Effects of Primary Mast Cell Disease on Hemostasis and Erythropoiesis
Abstract
:1. Introduction
2. Impact on Hemostasis and Thrombosis
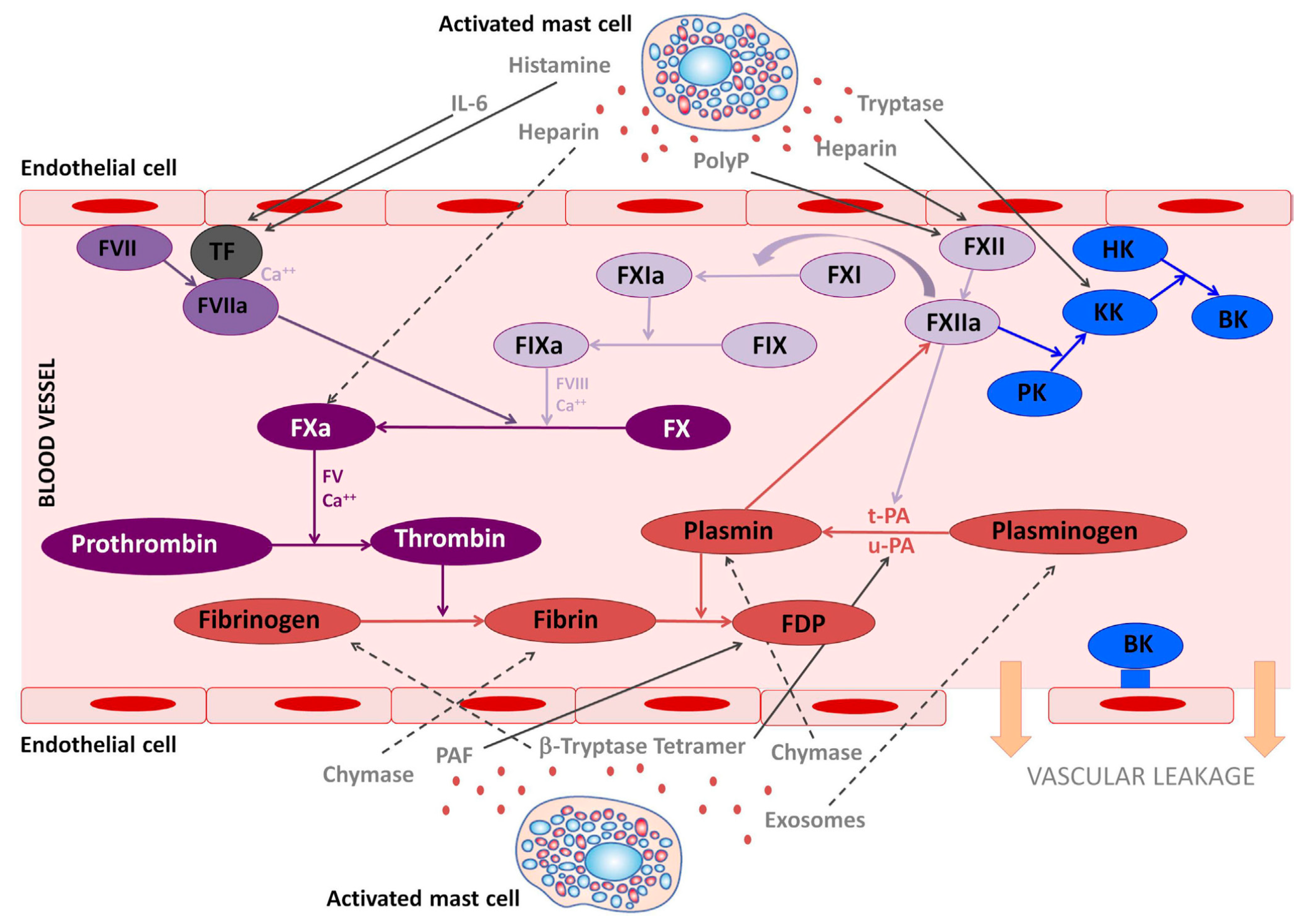
2.1. Mast Cells as Parts of Bleeding Diatheses
2.1.1. Laboratory Diagnostics
2.1.2. Treatment Options in Mast Cell Mediator-Induced Bleeding
2.2. Mast Cells as Parts of Increased Thrombophilia
Therapeutic Procedures in Patients with Mast Cell Disease and Thrombophilia
3. Impact on Erythropoiesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afrin, L.B.; Butterfield, J.H.; Raithel, M.; Molderings, G.J. Often seen, rarely recognized: Mast cell activation disease—A guide to diagnosis and therapeutic options. Ann. Med. 2016, 48, 190–201. [Google Scholar] [CrossRef]
- Cardamone, C.; Parente, R.; Feo, G.D.; Triggiani, M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol. Lett. 2016, 178, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Ibelgaufts, H. “Mast Cells” in COPE: Cytokines and Cells Online Pathfinder Encyclopaedia. 2020. Available online: http://www.cells-talk.com/ (accessed on 22 June 2021).
