Drug Delivery Systems for the Treatment of Knee Osteoarthritis: A Systematic Review of In Vivo Studies
Abstract
:1. Introduction
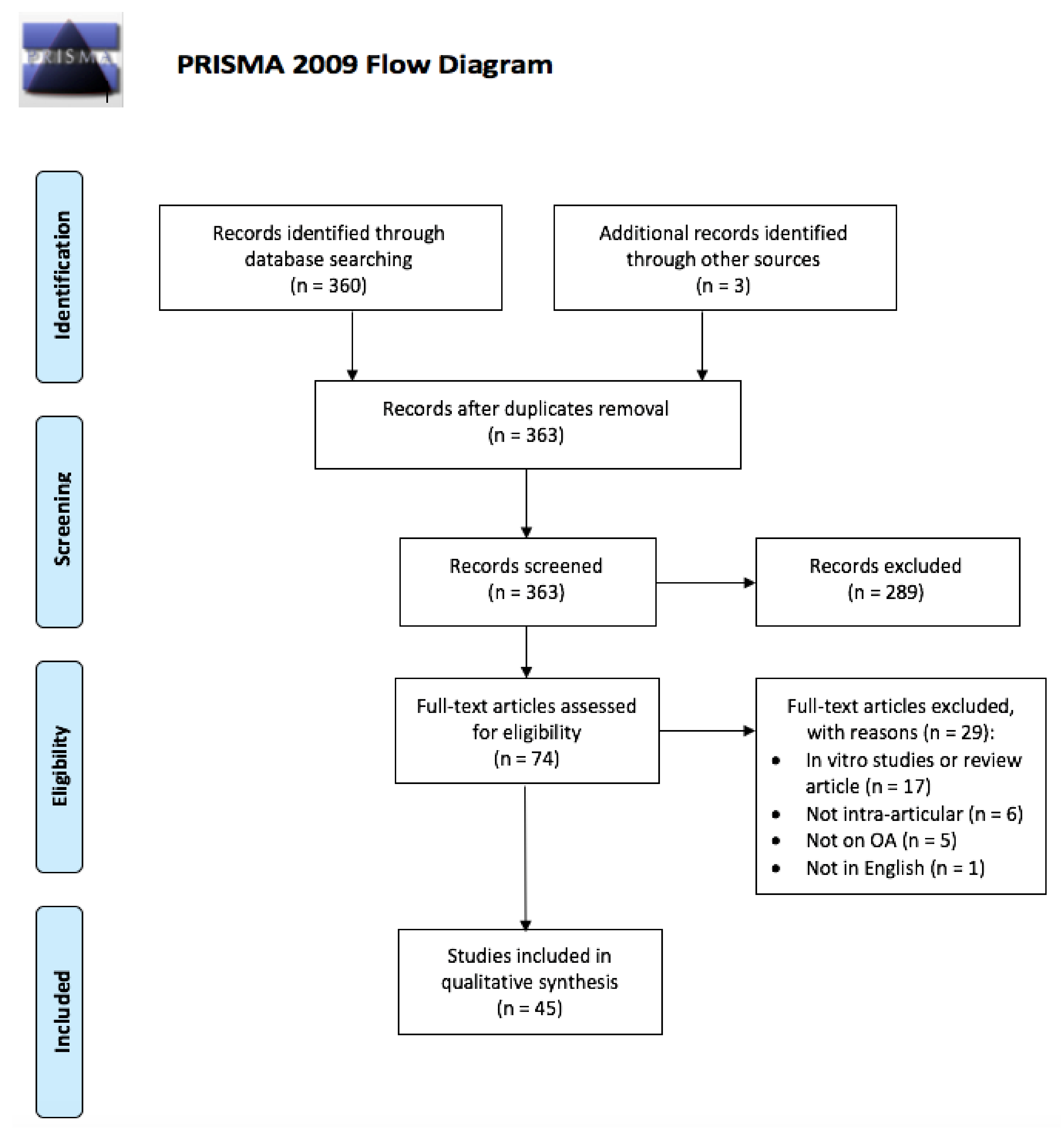
2. Methods
3. Results
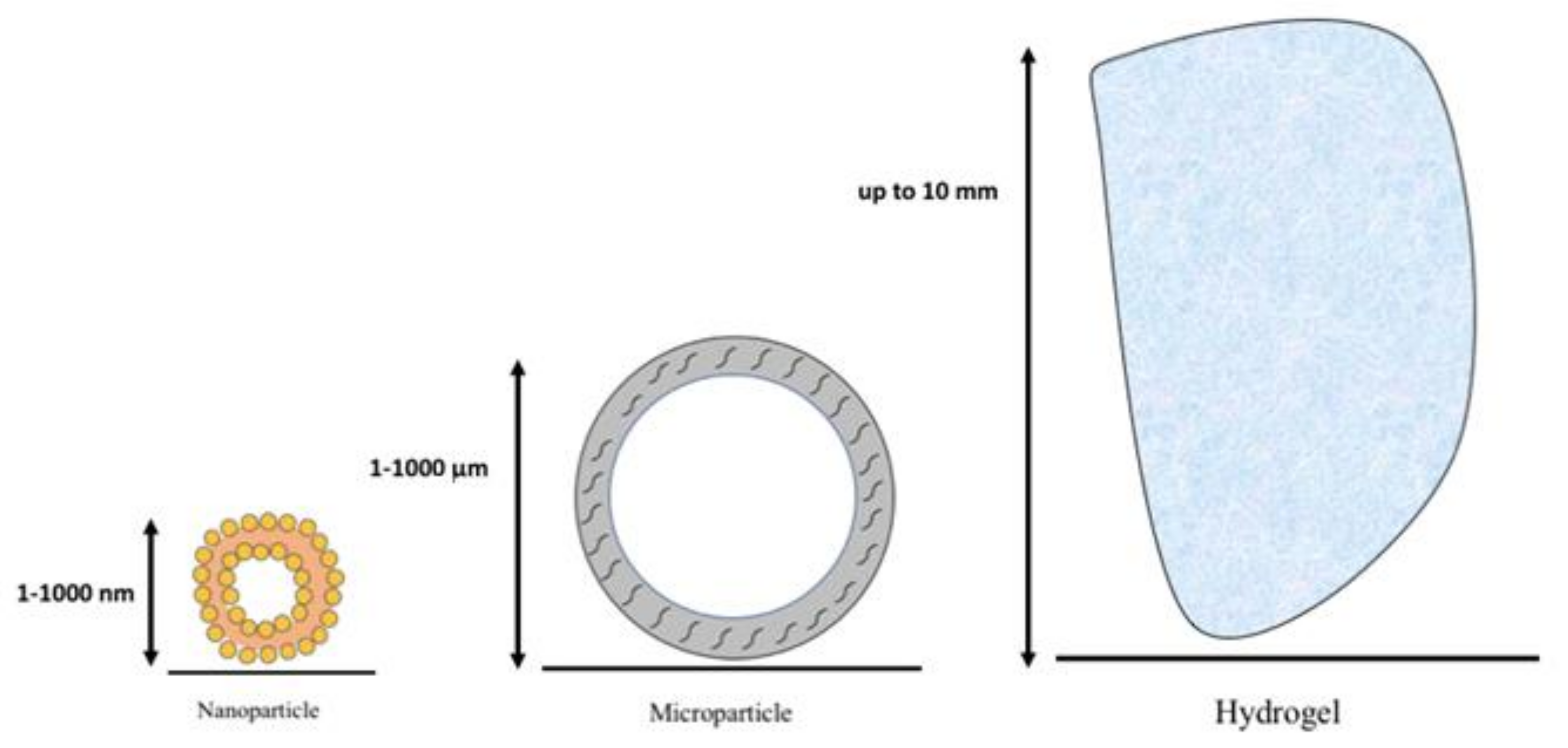
3.1. Drug Delivery Systems
3.1.1. Nanoparticles
3.1.2. Microparticles
3.1.3. Hydrogels
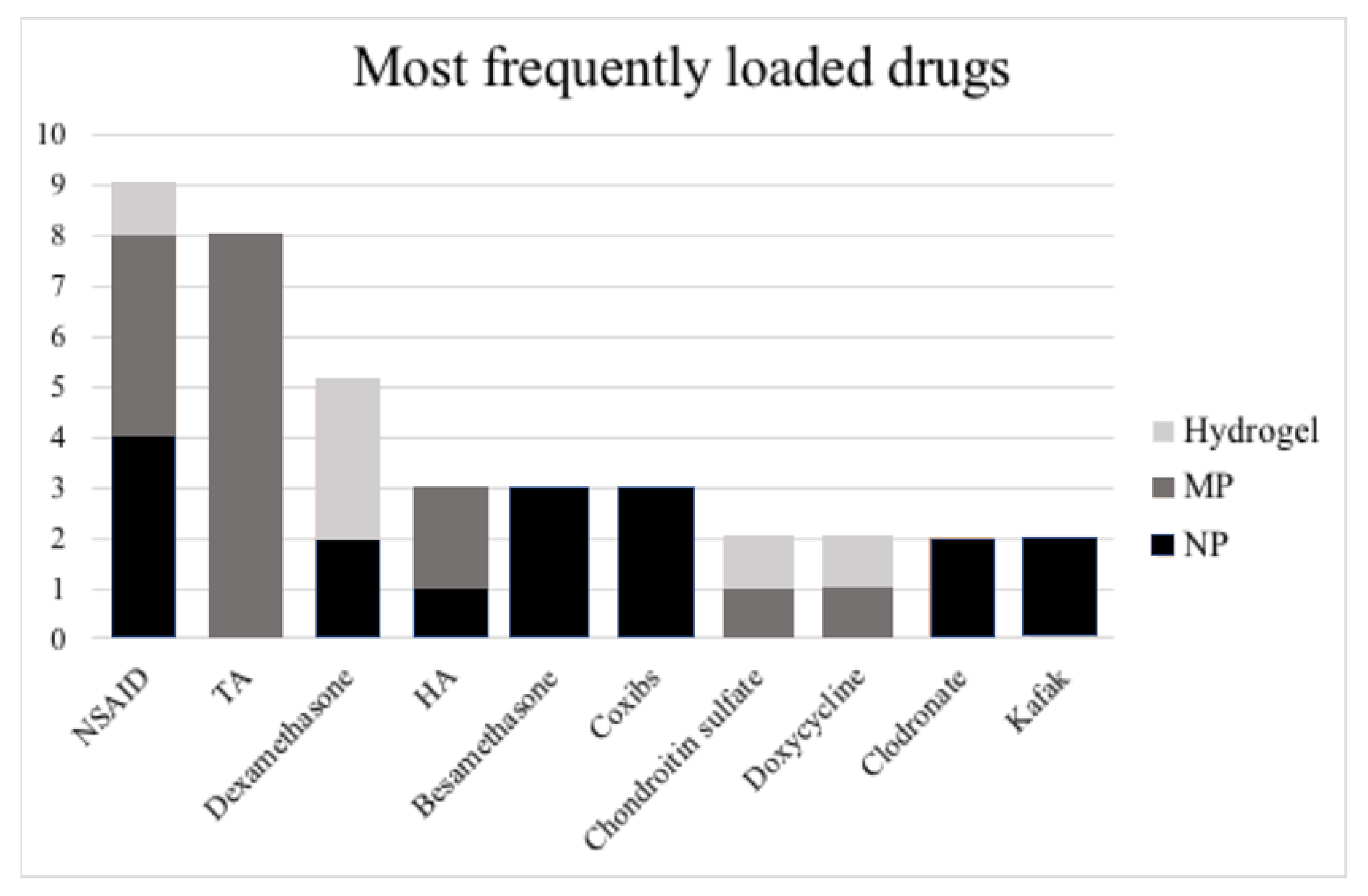
3.2. Types of Pharmacological Agents Delivered
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HA | Hyaluronic Acid |
| IA | Intra-Articular |
| MPs | Microparticles |
| MMP-3 | Matrix-Metalloprotenase-3 |
| NPs | Nanoparticles |
| OA | Osteoarthritis |
| PEG | Polyethylene Glycol |
| PLA | Poly Lactic Acid |
| PLGA | Poly Lactic-co-Glycolic Acid |
References
- Bannuru, R.R.; Schmid, C.H.; Kent, D.M.; Vaysbrot, E.E.; Wong, J.B.; McAlindon, T.E. Comparative Effectiveness of Pharmacologic Interventions for Knee Osteoarthritis: A Systematic Review and Network Meta-Analysis. Ann. Intern. Med. 2015, 162, 46–54. