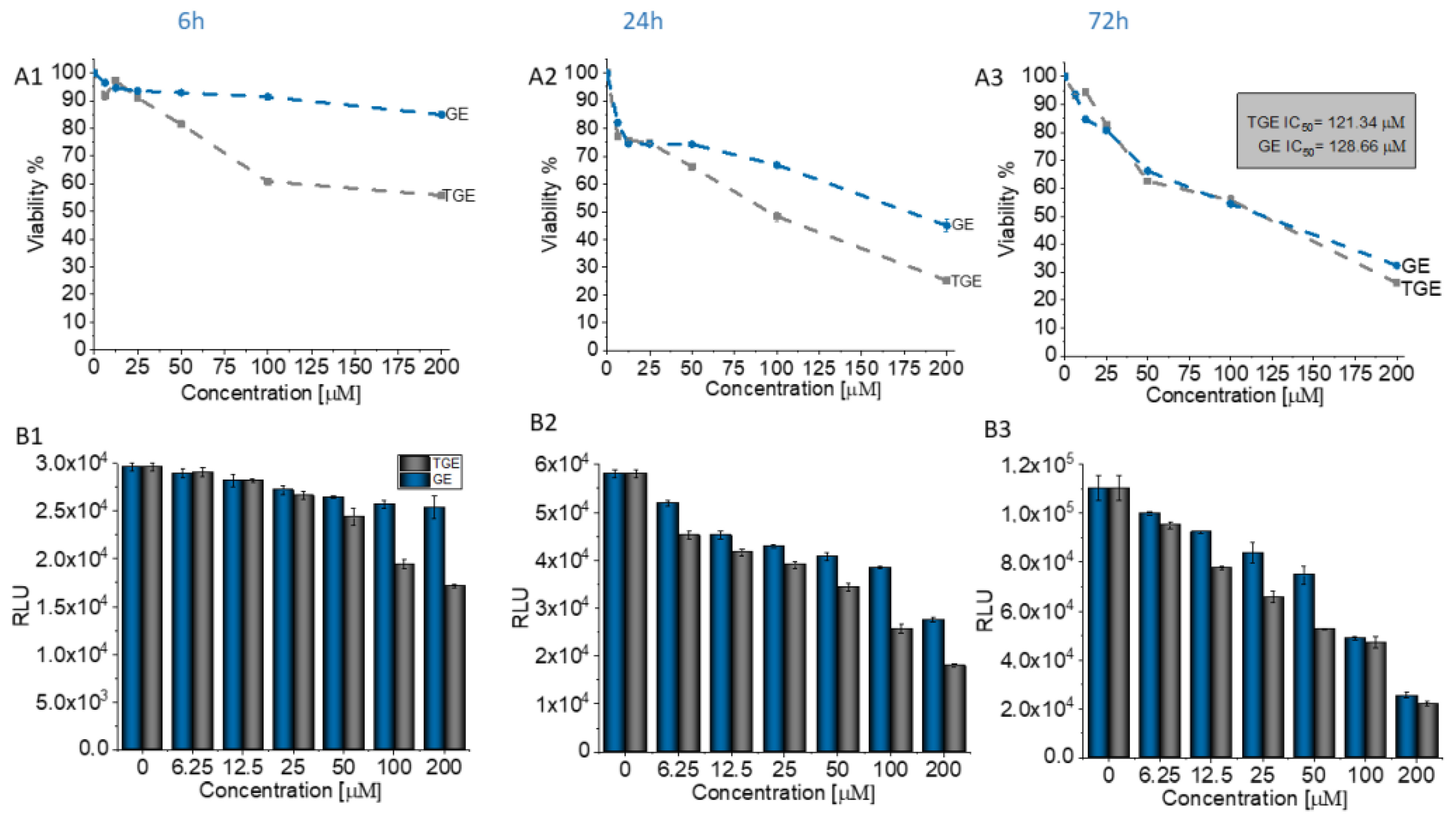
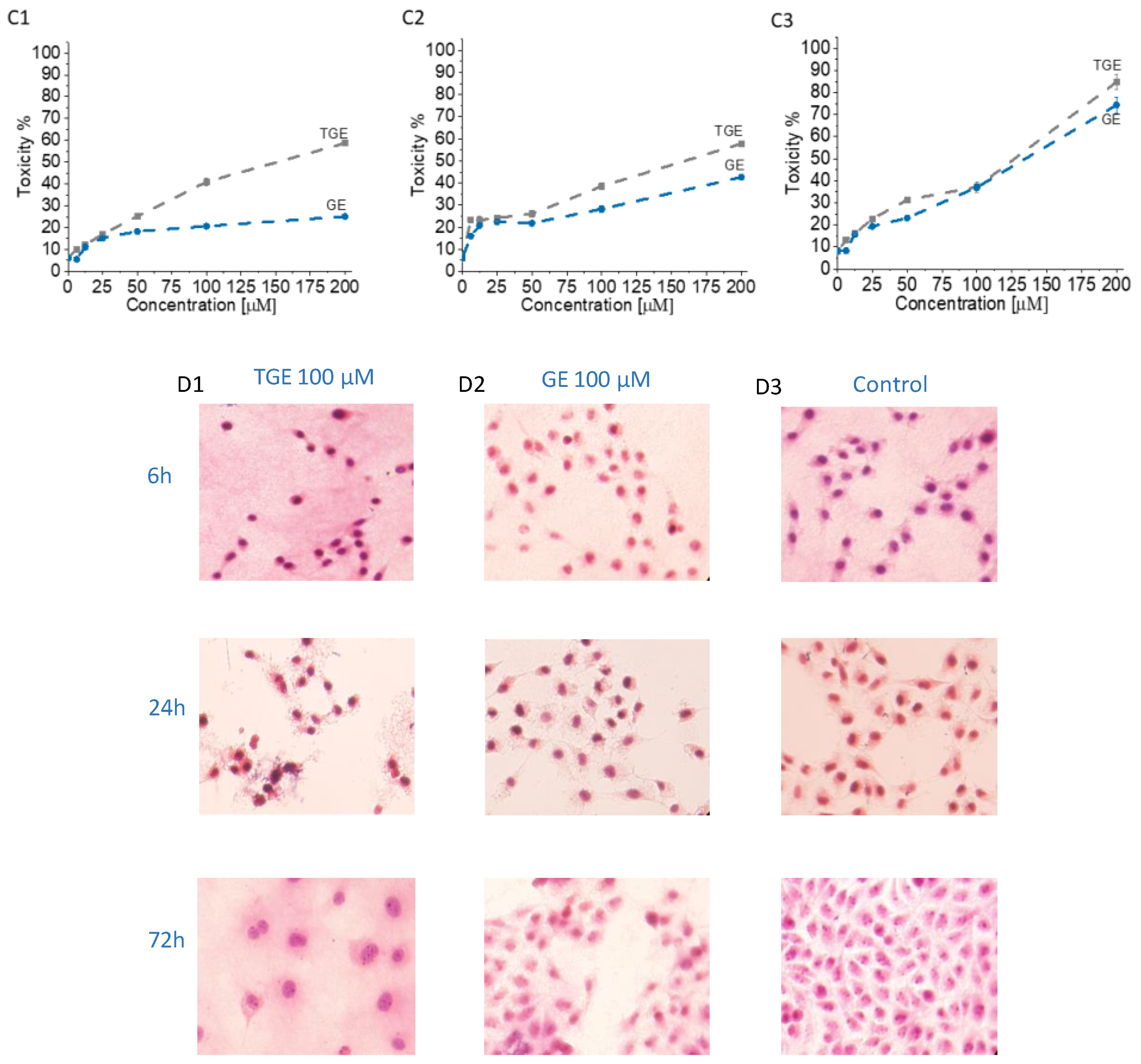
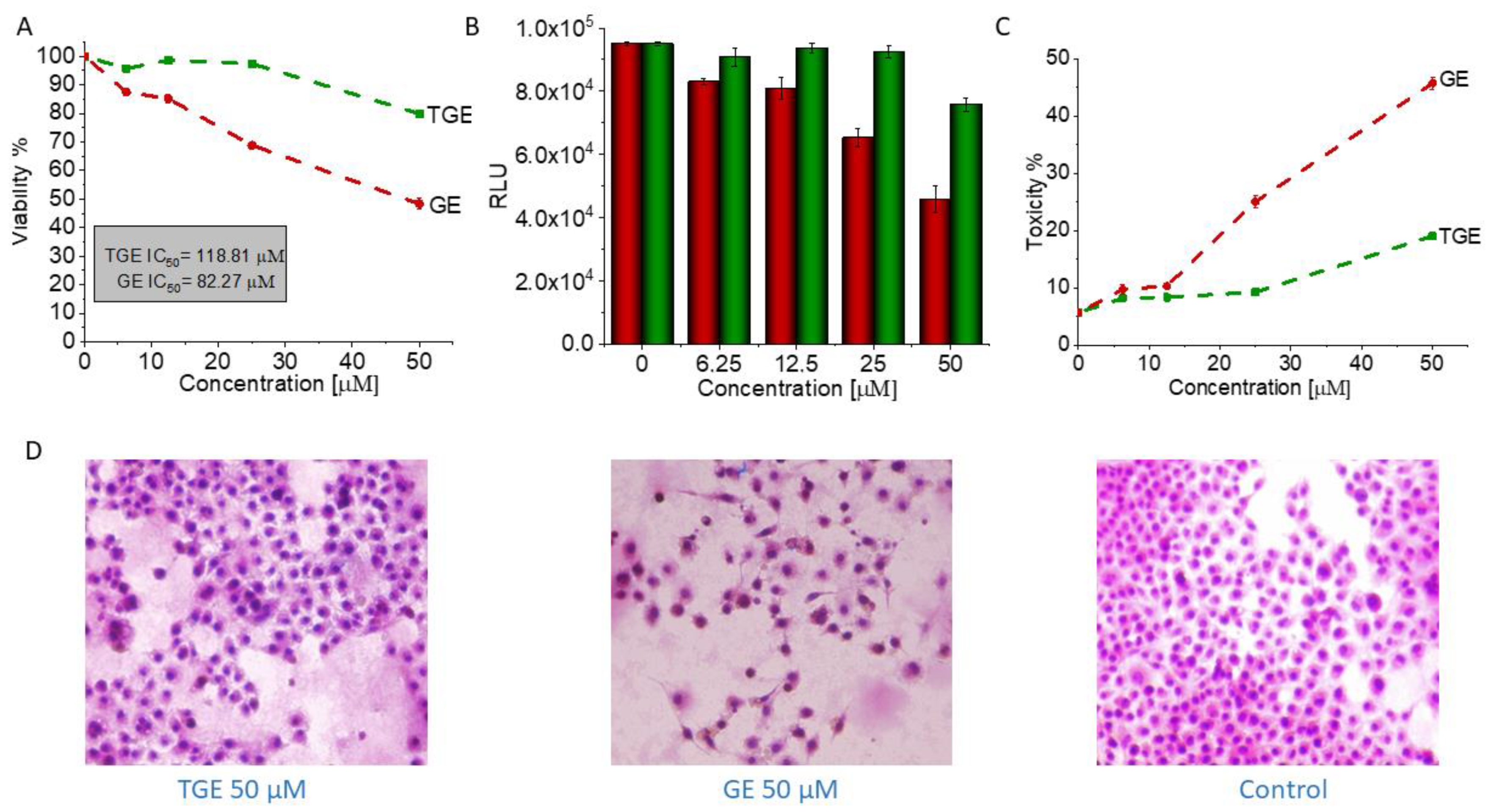
3.5. In Vitro Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, P.; Wang, R.; Zhang, X.; DeLa Torre, E.; Leon, F.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.-H. Design, Synthesis, and Evaluation of Genistein Analogues as Anti-Cancer Agents. Anti-Cancer Agents Med. Chem. 2015, 15, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Zagórska-Dziok, M.; Kleczkowska, P.; Olędzka, E.; Figat, R.; Sobczak, M. Poly(Chitosan-ester-ether-urethane) hydrogels as highly controlled genistein release systems. Int. J. Mol. Sci. 2021, 22, 3339. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Ho, C.-T.; Chen, Y.; Wang, Y.; Wei, Z.; Dong, M.; Huang, Q. Synthesis, Characterization, and Evaluation of Genistein-Loaded Zein/Carboxymethyl Chitosan Nanoparticles with Improved Water Dispersibility, Enhanced Antioxidant Activity, and Controlled Release Property. Foods 2020, 9, 1604. [Google Scholar] [CrossRef]
- Stolarczyk, E.U.; Stolarczyk, K.; Łaszcz, M.; Kubiszewski, M.; Maruszak, W.; Olejarz, W.; Bryk, D. Synthesis and characterization of genistein conjugated with gold nanoparticles and the study of their cytotoxic properties. Eur. J. Pharm. Sci. 2017, 96, 176–185. [Google Scholar] [CrossRef]