- Molderings, G.J.; Haenisch, B.; Bogdanow, M.; Fimmers, R.; Nöthen, M.M. Familial occurrence of systemic mast cell activation disease. PLoS ONE 2013, 8, e76241. [Google Scholar] [CrossRef] [PubMed]
- Maitland, A.; Brock, I.; Reed, W. Immune dysfunction, both mast cell activation disorders and primary immune deficiency, is common among patients with hypermobile spectrum disorder (HSD) or hypermobile type Ehlers Danlos Syndrome (hEDS). In Proceedings of the EDS ECHO Summit, 2–3 October 2020; Available online: https://www.ehlers-danlos.com/eds-echo-summit-2020/ (accessed on 22 June 2021).
- Luskin, K.T.; White, A.A.; Lyons, J.J. The genetic basis and clinical impact of hereditary alpha-tryptasemia. J. Allergy Clin. Immunol. Pract. 2021, 9, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Sobalska-Kwapis, M.; Strapagiel, D.; Lange, M.; Górska, A.; Elberink, J.N.G.O.; van Doormaal, J.; Słomka, M.; Kalinowski, L.; Gruchała-Niedoszytko, M.; et al. Results from a genome-wide association study (GWAS) in Mastocytosis Reveal New Gene Polymorphisms Associated with WHO subgroups. Int. J. Mol. Sci. 2020, 21, 5506. [Google Scholar] [CrossRef] [PubMed]
- Galatà, G.; García-Montero, A.C.; Kristensen, T.; Dawoud, A.A.Z.; Muñoz-González, J.I.; Meggendorfer, M.; Guglielmelli, P.; Hoade, Y.; Alvarez-Twose, I.; Gieger, C.; et al. Genome-wide association study identifies novel susceptibility loci for KIT D816V positive mastocytosis. Am. J. Hum. Genet. 2021, 108, 284–294. [Google Scholar] [CrossRef]
- Altmüller, J.; Haenisch, B.; Kawalia, A.; Menzen, M.; Nöthen, M.M.; Fier, H.; Molderings, G.J. Mutational profiling in the peripheral blood leukocytes of patients with systemic mast cell activation syndrome using next-generation sequencing. Immunogenetics 2017, 69, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B.; Self, S.; Menk, J.; Lazarchick, J. Characterization of mast cell activation syndrome. Am. J. Med. Sci. 2017, 353, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Afrin, L.B. Polycythemia from mast cell activation syndrome: Lessons learned. Am. J. Med. Sci. 2011, 342, 44–49. [Google Scholar] [CrossRef]
- Afrin, L.B. Mast cell activation disorder masquerading as pure red cell aplasia. Int. J. Hematol. 2010, 91, 907–908. [Google Scholar] [CrossRef] [PubMed]
- Frieling, T.; Meis, K.; Kolck, U.W.; Homann, J.; Hülsdonk, A.; Haars, U.; Hertfelder, H.J.; Oldenburg, J.; Seidel, H.; Molderings, G.J. Evidence for mast cell activation in patients with therapy-resistant irritable bowel syndrome. Z. Gastroenterol. 2011, 49, 191–194. [Google Scholar] [CrossRef]
- Rafii, M.; Firooznia, H.; Golimbu, C.; Balthazar, E. Pathologic fracture in systemic mastocytosis: Radiographic spectrum and review of the literature. Clin. Orthop. Relat. Res. 1983, 180, 260–267. [Google Scholar] [CrossRef]
- Marshall, A.; Kavanagh, R.T.; Crisp, A.J. The effect of pamidronate on lumbar spine bone density and pain in osteoporosis secondary to systemic mastocytosis. Br. J. Rheumatol. 1997, 36, 393–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilarte, M.; Sala-Cunill, A.; Luengo, O.; Labrador-Horrillo, M.; Cardona, V. The mast cell, contact, and coagulation system connection in anaphylaxis. Front. Immunol. 2017, 8, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, H.; Molderings, G.J.; Oldenburg, J.; Meis, K.; Kolck, U.W.; Homann, J.; Hertfelder, H.J. Bleeding diathesis in patients with mast cell activation disease. Thromb. Haemost. 