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee Osteoarthritis: Pathophysiology and Current Treatment Modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI Guidelines for the Non-Surgical Management of Knee Osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P.; et al. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jevsevar, D.S. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline, 2nd Edition. J. Am. Acad. Orthop. Surg. 2013, 21, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Gentile, P.; Marcarelli, M.; Balli, M.; Ronzoni, F.L.; Benedetti, L.; Cusella De Angelis, M.G. In Vitro and In Vivo Studies of Alar-Nasal Cartilage Using Autologous Micro-Grafts: The Use of the Rigenera® Protocol in the Treatment of an Osteochondral Lesion of the Nose. Pharmaceuticals 2017, 10, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Q.; Long, H.; Lin, J.; Du, D.; Zhou, J.; Chen, J.; Li, S.; Zhang, Y.; Cheng, Y.; Ma, X.; et al. Quality of Life and Treatment Satisfaction with Pharmacological Interventions in Chinese Adults with Chronic Pain Due to Osteoarthritis. BMC Musculoskelet. Disord. 2021, 22, 178. [Google Scholar] [CrossRef]
- Allen, K.D.; Adams, S.B.; Setton, L.A. Evaluating Intra-Articular Drug Delivery for the Treatment of Osteoarthritis in a Rat Model. Tissue Eng. Part B Rev. 2010, 16, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Kou, L.; Xiao, S.; Sun, R.; Bao, S.; Yao, Q.; Chen, R. Biomaterial-Engineered Intra-Articular Drug Delivery Systems for Osteoarthritis Therapy. Drug Deliv. 2019, 26, 870–885. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS 80004-1:2015(En), Nanotechnologies—Vocabulary—Part 1: Core Terms. Available online: https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-1:ed-2:v1:en (accessed on 5 April 2021).