- Vodnik, V.V.; Mojić, M.; Stamenović, U.; Otoničar, M.; Ajdžanović, V.; Maksimović-Ivanić, D.; Mijatović, S.; Marković, M.M.; Barudžija, T.; Filipović, B.; et al. Development of genistein-loaded gold nanoparticles and their antitumor potential against prostate cancer cell lines. Mater. Sci. Eng. C 2021, 124, 112078. [Google Scholar] [CrossRef] [PubMed]
- Dinkel, R.; Braunschweig, B.; Peukert, W. Fast and slow ligand exchange at the surface of colloidal gold nanoparticles. J. Phys. Chem. C 2016, 120, 1673–1682. [Google Scholar] [CrossRef]
- Sidoryk, K.; Michalak, O.; Kubiszewski, M.; Leś, A.; Cybulski, M.; Stolarczyk, E.U.; Doubsky, J. Synthesis of thiol derivatives of biological active compounds for nanotechnology application. Molecules 2020, 25, 3470. [Google Scholar] [CrossRef]
- Jiang, C.; Elliott, J.M.; Cardin, D.J.; Tsang, S.C. An electrochemical study of 4-aminothiophenol/Pt nanoparticle multilayers on gold electrodes. Langmuir 2008, 25, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Lukkari, J.; Kleemola, K.; Meretoja, M.; Ollonqvist, T.; Kankare, J. Electrochemical post-self-assembly transformation of 4-aminothiophenol monolayers on gold electrodes. Langmuir 1998, 14, 1705–1715. [Google Scholar] [CrossRef]