2011, 106, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Karhausen, J.; Choi, H.W.; Maddipati, K.R.; Mathew, J.P.; Ma, Q.; Boulaftali, Y.; Lee, R.H.; Bergmeier, W.; Abraham, S.N. Platelets trigger perivascular mast cell degranulation to cause inflammatory responses and tissue injury. Sci. Adv. 2020, 6, eaay6314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalhosa, A.B.; Aouba, A.; Damaj, G.; Canioni, D.; Brouzes, C.; Gyan, E.; Durupt, S.; Durieu, I.; Cathebras, P.; Costédoat-Chalumeau, N.; et al. A French national survey on clotting disorders in mastocytosis. Medicine 2015, 94, e1414. [Google Scholar] [CrossRef] [PubMed]
- Sucker, C.; Mansmann, G.; Steiner, S.; Gattermann, N.; Schmitt-Graeff, A.; Loncar, R.; Scharf, R.E.; Stockschlader, M. Fatal bleeding due to a heparin-like anticoagulant in a 37-year-old woman suffering from systemic mastocytosis. Clin. Appl. Thromb. Hemost. 2008, 14, 360–364. [Google Scholar] [CrossRef]
- Koenig, M.; Morel, J.; Reynaud, J.; Varvat, C.; Cathébras, P. An unusual cause of spontaneous bleeding in the intensive care unit-mastocytosis: A case report. Cases J. 2008, 1, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Ancillo, A.; Gil-Adrados, A.C.; Domínguez-Noche, C.; Jurado-Palomo, J. Bleeding and shock in a 44-year-old woman with systemic mastocytosis. J. Investig. Allergol. Clin. Immunol. 2013, 23, 517–518. [Google Scholar]
- Ahmed, M.; Kesavan, M.; Jilani, B.N.; Ahmed, S.; Deeb, L. Systemic mastocytosis as an unconventional cause of variceal bleeding: Think outside the box. Cureus 2016, 8, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto-García, A.; Castells, M.C.; Hansbro, P.M.; Stevens, R.L. Mast cell-restricted tetramer-forming tryptases and their beneficial roles in hemostasis and blood coagulation. Immunol. Allergy Clin. N. Am. 2014, 34, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Studer, A. Occurrence and significance of endogenous heparin. Experientia 1954, 10, 148–152. [Google Scholar] [CrossRef]
- Jaques, L.B. Endogenous heparin. Semin. Thromb. Hemost. 1978, 4, 326–349. [Google Scholar] [CrossRef] [PubMed]
- Vysniauskaite, M.; Hertfelder, H.J.; Oldenburg, J.; Dreßen, P.; Brettner, S.; Homann, J.; Molderings, G.J. Determination of plasma heparin level improves identification of systemic mast cell activation disease. PLoS ONE 2015, 10, e0124912. [Google Scholar] [CrossRef]
- Mital, A.; Prejzner, W.; Hellmann, A. Acquired von Willebrand syndrome during systemic mastocytosis: An analysis of 21 cases. Pol. Arch. Intern. Med. 2018, 128, 491–493. [Google Scholar] [CrossRef] [Green Version]
- Majeranowski, A.; Mital, A.; Zawilski, J.; Czarnogorski, M.; Janowiak-Majeranowska, A. Acquired von Willebrand syndrome associated with indolent systemic mastocytosis. J. Transf. Med. 2020, 13, 151–152. [Google Scholar] [CrossRef]
- Vadasz, Z.; Toubi, E. Hemostasis in allergy. Semin. Thromb. Hemost. 2018, 44, 669–675. [Google Scholar] [PubMed]
- Sillaber, C.; Baghestanian, M.; Bevec, D.; Willheim, M.; Agis, H.; Kapiotis, S.; Füreder, W.; Bankl, H.C.; Kiener, H.P.; Speiser, W.; et al. The mast cell as site of tissue-type plasminogen activator expression and fibrinolysis. J. Immunol. 1999, 162, 1032–1041. [Google Scholar]