- Zhang, Y. Relationship between Size and Function of Natural Substance Particles. Nano Biomed. Eng. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles-From Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Charmi, G.; Matyjaszewski, K.; Banquy, X.; Pietrasik, J. Recent Developments in Natural and Synthetic Polymeric Drug Delivery Systems Used for the Treatment of Osteoarthritis. Acta Biomater. 2021, 123, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.S.; Farhana, A. Histology, Cell. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Maudens, P.; Seemayer, C.A.; Pfefferlé, F.; Jordan, O.; Allémann, E. Nanocrystals of a Potent P38 MAPK Inhibitor Embedded in Microparticles: Therapeutic Effects in Inflammatory and Mechanistic Murine Models of Osteoarthritis. J. Control. Release 2018, 276, 102–112. [Google Scholar] [CrossRef]
- Paik, J.; Duggan, S.T.; Keam, S.J. Triamcinolone Acetonide Extended-Release: A Review in Osteoarthritis Pain of the Knee. Drugs 2019, 79, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Wang, B.; Hu, C.; Zhao, J. An Overview of Hydrogel-Based Intra-Articular Drug Delivery for the Treatment of Osteoarthritis. Colloids Surf. B Biointerfaces 2017, 154, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Li, J.; Yuan, L.; Chen, J.; Wang, Z.; Wang, Y.; Guo, C.; Mo, X.; Yan, Z. Intra-Articular Injection of Kartogenin-Conjugated Polyurethane Nanoparticles Attenuates the Progression of Osteoarthritis. Drug Deliv. 2018, 25, 1004–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higaki, M.; Ishihara, T.; Izumo, N.; Takatsu, M.; Mizushima, Y. Treatment of Experimental Arthritis with Poly(D, L-Lactic/Glycolic Acid) Nanoparticles Encapsulating Betamethasone Sodium Phosphate. Ann. Rheum. Dis. 2005, 64, 1132–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horisawa, E.; Hirota, T.; Kawazoe, S.; Yamada, J.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Prolonged Anti-Inflammatory Action of DL-Lactide/Glycolide Copolymer Nanospheres Containing Betamethasone Sodium Phosphate for an Intra-Articular Delivery System in Antigen-Induced Arthritic Rabbit. Pharm. Res. 2002, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.; Salama, A.H.; Mahmoud, A.A. Development and Optimization of Self-Assembling Nanosystem for Intra-Articular Delivery of Indomethacin. Int. J. Pharm. 2016, 515, 657–668. [Google Scholar] [CrossRef]
- Kang, C.; Jung, E.; Hyeon, H.; Seon, S.; Lee, D. Acid-Activatable Polymeric Curcumin Nanoparticles as Therapeutic Agents for Osteoarthritis. Nanomedicine 2020, 23, 102104. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-L.; Kim, J.-E.; Im, G.-I. Thermoresponsive Nanospheres with Independent Dual Drug Release Profiles for the Treatment of Osteoarthritis. Acta Biomater. 2016, 39, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Ho, M.J.; Kim, S.H.; Cho, H.R.; Kim, H.S.; Choi, Y.S.; Choi, Y.W.; Kang, M.J. Increased Localized Delivery of Piroxicam by Cationic Nanoparticles after Intra-Articular Injection. Drug Des. Dev. Ther. 2016, 10, 3779–3787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Gu, L.; Ren, L.; Chen, J.; Li, T.; Wang, X.; Yang, J.; Chen, C.; Sun, L. Intra-Articular Injection of Etoricoxib-Loaded PLGA-PEG-PLGA Triblock Copolymeric Nanoparticles Attenuates Osteoarthritis Progression. Am. J. Transl. Res. 2019, 11, 6775–6789. [Google Scholar] [PubMed]
- Liu, X.; Corciulo, C.; Arabagian, S.; Ulman, A.; Cronstein, B.N. Adenosine-Functionalized Biodegradable PLA-b-PEG Nanoparticles Ameliorate Osteoarthritis in Rats. Sci. Rep. 2019, 9, 7430. [Google Scholar] [CrossRef] [PubMed]
- Villamagna, I.J.; Gordon, T.N.; Hurtig, M.B.; Beier, F.; Gillies, E.R. Poly(Ester Amide) Particles for Controlled Delivery of Celecoxib. J. Biomed. Mater. Res. A 2019, 107, 1235–1243. [Google Scholar] [CrossRef]
- Zerrillo, L.; Que, I.; Vepris, O.; Morgado, L.N.; Chan, A.; Bierau, K.; Li, Y.; Galli, F.; Bos, E.; Censi, R.; et al. PH-Responsive Poly(Lactide-Co-Glycolide) Nanoparticles Containing near-Infrared Dye for Visualization and Hyaluronic Acid for Treatment of Osteoarthritis. J. Control. Release 2019, 309, 265–276. [Google Scholar] [CrossRef]