- Porter, M.D.; Bright, T.B.; Allara, D.L.; Chidsey, C.E. Spontaneously Organized Molecular Assemblies. 4. Structural Characterization of n-Alkyl Thiol Monolayers on Gold by Optical Ellipsometry, Infrared Spectroscopy, and Electrochemistry. J. Am. Chem. Soc. 1987, 109, 3559–3568. [Google Scholar] [CrossRef]
- Marshall, G.M.; Bensebaa, F.; Dubowski, J.J. Observation of surface enhanced IR absorption coefficient in alkanethiol based self-assembled monolayers on GaAs(001). J. Appl. Phys. 2009, 105, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Peng, D.K.; Lahann, J. Chemical, Electrochemical, and Structural Stability of Low-Density Self-Assembled Monolayers. Langmuir 2007, 23, 10184–10189. [Google Scholar] [CrossRef]
- Nuzzo, R.G.; Fusco, F.A.; Allara, D.L. Spontaneously Organized Molecular Assemblies. 3. Preparation and Properties of Solution Adsorbed Monolayers of Organic Disulfides on Gold Surfaces. J. Am. Chem. Soc. 1987, 109, 2358–2368. [Google Scholar] [CrossRef]
- Sato, Y.; Mizutani, F. Formation and characterization of aromatic selenol and thiol monolayers on gold: In-situ IR studies and electrochemical measurements. Phys. Chem. Chem. Phys. 2004, 6, 1328–1331. [Google Scholar] [CrossRef]