- Valent, P.; Baghestanian, M.; Bankl, H.C.; Sillaber, C.; Sperr, W.R.; Wojta, J.; Binder, B.R.; Lechner, K. New aspects in thrombosis research: Possible role of mast cells as profibrinolytic and antithrombotic cells. Thromb. Haemost. 2002, 87, 786–790. [Google Scholar]
- Kitamura, Y.; Taguchi, T.; Yokoyama, M.; Inoue, M.; Yamatodani, A.; Asano, H.; Koyama, T.; Kanamaru, A.; Hatanaka, K.; Wershil, B.K.; et al. Higher susceptibility of mast-cell-deficient W/WV mutant mice to brain thromboembolism and mortality caused by intravenous injection of India ink. Am. J. Pathol. 1986, 122, 469–480. [Google Scholar] [PubMed]
- Dwyer, D.; Barrett, N.; Austen, K.F. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, K.; Aoki, N. Up-regulation of thrombomodulin by activation of histamine H1-receptors in human umbilical-vein endothelial cells in vitro. Biochem. J. 1991, 276, 739–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzi, O.; Frieden, M.; Villemin, P.; Fournier, M.; Foti, M.; Vischer, U.M. Protein kinase C-delta mediates von Willebrand factor secretion from endothelial cells in response to vascular endothelial growth factor (VEGF) but not histamine. J. Thromb. Haemost. 2008, 6, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Santell, L. Stimulation and desensitization of tissue plasminogen activator release from human endothelial cells. J. Biol. Chem. 1988, 263, 9360–9365. [Google Scholar] [CrossRef]
- Cooper, B. Diminished platelet adenylate cyclase activation by prostaglandin D2 in acute thrombosis. Blood 1979, 54, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Braune, S.; Küpper, J.H.; Jung, F. Effect of prostanoids on human platelet function: An overview. Int. J. Mol. Sci. 2020, 21, 9020. [Google Scholar] [CrossRef]
- Prieto-García, A.; Zheng, D.; Adachi, R.; Xing, W.; Lane, W.S.; Chung, K.; Anderson, P.; Hansbro, P.M.; Castells, M.; Steven, R.L. Mast cell restricted mouse and human tryptase·heparin complexes hinder thrombin-induced coagulation of plasma and the generation of fibrin by proteolytically destroying fibrinogen. J. Biol. Chem. 2012, 287, 7834–7844. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.H.; Tefferi, A.; Lasho, T.L.; Finke, C.; Patnaik, M.; Butterfield, J.H.; McClure, R.F.; Li, C.Y.; Pardanani, A. Systemic mastocytosis in 342 consecutive adults: Survival studies and prognostic factors. Blood 2009, 113, 5727–5736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Libby, P.; Ridker, P.M. COVID-19-A vascular disease. Trends Cardiovasc. Med. 2021, 31, 1–5. [Google Scholar] [CrossRef]
- Sido, B.; Homann, J.; Hertfelder, H.J.; Zienkiewicz, T.; Christians, K.P.; Schablin, P.; Mücke, M.; Molderings, G.J. Surgical interventions in patients with systemic mast cell activation disease: Recommendations for perioperative management. Chirurg 2019, 90, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.L.; Fox, C.C.; Lichtenstein, L.M.; Austen, K.F. Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc. Natl. Acad. Sci. USA 1988, 85, 2284–2287. [Google Scholar] [CrossRef] [Green Version]
- Contant, G.; Gouault-Heilmann, M.; Martinoli, J.L. Heparin inactivation during blood storage: Its prevention by blood collection in citric acid, theophylline, adenosine, dipyridamole-C.T.A.D. mixture. Thromb. Res. 1983, 31, 365–374. [Google Scholar] [CrossRef]