- Zhou, F.; Mei, J.; Yang, S.; Han, X.; Li, H.; Yu, Z.; Qiao, H.; Tang, T. Modified ZIF-8 Nanoparticles Attenuate Osteoarthritis by Reprogramming the Metabolic Pathway of Synovial Macrophages. ACS Appl. Mater. Interfaces 2020, 12, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.-H.; Qiu, B.; Deng, R.-H.; Li, H.-J.; Xu, X.-F.; Shang, X.-F. Chondroprotective Effects of Hyaluronic Acid-Chitosan Nanoparticles Containing Plasmid DNA Encoding Cytokine Response Modifier A in a Rat Knee Osteoarthritis Model. Cell. Physiol. Biochem. 2018, 47, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Yudoh, K.; Shishido, K.; Murayama, H.; Yano, M.; Matsubayashi, K.; Takada, H.; Nakamura, H.; Masuko, K.; Kato, T.; Nishioka, K. Water-Soluble C60 Fullerene Prevents Degeneration of Articular Cartilage in Osteoarthritis via down-Regulation of Chondrocyte Catabolic Activity and Inhibition of Cartilage Degeneration during Disease Development. Arthritis Rheum. 2007, 56, 3307–3318. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, P.; Zhang, J.; Ye, W.; Wei, Q. Restoration Effect and Tribological Behavior of Hyaluronic Acid Reinforced with Graphene Oxide in Osteoarthritis. J. Nanosci. Nanotechnol. 2019, 19, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, C.; Liu-Bryan, R.; Magrini, A.; Rosato, N.; Bottini, N.; Bottini, M. Polyethylene-Glycol-Modified Single-Walled Carbon Nanotubes for Intra-Articular Delivery to Chondrocytes. ACS Nano 2014, 8, 12280–12291. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Jiang, D.; Wang, Z.; Wu, G.; Miao, L.; Huang, L. Intra-Articular Delivery of Liposomal Celecoxib-Hyaluronate Combination for the Treatment of Osteoarthritis in Rabbit Model. Int. J. Pharm. 2013, 441, 285–290. [Google Scholar] [CrossRef]
- Sarkar, A.; Carvalho, E.; D’souza, A.A.; Banerjee, R. Liposome-Encapsulated Fish Oil Protein-Tagged Gold Nanoparticles for Intra-Articular Therapy in Osteoarthritis. Nanomedicine 2019, 14, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, Y.; Cui, F.; Du, X.; Shang, G.; Xiao, W.; Yang, X.; Cui, Q. Antioxidative Nanofullerol Inhibits Macrophage Activation and Development of Osteoarthritis in Rats. Int. J. Nanomed. 2019, 14, 4145–4155. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, X.-Z.; Alcântara, C.C.J.; Sevim, S.; Hoop, M.; Terzopoulou, A.; de Marco, C.; Hu, C.; de Mello, A.J.; Falcaro, P.; et al. MOFBOTS: Metal-Organic-Framework-Based Biomedical Microrobots. Adv. Mater. 2019, 31, e1901592. [Google Scholar] [CrossRef] [PubMed]
- Abou-ElNour, M.; Ishak, R.A.H.; Tiboni, M.; Bonacucina, G.; Cespi, M.; Casettari, L.; Soliman, M.E.; Geneidi, A.S. Triamcinolone Acetonide-Loaded PLA/PEG-PDL Microparticles for Effective Intra-Articular Delivery: Synthesis, Optimization, in Vitro and in Vivo Evaluation. J. Control. Release 2019, 309, 125–144. [Google Scholar] [CrossRef]
- Bodick, N.; Williamson, T.; Strand, V.; Senter, B.; Kelley, S.; Boyce, R.; Lightfoot-Dunn, R. Local Effects Following Single and Repeat Intra-Articular Injections of Triamcinolone Acetonide Extended-Release: Results from Three Nonclinical Toxicity Studies in Dogs. Rheumatol. Ther. 2018, 5, 475–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conaghan, P.G.; Cohen, S.B.; Berenbaum, F.; Lufkin, J.; Johnson, J.R.; Bodick, N. Brief Report: A Phase IIb Trial of a Novel Extended-Release Microsphere Formulation of Triamcinolone Acetonide for Intraarticular Injection in Knee Osteoarthritis. Arthritis Rheumatol. 2018, 70, 204–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conaghan, P.G.; Hunter, D.J.; Cohen, S.B.; Kraus, V.B.; Berenbaum, F.; Lieberman, J.R.; Jones, D.G.; Spitzer, A.I.; Jevsevar, D.S.; Katz, N.P.; et al. Effects of a Single Intra-Articular Injection of a Microsphere Formulation of Triamcinolone Acetonide on Knee Osteoarthritis Pain. J. Bone Jt. Surg. Am. 2018, 100, 666–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liu, Y.; Wen, Y.; Yu, Q.; Liu, J.; Zhao, Y.; Liu, J.; Ye, G. A Photothermal-Triggered Nitric Oxide Nanogenerator Combined with SiRNA for Precise Therapy of Osteoarthritis by Suppressing Macrophage Inflammation. Nanoscale 2019, 11, 6693–6709. [Google Scholar] [CrossRef]
- Kraus, V.B.; Conaghan, P.G.; Aazami, H.A.; Mehra, P.; Kivitz, A.J.; Lufkin, J.; Hauben, J.; Johnson, J.R.; Bodick, N. Synovial and Systemic Pharmacokinetics (PK) of Triamcinolone Acetonide (TA) Following Intra-Articular (IA) Injection of an Extended-Release Microsphere-Based Formulation (FX006) or Standard Crystalline Suspension in Patients with Knee Osteoarthritis (OA). Osteoarthr. Cartil. 2018, 26, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Ho, M.J.; Jeong, H.T.; Im, S.H.; Kim, H.T.; Lee, J.E.; Park, J.S.; Cho, H.R.; Kim, D.Y.; Choi, Y.W.; Lee, J.W.; et al. Design and In Vivo Pharmacokinetic Evaluation of Triamcinolone Acetonide Microcrystals-Loaded PLGA Microsphere for Increased Drug Retention in Knees after Intra-Articular Injection. Pharmaceutics 2019, 11, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Bendele, A.M.; Blanks, R.C.; Bodick, N. Sustained Efficacy of a Single Intra-Articular Dose of FX006 in a Rat Model of Repeated Localized Knee Arthritis. Osteoarthr. Cartil. 