- Skoda, M.W.A.; Jacobs, R.M.J.; Willis, J.; Schreiber, F. Hydration of oligo(ethylene glycol) self-assembled monolayers studied using polarization modulation infrared spectroscopy. Langmuir 2007, 23, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Corno, M.; Rimola, A.; Bolis, V.; Ugliengo, P. Hydroxyapatite as a key biomaterial: Quantum-mechanical simulation of its surfaces in interaction with biomolecules. Phys. Chem. Chem. Phys. 2010, 12, 6309–6329. [Google Scholar] [CrossRef]
- Stevens, C.A.; Safazadeh, L.; Berron, B.J. Thiol-yne adsorbates for stable, low-density, self-assembled monolayers on gold. Langmuir 2014, 30, 1949–1956. [Google Scholar] [CrossRef]
- Tao, Y.T.; Wu, C.C.; Eu, J.Y.; Lin, W.L.; Wu, K.C.; Chen, C.H. Structure evolution of aromatic-derivatized thiol monolayers on evaporated gold. Langmuir 1997, 13, 4018–4023. [Google Scholar] [CrossRef]
- Wan, L.J.; Terashima, M.; Noda, H.; Osawa, M. Molecular orientation and ordered structure of benzenethiol adsorbed on gold(111). J. Phys. Chem. B 2000, 104, 3563–3569. [Google Scholar] [CrossRef]
- Kim, I. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Rizzo, G. The antioxidant role of soy and soy foods in human health. Antioxidants 2020, 9, 635. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03. Available online: http://gaussian.com (accessed on 13 August 2021).