- Yang, W.; Chen, J.; Zhou, L. Effects of shear stress on intracellular calcium change and histamine release in rat basophilic leukemia (RBL-2H3) cells. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, V.; Wilson, C.G.; Carver, W.; Goldsmith, E.C. Mechanical loading promotes mast cell degranulation via RGD-integrin dependent pathways. J. Biomech. 2013, 46, 788–795. [Google Scholar] [CrossRef] [Green Version]
- Afrin, L.B.; Dempsey, T.T.; Rosenthal, L.S.; Dorff, S.R. Successful mast-cell-targeted treatment of chronic dyspareunia, vaginitis, and dysfunctional uterine bleeding. J. Obstet. Gynaecol. 2019, 39, 664–669. [Google Scholar] [CrossRef]
- Pfizer Canada Inc. Cyklokapron®—Product Monograph; Pfizer Canada Inc.: Kirkland, QC, Canada, 2013; pp. 1–22. [Google Scholar]
- Dunn, C.J.; Goa, K.L. Tranexamic acid: A review of its use in surgery and other indications. Drugs 1999, 57, 1005–1032. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Iribarren, J.L.; Lorente, L.; Rodriguez, J.M.; Hernandez, D.; Nassar, I.; Perez, R.; Brouard, M.; Milena, A.; Martinez, R.; et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: A case control study followed by a randomized double-blind controlled trial. Crit. Care 2007, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, J.J.; Iribarren, J.L.; Brouard, M.; Hernández, D.; Palmero, S.; Jiménez, A.; Lorente, L.; Machado, P.; Borreguero, J.M.; Raya, J.M.; et al. Safety and effectiveness of two treatment regimes with tranexamic acid to minimize inflammatory response in elective cardiopulmonary bypass patients: A randomized double-blind, dose-dependent, phase IV clinical trial. J. Cardiothorac. Surg. 2011, 6, 138. [Google Scholar] [CrossRef] [Green Version]
- Sheffer, A.L.; Fearon, D.T.; Austen, K.F.; Rosen, F.S. Tranexamic acid: Preoperative prophylactic therapy for patients with hereditary angioneurotic edema. J. Allergy Clin. Immunol. 1977, 60, 8–40. [Google Scholar] [CrossRef]
- McCormack, P.L. Tranexamic acid: A review of its use in the treatment of hyperfibrinolysis. Drugs 2012, 72, 585–617. [Google Scholar] [CrossRef] [PubMed]
- Kovanen, P.T. Mast cells: Multipotent local effector cells in atherothrombosis. Immunol. Rev. 2007, 217, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Bot, I.; Biessen, E.A. Mast cells in atherosclerosis. Thromb. Haemost. 2011, 106, 820–826. [Google Scholar] [PubMed] [Green Version]
- Morrow, J.D.; Oates, J.A.; Roberts, L.J., 2nd; Zackert, W.E.; Mitchell, T.A.; Lazarus, G.; Guzzo, C. Increased formation of thromboxane in vivo in humans with mastocytosis. J. Investig. Dermatol. 1999, 113, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Ponomaryov, T.; Payne, H.; Fabritz, L.; Wagner, D.D.; Brill, A. Mast cells granular contents are crucial for deep vein thrombosis in mice. Circ. Res. 2017, 121, 941–950. [Google Scholar] [CrossRef]
- Kindblom, L.G. Factor VIII related antigen and mast cells. Acta Pathol. Microbiol. Immunol. Scand. A 1982, 90, 437–439. [Google Scholar] [CrossRef]
- Akiyama, M.; Watanabe, Y.; Nishikawa, T. Immunohistochemical characterization of human cutaneous mast cells in urticaria pigmentosa (cutaneous mastocytosis). Acta Pathol. Jpn. 1991, 41, 344–349. [Google Scholar] [CrossRef]
- Lipitsä, T.; Siiskonen, H.; Naukkarinen, A.; Harvima, I.T. Mast cell chymase degrades fibrinogen and fibrin. Br. J. Dermatol. 2019, 181, 296–303. [Google Scholar] [CrossRef]
- Morrissey, J.H.; Smith, S.A. Polyphosphate as modulator of hemostasis, thrombosis, and inflammation. J. Thromb. Haemost. 2015, 13, S92–S97. [Google Scholar] [CrossRef] [Green Version]