2015, 23, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Russell, S.J.; Sala, R.; Conaghan, P.G.; Habib, G.; Vo, Q.; Manning, R.; Kivitz, A.; Davis, Y.; Lufkin, J.; Johnson, J.R.; et al. Triamcinolone Acetonide Extended-Release in Patients with Osteoarthritis and Type 2 Diabetes: A Randomized, Phase 2 Study. Rheumatology 2018, 57, 2235–2241. [Google Scholar] [CrossRef] [Green Version]
- Rudnik-Jansen, I.; Schrijver, K.; Woike, N.; Tellegen, A.; Versteeg, S.; Emans, P.; Mihov, G.; Thies, J.; Eijkelkamp, N.; Tryfonidou, M.; et al. Intra-Articular Injection of Triamcinolone Acetonide Releasing Biomaterial Microspheres Inhibits Pain and Inflammation in an Acute Arthritis Model. Drug Deliv. 2019, 26, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Bi, X.; Li, H.; Huang, G. Enhanced Targeting Efficiency of PLGA Microspheres Loaded with Lornoxicam for Intra-Articular Administration. Drug Deliv. 2011, 18, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, G. Intra-Articular Lornoxicam Loaded PLGA Microspheres: Enhanced Therapeutic Efficiency and Decreased Systemic Toxicity in the Treatment of Osteoarthritis. Drug Deliv. 2012, 19, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Aydin, O.; Korkusuz, F.; Korkusuz, P.; Tezcaner, A.; Bilgic, E.; Yaprakci, V.; Keskin, D. In Vitro and in Vivo Evaluation of Doxycycline-Chondroitin Sulfate/PCLmicrospheres for Intraarticular Treatment of Osteoarthritis. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Tellier, L.E.; Treviño, E.A.; Brimeyer, A.L.; Reece, D.S.; Willett, N.J.; Guldberg, R.E.; Temenoff, J.S. Intra-Articular TSG-6 Delivery from Heparin-Based Microparticles Reduces Cartilage Damage in a Rat Model of Osteoarthritis. Biomater. Sci. 2018, 6, 1159–1167. [Google Scholar] [CrossRef]
- Park, J.W.; Yun, Y.-P.; Park, K.; Lee, J.Y.; Kim, H.-J.; Kim, S.E.; Song, H.-R. Ibuprofen-Loaded Porous Microspheres Suppressed the Progression of Monosodium Iodoacetate-Induced Osteoarthritis in a Rat Model. Colloids Surf. B Biointerfaces 2016, 147, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Matsushita, T.; Tabata, Y.; Saito, T.; Matsumoto, T.; Nagai, K.; Kuroda, R.; Kurosaka, M. Intra-Articular Administration of Gelatin Hydrogels Incorporating Rapamycin-Micelles Reduces the Development of Experimental Osteoarthritis in a Murine Model. Biomaterials 2014, 35, 9904–9911. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Matsushita, T.; Nishida, K.; Takayama, K.; Nagai, K.; Araki, D.; Matsumoto, T.; Tabata, Y.; Kuroda, R. Attenuation of Osteoarthritis Progression in Mice Following Intra-Articular Administration of Simvastatin-Conjugated Gelatin Hydrogel. J. Tissue Eng. Regen. Med. 2019, 13, 423–432. [Google Scholar] [CrossRef]
- Tsubosaka, M.; Kihara, S.; Hayashi, S.; Nagata, J.; Kuwahara, T.; Fujita, M.; Kikuchi, K.; Takashima, Y.; Kamenaga, T.; Kuroda, Y.; et al. Gelatin Hydrogels with Eicosapentaenoic Acid Can Prevent Osteoarthritis Progression in Vivo in a Mouse Model. J. Orthop. Res. 2020, 38, 2157–2169. [Google Scholar] [CrossRef]
- Lu, H.-T.; Sheu, M.-T.; Lin, Y.-F.; Lan, J.; Chin, Y.-P.; Hsieh, M.-S.; Cheng, C.-W.; Chen, C.-H. Injectable Hyaluronic-Acid-Doxycycline Hydrogel Therapy in Experimental Rabbit Osteoarthritis. BMC Vet. Res. 2013, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- García-Fernández, L.; Olmeda-Lozano, M.; Benito-Garzón, L.; Pérez-Caballer, A.; San Román, J.; Vázquez-Lasa, B. Injectable Hydrogel-Based Drug Delivery System for Cartilage Regeneration. Mater. Sci. Eng. C 2020, 110, 110702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, S.; Dou, H.; Liu, Q.; Shu, G.; Lin, J.; Zhang, W.; Peng, G.; Zhong, Z.; Fu, H. Novel Glucosamine-Loaded Thermosensitive Hydrogels Based on Poloxamers for Osteoarthritis Therapy by Intra-Articular Injection. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111352. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.H.; Chan, S.-W.; Li, J.; Goh, J.C.H.; Li, L.; Ren, X.F.; Lee, E.H. Intra-Articular Delivery of Chondroitin Sulfate for the Treatment of Joint Defects in Rabbit Model. J. Mol. Hist. 2007, 38, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.-W.; Fu, S.-C.; Cheuk, Y.-C.; Chu, I.-M.; Chan, K.-M.; Qin, L.; Yung, S.-H.; Kevin Ho, K.-W. Intra-Articular Delivery of Quercetin Using Thermosensitive Hydrogel Attenuate Cartilage Degradation in an Osteoarthritis Rat Model. Cartilage 2020, 11, 490–499. [Google Scholar] [CrossRef]