- Hayes, W.A.; Shannon, C. Electrochemistry of surface-confined mixed monolayers of 4-aminothiophenol and thiophenol on Au. Langmuir 1996, 12, 3688–3694. [Google Scholar] [CrossRef]
- Brust, M.; Fink, J.; Bethell, D.; Schiffrin, D.J.; Kiely, C. Synthesis and reactions of functionalised gold nanoparticles. J. Chem. Soc. Chem. Commun. 1995, 1655–1656. [Google Scholar] [CrossRef]
- Jin, Q.; Rodriguez, J.A.; Li, C.Z.; Darici, Y.; Tao, N.J. Self-assembly of aromatic thiols on Au(111). Surf. Sci. 1999, 425, 101–111. [Google Scholar] [CrossRef]
- Johnson, S.R.; Evans, S.D.; Mahon, S.W.; Ulman, A. Alkanethiol molecules containing an aromatic moiety self-assembled onto gold clusters. Langmuir 1997, 13, 51–57. [Google Scholar] [CrossRef]
- Stolarczyk, K.; Bilewicz, R. Catalytic oxidation of ascorbic acid on 2D and 3D monolayers of 4-hydroxythiophenol. Electroanalysis 2004, 16, 1609–1615. [Google Scholar] [CrossRef]
- Stolarczyk, K.; Pałys, B.; Bilewicz, R. Catalytic properties of 4-hydroxythiophenol protected gold nanoclusters supported on gold electrodes. J. Electroanal. Chem. 2004, 564, 93–98. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Sinniah, K.; Cheng, J.; Terrettaz, S.; Reutt-Robey, J.E.; Miller, G.J. Self-assembled ω-hydroxyalkanethiol monolayers with internal functionalities: Electrochemical and infrared structural characterizations of ether-containing monolayers. J. Phys. Chem. 1995, 99, 14500–14505. [Google Scholar] [CrossRef]
- Crupi, V.; Majolino, D.; Paciaroni, A.; Rossi, B.; Stancanelli, R.; Venuti, V.; Viliani, G. The effect of hydrogen bond on the vibrational dynamics of genistein free and complexed with β-cyclodextrins. J. Raman Spectrosc. 2010, 41, 764–770. [Google Scholar] [CrossRef]
- Mrksich, M. Mass spectrometry of self-assembled monolayers: A new tool for molecular surface science. ACS Nano 2008, 2, 7–18. [Google Scholar] [CrossRef]