- Mailer, R.K.W.; Hänel, L.; Allende, M.; Renné, T. Polyphosphate as a target for interference with inflammation and thrombosis. Front. Med. 2019, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Pertiwi, K.R.; de Boer, O.J.; Mackaaij, C.; Pabittei, D.R.; de Winter, R.J.; Li, X.; van der Wal, A.C. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J. Pathol. 2019, 247, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Von Kockritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef]
- Brown, T.P.; Forouzan, O.; Shevkoplyas, S.S.; Khismatullin, D.B. Histamine reduces GPIbα-mediated adhesion of platelets to TNF-α-activated vascular endothelium. Thromb. Res. 2013, 131, 150–157. [Google Scholar] [CrossRef]
- Wojta, J.; Huber, K.; Valent, P. New aspects in thrombotic research: Complement induced switch in mast cells from a profibrinolytic to a prothrombotic phenotype. Pathophysiol. Haemost. Thromb. 2003, 33, 438–441. [Google Scholar] [CrossRef]
- Molderings, G.J.; Haenisch, B.; Brettner, S.; Homann, J.; Menzen, M.; Dumoulin, F.L.; Panse, J.; Butterfield, J.; Afrin, L.B. Pharmacological treatment options for mast cell activation disease. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 671–694. [Google Scholar] [CrossRef] [Green Version]
- Afrin, L.B.; Spruill, L.S.; Schabel, S.I.; Young-Pierce, J.L. Improved metastatic uterine papillary serous cancer outcome with treatment of mast cell activation syndrome. Oncology 2014, 28, 129–131. [Google Scholar] [PubMed]
- Meng, X.M. Inflammatory mediators and renal fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 381–406. [Google Scholar]
- Vibhushan, S.; Bratti, M.; Montero-Hernández, J.E.; El Ghoneimi, A.; Benhamou, M.; Charles, N.; Daugas, E.; Blank, U. Mast cell chymase and kidney disease. Int. J. Mol. Sci. 2020, 22, 302. [Google Scholar] [CrossRef] [PubMed]
- Greiner, G.; Witzeneder, N.; Berger, A.; Schmetterer, K.; Eisenwort, G.; Schiefer, A.I.; Roos, S.; Popow-Kraupp, T.; Müllauer, L.; Zuber, J.; et al. CCL2 is a KIT D816V-dependent modulator of the bone marrow microenvironment in systemic mastocytosis. Blood 2017, 129, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Yokote, T.; Hiraoka, N.; Akioka, T.; Nishiwaki, U.; Miyoshi, T.; Iwaki, K.; Fumimoto, A.; Masuda, Y.; Hatooka, J.; et al. Transforming growth factor β- and interleukin 13-producing mast cells are associated with fibrosis in bone marrow. Hum. Pathol. 2017, 62, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Puri, N. A new role for mast cells as scavengers for clearance of erythrocytes damaged due to oxidative stress. Immunol. Lett. 2018, 199, 3–35. [Google Scholar] [CrossRef] [PubMed]
- Karam, D.; Swiatkowski, S.; Ravipati, M.; Agrawal, B. Aggressive systemic mastocytosis in association with pure red rell aplasia. Case Rep. Hematol. 2018, 2018, 6928571. [Google Scholar] [PubMed]
| Primary Mast Cell Disease | ||||
|---|---|---|---|---|
| Systemic Mastocytosis (SM) | Mast Cell Activation Syndrome (MCAS) | Cutaneous Mastocytosis (CM) | Mast Cell Sarcoma | Hereditary Hypertryptasemia |
| Clinical variants | ||||
|
|
| ||
| Affected Erythrocytic Parameter | Type/Direction of Aberrancy | Subtype/Mechanism/Comments |
|---|---|---|