- Stefani, R.M.; Lee, A.J.; Tan, A.R.; Halder, S.S.; Hu, Y.; Guo, X.E.; Stoker, A.M.; Ateshian, G.A.; Marra, K.G.; Cook, J.L.; et al. Sustained Low-Dose Dexamethasone Delivery via a PLGA Microsphere-Embedded Agarose Implant for Enhanced Osteochondral Repair. Acta Biomater. 2020, 102, 326–340. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Xu, B.-X.; Fan, K.-J.; Li, Y.-W.; Wu, J.; Wang, T.-Y. Dexamethasone-Loaded Thermosensitive Hydrogel Suppresses Inflammation and Pain in Collagen-Induced Arthritis Rats. Drug Des. Dev. Ther. 2020, 14, 4101–4113. [Google Scholar] [CrossRef] [PubMed]
- Mou, D.; Yu, Q.; Zhang, J.; Zhou, J.; Li, X.; Zhuang, W.; Yang, X. Intra-Articular Injection of Chitosan-Based Supramolecular Hydrogel for Osteoarthritis Treatment. Tissue Eng. Regen. Med. 2021, 18, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Ummarino, A.; Gambaro, F.M.; Kon, E.; Torres Andón, F. Therapeutic Manipulation of Macrophages Using Nanotechnological Approaches for the Treatment of Osteoarthritis. Nanomaterials 2020, 10, 1562. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; van Weeren, P.R.; Dhert, W.J.A.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid and Dextran-Based Semi-IPN Hydrogels as Biomaterials for Bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Table | Articles | Drug | Material | Animal Model | Results | Ref |
|---|---|---|---|---|---|---|
| Polymeric NPs | Fan W 2018 | Kartogenin | PEG-HDI-N-BOC Serinol | Rat | Better histological and OARSI score at 12 weeks compared to control | [22] |
| Higaki M 2005 | Betamethasone | PLGA | Rat | Decrease in inflammatory cells after 7 days | [23] | |
| Horisawa E 2002 | Betamethasone | PLGA | Rabbit | Decreased joint swelling for 21 d | [24] | |
| Kamel R 2016 | Indomethacin | Self assembling PLGA | Rat | ↓ diameter; favorable histology; ↓ TNF-α in serum | [25] | |
| Kang C 2020 | Curcumin | Acid-activable PAE | Rat | ↓ TNF-α and IL-1β production, favorable histology | [25] | |
| Kang M 2016 | Diclofenac/Kartogenin | Pluronic | Rat | ↓ of OARSI score | [26] | |
| Kim SR 2016 | Piroxicam | PLGA + Eudragit RL | Rat | Prolonged retention into joint compared to NPs without Eudragit RL | [27] | |
| Liu P 2019 | Etoricoxib | PLGA/PEG | Rat | Favorable μCT; ↓ MMP-13 and ADAMTS-5; ↑ collagen and aggrecan | [28] | |
| Liu X 2019 | Adenosine | PLGA-PEG | Rat | ↓ OARSI score | [30] | |
| Villamagna IJ 2019 | Celecoxib | PEAs | Sheep | ↓ joint effusion; ↓ WBC | [31] | |
| Zerrillo L 2019 | HA | PLGA | Rat | NP are still in the knee after 35 days | [32] | |
| Zhou F 2015 | Berberine chloride | Chitosan | Rat | Higher anti-apoptosis activity and prolonged i.a. drug retention | [33] | |
| Zhou PH 2018 | CrmA | HA and Chitosan | Rat | ↓ OARSI score; ↓ IL-1β, MMP-3, MMP-13; collagen conserved | [34] | |
| Liposomes | Dong J 2013 | celecoxib | Liposome + hyaluronic acid | Rabbit | Favorable histology | [38] |
| Carbon-based NPs | Yudoh K 2007 | KAFAK | Fullerene | Rabbit | Favorable histology | [35] |
| Liu A 2019 | Hyaluronan conjugation | Graphene oxide | Rat | ↓ MMP-3 concentration in the joint | [36] | |
| Sacchetti C. 2014 | Antisense oligomers | Carbon nanotubes | Rat | Inhibition of protein synthesis in chondrocytes and reduction in inflammation | [37] | |
| Metal-based NPs | Sarkar A 2019 | Fish oil protein, both in DPPC liposomes | Gold | Rat | ↓ IL-1β, IL-12, PGE2, TNF-α | [39] |
| Other NPs | Zhou F 2020 | S-methylisothiourea Catalase Anti-CD16/32 | ZIF-8 (MOF) | Rat | Favorable histology and X-ray | [33] |
| Articles | Drug | Material | Animal Model | Results | Ref |
|---|---|---|---|---|---|
| Abou Elnour M 2019 | Triamcinolone acetonide (TA) | PLGA | Rat | MP performed higher in inflammation suppression, compared to the free drug suspension | [46] |
| Allah HA 2016 | Lornoxicam | Chitosan | Rat | Persistent inhibition of knee swelling and lower IL-6 levels until 14 days | [35] |
| Aydin O 2015 | Doxycycline (D) and doxycycline-chondroitin sulfate (D-CS) | poly-ɛ-caprolactone (PCL) | Rabbit | Radiographic scores of D MS and D-CS MS groups improved after 8 weeks when compared to OA groups | [58] |
| Bodick N 2018 | Triamcinolone acetonide | PLGA | Dog | Toxicity study, the synovial FBR to PLGA microspheres was focal and transient | [47] |