- Ha, T.K.; Oh, H.B.; Chung, J.; Lee, T.G.; Han, S.Y. Investigation of the MALDI process used to characterize self-assembled monolayers of alkanethiolates on gold. Langmuir 2009, 25, 3692–3697. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Honig, D.M.; Metzler, M. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo. J. Agric. Food Chem. 2001, 49, 3024–3033. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Valcic, S.; Cornejo, S.; Nair, M.G.; Timmermann, B.N.; Liebler, D.C. Reactions of genistein with alkylperoxyl radicals. Chem. Res. Toxicol. 2000, 13, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; De Freitas, V. Tannins in food: Insights into the molecular perception of astringency and bitter taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Da Santos, C.C.; Pimenta, T.C.; Thomasini, R.L.; Verly, R.M.; Franco, D.L.; Ferreira, L.F. Electropolymerization of phenol and aniline derivatives: Synthesis, characterization and application as electrochemical transducers. J. Electroanal. Chem. 2019, 846, 113163. [Google Scholar] [CrossRef]
- Zhang, W.; Bao, L.; Zhang, X.; He, J.; Wei, G. Electropolymerization Treatment of Phenol Wastewater and the Reclamation of Phenol. Water Environ. Res. 2012, 84, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Khasanov, V.; Kravtsova, S.; Dychko, K.; Khasanov, V. Investigation of intermediate oxidation products of genistein and luteolin generated in aerobic conditions. Key Eng. Mater. 2015, 670, 171–176. [Google Scholar] [CrossRef]
- Niu, L.T.; Li, G.G.; Li, H.F.; Cui, F.; Zhang, J.; Huang, Y.; Chen, K.; Zhang, J.F.; Li, W.F.; Liu, W.L. A Novel Genistein Electrochemical Sensor Based on Molecularly Imprinted Polycarbazole/Carboxylated Multiwalled Carbon Nanotubes Nanocomposite. Chin. J. Anal. Chem. 2019, 47, e19095–e19103. [Google Scholar] [CrossRef]