| Quantity | Erythrocytosis/polycythemia | Polycythemia driven by chronic aberrant mast cell expression of erythropoietic mediators (e.g., erythropoietin, activin A, etc.) is not uncommon; rarely, male MCAS patients may drive polycythemia through androgen use to treat chronic fatigue; erythropoietin-secreting tumors induced by mast cell activation are rare. |
| Polycythemia vera is rare, but mast cell disease of any type does confer increased risk for any type of acute and chronic hematologic malignancies. | ||
| Erythropenia/anemia | Anemia of chronic inflammation is common. | |
| Anemia of iron deficiency driven by insufficient iron intake (due to dietary and medication intolerances) or insufficient iron absorption (due to disease-driven or iatrogenic gastric acid insufficiency and/or duodenal inflammation) or bleeding (see below) is common. | ||
| Anemia of copper deficiency due to disease-driven or iatrogenic gastric acid insufficiency is uncommon. | ||
| Chronic kidney disease (and related anemia) is common, but the proportion of such disease driven by mast cell disease (dominantly MCAS) has not yet been identified. | ||
| Gastrointestinal tract bleeding (due to mast cell activation-driven ulcer disease or vascular malformations, occasionally even tumors) is not uncommon. | ||
| Genitourinary tract bleeding (principally menorrhagia due to aberrant heparin release and fibrinolysis from dysfunctional endometrial mast cells) is very common in MCAS. | ||
| Anemia due to marrow compromise by high tumor load is common in the rare disease of systemic mastocytosis. | ||
| Sequestration (e.g., hepatosplenomegaly) is rare. | ||
| Quality | Macrocytic | Mild macrocytosis is common in MCAS and systemic mastocytosis, likely from premature release from marrow of maturing erythrocytes due to aberrant mast cell mediator expression; hemolysis, sequestration, massive splenomegaly, cobalamin or folate deficiency, or hematologic malignancy are seen rarely or uncommonly. |
| Microcytic | Mild to moderate microcytosis is common in MCAS, most likely from iron deficiency, rarely also from copper deficiency. | |
| Dyspoietic | Ineffective erythropoiesis (as in myelodysplastic syndrome) is uncommon. | |
| Hemolytic | Hemolysis (autoimmune or non-immune, congenital or acquired, acute or chronic) is rare. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidel, H.; Hertfelder, H.-J.; Oldenburg, J.; Kruppenbacher, J.P.; Afrin, L.B.; Molderings, G.J. Effects of Primary Mast Cell Disease on Hemostasis and Erythropoiesis. Int. J. Mol. Sci. 2021, 22, 8960. https://doi.org/10.3390/ijms22168960
Seidel H, Hertfelder H-J, Oldenburg J, Kruppenbacher JP, Afrin LB, Molderings GJ. Effects of Primary Mast Cell Disease on Hemostasis and Erythropoiesis. International Journal of Molecular Sciences. 2021; 22(16):8960. https://doi.org/10.3390/ijms22168960
Chicago/Turabian StyleSeidel, Holger, Hans-Jörg Hertfelder, Johannes Oldenburg, Johannes P. Kruppenbacher, Lawrence B. Afrin, and Gerhard J. Molderings. 2021. "Effects of Primary Mast Cell Disease on Hemostasis and Erythropoiesis" International Journal of Molecular Sciences 22, no. 16: 8960. https://doi.org/10.3390/ijms22168960
APA StyleSeidel, H., Hertfelder, H.-J., Oldenburg, J., Kruppenbacher, J. P., Afrin, L. B., & Molderings, G. J. (2021). Effects of Primary Mast Cell Disease on Hemostasis and Erythropoiesis. International Journal of Molecular Sciences, 22(16), 8960. https://doi.org/10.3390/ijms22168960