| Chen H 2019 | Hydroxychloroquine | PLGA | Rat | Drug detectable inside the joint up to week 2 | [50] |
| Conaghan GP 2018 | Triamcinolone acetonide | PLGA | Human | Drug detectable inside the joint up to week 12 | [48] |
| Conaghan GP 2018 | Triamcinolone acetonide | PLGA | Human | Drug detectable inside the joint up to week 13 | [49] |
| Ho Jin M 2019 | Triamcinolone Acetonide Microcrystals | PLGA | Rat | The novel MS was physicochemically stable, with no changes in drug crystallinity and release profile over 12 months | [52] |
| Kraus VB 2017 | Triamcinolone acetonide | PLGA | Human | Drug detectable inside the joint up to week 12 | [51] |
| Kumar A 2015 | Triamcinolone acetonide | PLGA FX006 | Rat | Improved histological joint scores and better gait (pain) scores | [53] |
| Park JW 2016 | Ibuprofen | PLGA, PVA | Rat | Reduction in IL-1β, IL-6, IL-17, TNF-α, ADAMTS-5 and MMP3 | [60] |
| Rudnik-Jansen I 2019 | triamcinolone acetonide | PLGA-PEA | Rat | A single intra-articular injection of TAA-loaded PEA microspheres reduced joint swelling and induced longer pain relief compared to bolus injection. | [55] |
| Russel SJ 2018 | Triamcinolone acetonide | PLGA | Human | In diabetic patients, who represented the study population, intra-articular PLGA-TA provided better post-injective glycemic control than standard TA | [54] |
| Tellier EL 2018 | TNF-α stimulated gene-6 (TSG-6) | Heparin based MP | Rat | After 21 days, cartilage thickness, volume, and attenuation were significantly increased | [59] |
| Zhang Z 2011 | Lornoxicam | PLGA | Rat | Longer retention in the joint with MP system | [56] |
| Zhang Z 2012 | Lornoxicam | PLGA | Rat | Retention of drug in the joint for >96 h | [57] |
| Articles | Drug | Material | Animal Model | Results | Ref |
|---|---|---|---|---|---|
| Garcia-Fernandez L 2020 | Naproxen or Dexamethasone | Dextran and HA | Rabbit | Dexamethasone group showed higher collagen type II levels and better recovery | [65] |
| Hui JH 2007 | Chondroitin-sulfate | α-Chondroitin Sulfate-EG | Rabbit | Thicker layer composed of hyaline and fibrocartilage compared to control (saline) | [67] |
| Lu HT 2013 | Doxycycline | HA hydrogel | Rabbit | Lower grade of OA in the study group compared to control (saline) | [64] |
| Matsuzaki T 2014 | Rapamycin | Polylactic acid, gelatin | Rat | Delayed OA progression was maintained even at 16 weeks | [61] |
| Mok SW 2020 | Quercetin | PEG hydrogel | Rat | Released of Que could be sustained for >28 days. Higher OARSI score than control | [68] |
| Stefani RM 2020 | Dexamethasone | Agarose hydrogel loaded with MP | Dogs | Improved OARSI scores for proteoglycan, chondrocyte, and collagen pathology | [69] |
| Tanaka T 2019 | Simvastatitn | Polylactic acid gelatin | Rat | Decreased level cartilage-degrading enzymes and IL-1β and increased level type II collagen | [62] |
| Tsubosaka T 2020 | Eicosapentanoic acid | Polylactic acid gelatin | Rat | MMP-3-, MMP-13-, IL-1β-, and p-IKK α/β-positive cell ratio were significantly lower | [63] |
| Wang QS 2020 | Dexamethasone | Chitosan–glycerin-borax–hydrogel | Rat | Decreased OA scores and joint inflammation compared to control | [70] |
| Zhang T 2020 | Glucosamine | Poloxamer 407 and 188 hydrogel | Rabbit | Decreased swelling and inflammatory factors compared to control | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambaro, F.M.; Ummarino, A.; Torres Andón, F.; Ronzoni, F.; Di Matteo, B.; Kon, E. Drug Delivery Systems for the Treatment of Knee Osteoarthritis: A Systematic Review of In Vivo Studies. Int. J. Mol. Sci. 2021, 22, 9137. https://doi.org/10.3390/ijms22179137
Gambaro FM, Ummarino A, Torres Andón F, Ronzoni F, Di Matteo B, Kon E. Drug Delivery Systems for the Treatment of Knee Osteoarthritis: A Systematic Review of In Vivo Studies. International Journal of Molecular Sciences. 2021; 22(17):9137. https://doi.org/10.3390/ijms22179137
Chicago/Turabian StyleGambaro, Francesco Manlio, Aldo Ummarino, Fernando Torres Andón, Flavio Ronzoni, Berardo Di Matteo, and Elizaveta Kon. 2021. "Drug Delivery Systems for the Treatment of Knee Osteoarthritis: A Systematic Review of In Vivo Studies" International Journal of Molecular Sciences 22, no. 17: 9137. https://doi.org/10.3390/ijms22179137
APA StyleGambaro, F. M., Ummarino, A., Torres Andón, F., Ronzoni, F., Di Matteo, B., & Kon, E. (2021). Drug Delivery Systems for the Treatment of Knee Osteoarthritis: A Systematic Review of In Vivo Studies. International Journal of Molecular Sciences, 22(17), 9137. https://doi.org/10.3390/ijms22179137