- Li, F.; Wang, Q.; Ding, Z.; Tao, F. Microwave-assisted synthesis of diaryl ethers without catalyst. Org. Lett. 2003, 5, 2169–2171. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Hernandez-Montes, E.; Vauzour, D.; Schönthal, A.H.; Rice-Evans, C.; Cadenas, E.; Spencer, J.P.E. The intracellular genistein metabolite 5,7,3′,4′-tetrahydroxyisoflavone mediates G2-M cell cycle arrest in cancer cells via modulation of the p38 signaling pathway. Free Radic. Biol. Med. 2006, 41, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Kiriakidis, S.; Högemeier, O.; Starcke, S.; Dombrowski, F.; Hahne, J.C.; Pepper, M.; Jha, H.C.; Wernert, N. Novel tempeh (fermented soyabean) isoflavones inhibit in vivo angiogenesis in the chicken chorioallantoic membrane assay. Br. J. Nutr. 2005, 93, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [CrossRef]
- Markovits, J.; Linassier, C.; Fossé, P.; Couprie, J.; Pierre, J.; Jacquemin-Sablon, A.; Saucier, J.M.; Le Pecq, J.B.; Larsen, A.K. Inhibitory Effects of the Tyrosine Kinase Inhibitor Genistein on Mammalian DNA Topoisomerase II. Cancer Res. 1989, 49, 5111–5117. [Google Scholar]
- Popiołkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In vitro toxicity evaluation in the development of new anticancer drugs—Genistein glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef]
- Grosso, R.; De-Paz, M.-V. Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies—Reviewing Thiomers from Chitosan and Hyaluronic Acid. Pharmaceutics 2021, 13, 854. [Google Scholar] [CrossRef]
- Berube, G. Natural and Synthetic Biologically Active Dimeric Molecules: Anticancer Agents, Anti-HIV Agents, Steroid Derivatives and Opioid Antagonists. Curr. Med. Chem. 2005, 13, 131–154. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Atsumi, T.; Murakami, Y.; Kadoma, Y. Dimerization, ROS formation, and biological activity of o-methoxyphenols. Arch. Immunol. Ther. Exp. 2005, 53, 28–38. [Google Scholar]
- Paquin, A.; Reyes-Moreno, C.; Bérubé, G. Recent advances in the use of the dimerization strategy as a means to increase the biological potential of natural or synthetic molecules. Molecules 2021, 26, 2340. [Google Scholar] [CrossRef] [PubMed]

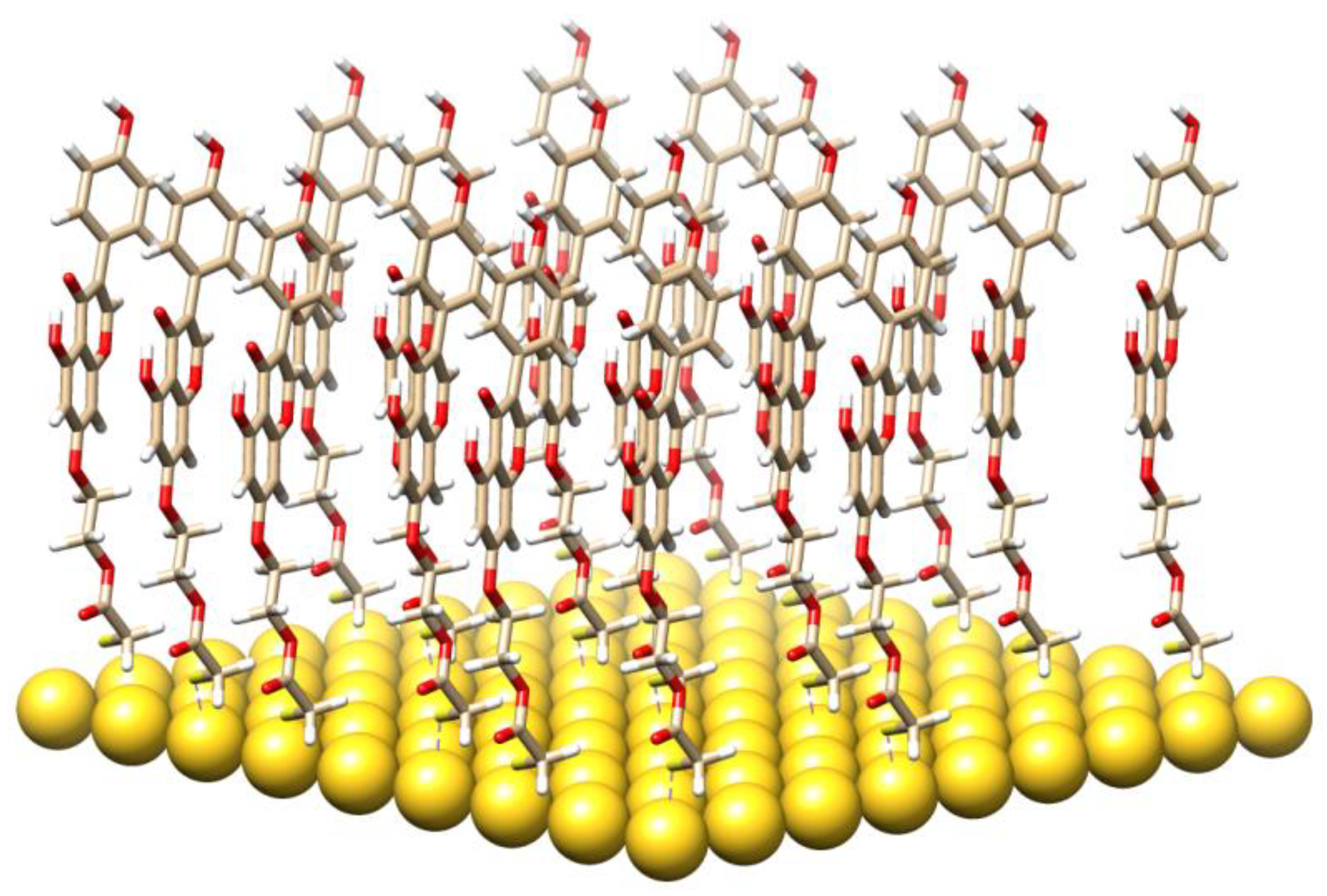
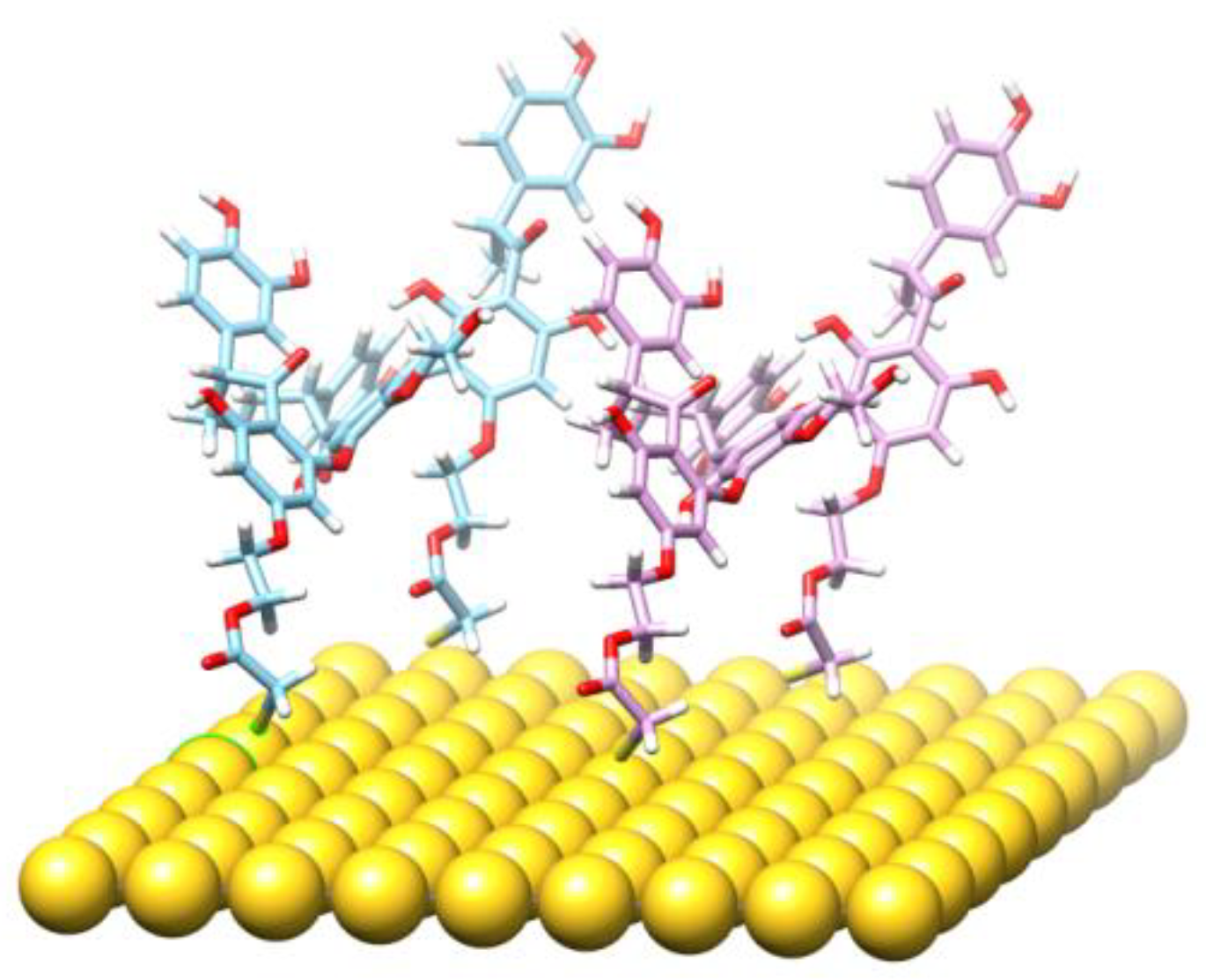
| System | IR | B3LYP | Comments |
|---|---|---|---|
| TGE | 1655 | 1689 | B3LYP for H-bonded TGE, Figure S2 |
| Au-TGE | 1661 | 1709 | B3LYP for broken H-bond, Figure S3 |
| Difference | 6 | 20 | - |
| Atom | Ring B | Atom | Ring A | ||
|---|---|---|---|---|---|
| B3LYP | H-F | B3LYP | H-F | ||
| O(at C4’) | 0.609 | 0.899 | O(at C5) | 0.624 | 0.928 |
| C4’ | −0.089 | −0.692 | C5 | −0.148 | −0.673 |
| C3’ | 0.231 | 0.784 | C5-4 (*) | 0.16 | 0.792 |
| C5’ | 0.221 | 0.781 | C6 | 0.311 | 0.748 |
| C2’ | −0.134 | −0.819 | C7 | −0.116 | −0.689 |
| C6’ | −0.133 | −0.833 | C8-1 | −0.113 | −0.722 |
| C8 | 0.272 | 0.729 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolarczyk, E.U.; Strzempek, W.; Łaszcz, M.; Leś, A.; Menaszek, E.; Sidoryk, K.; Stolarczyk, K. Anti-Cancer and Electrochemical Properties of Thiogenistein—New Biologically Active Compound. Int. J. Mol. Sci. 2021, 22, 8783. https://doi.org/10.3390/ijms22168783
Stolarczyk EU, Strzempek W, Łaszcz M, Leś A, Menaszek E, Sidoryk K, Stolarczyk K. Anti-Cancer and Electrochemical Properties of Thiogenistein—New Biologically Active Compound. International Journal of Molecular Sciences. 2021; 22(16):8783. https://doi.org/10.3390/ijms22168783
Chicago/Turabian StyleStolarczyk, Elżbieta U., Weronika Strzempek, Marta Łaszcz, Andrzej Leś, Elżbieta Menaszek, Katarzyna Sidoryk, and Krzysztof Stolarczyk. 2021. "Anti-Cancer and Electrochemical Properties of Thiogenistein—New Biologically Active Compound" International Journal of Molecular Sciences 22, no. 16: 8783. https://doi.org/10.3390/ijms22168783
APA StyleStolarczyk, E. U., Strzempek, W., Łaszcz, M., Leś, A., Menaszek, E., Sidoryk, K., & Stolarczyk, K. (2021). Anti-Cancer and Electrochemical Properties of Thiogenistein—New Biologically Active Compound. International Journal of Molecular Sciences, 22(16), 8783. https://doi.org/10.3390/ijms22168783






