Compromised Biomechanical Properties, Cell–Cell Adhesion and Nanotubes Communication in Cardiac Fibroblasts Carrying the Lamin A/C D192G Mutation
Abstract
:1. Introduction
2. Results
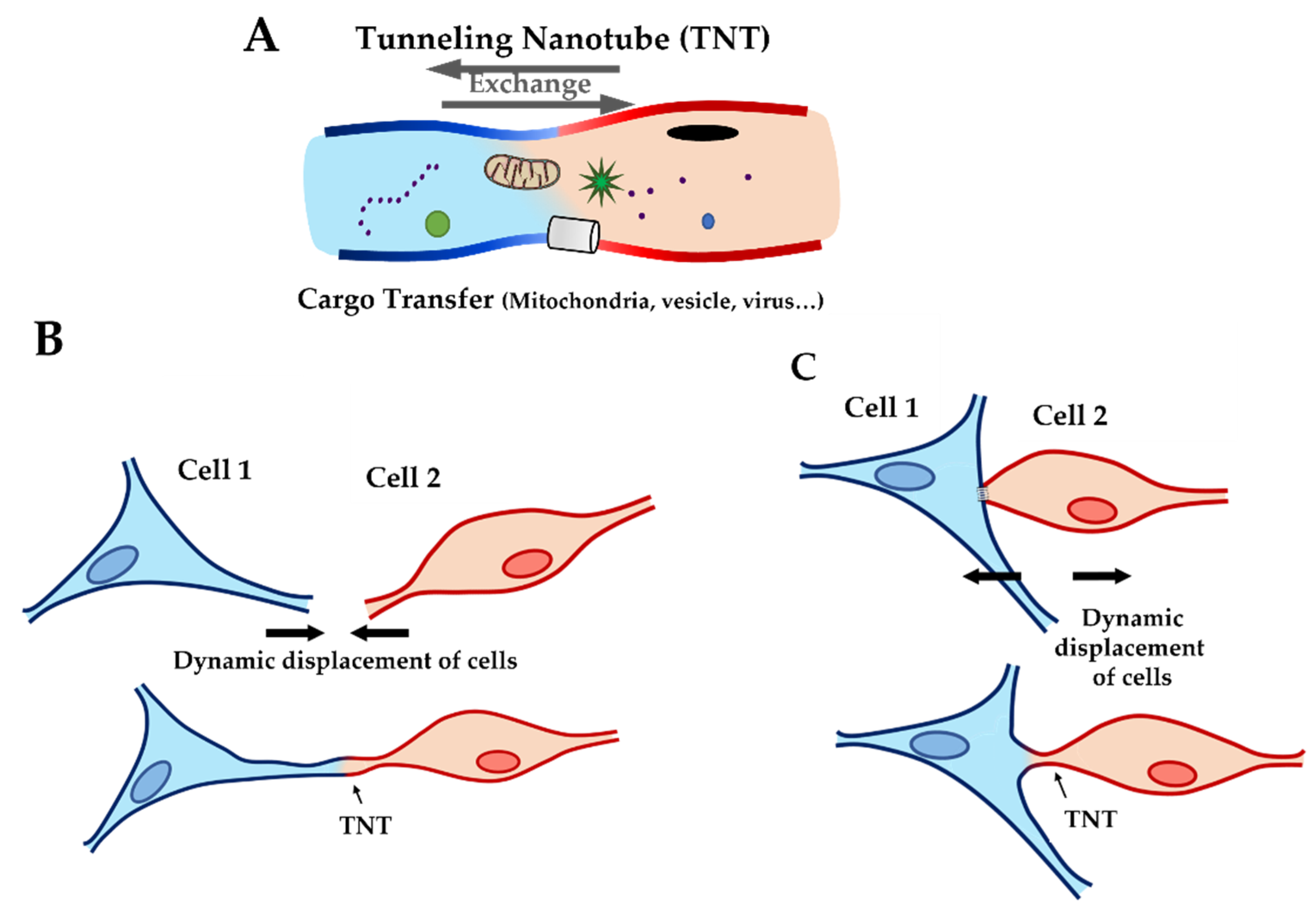
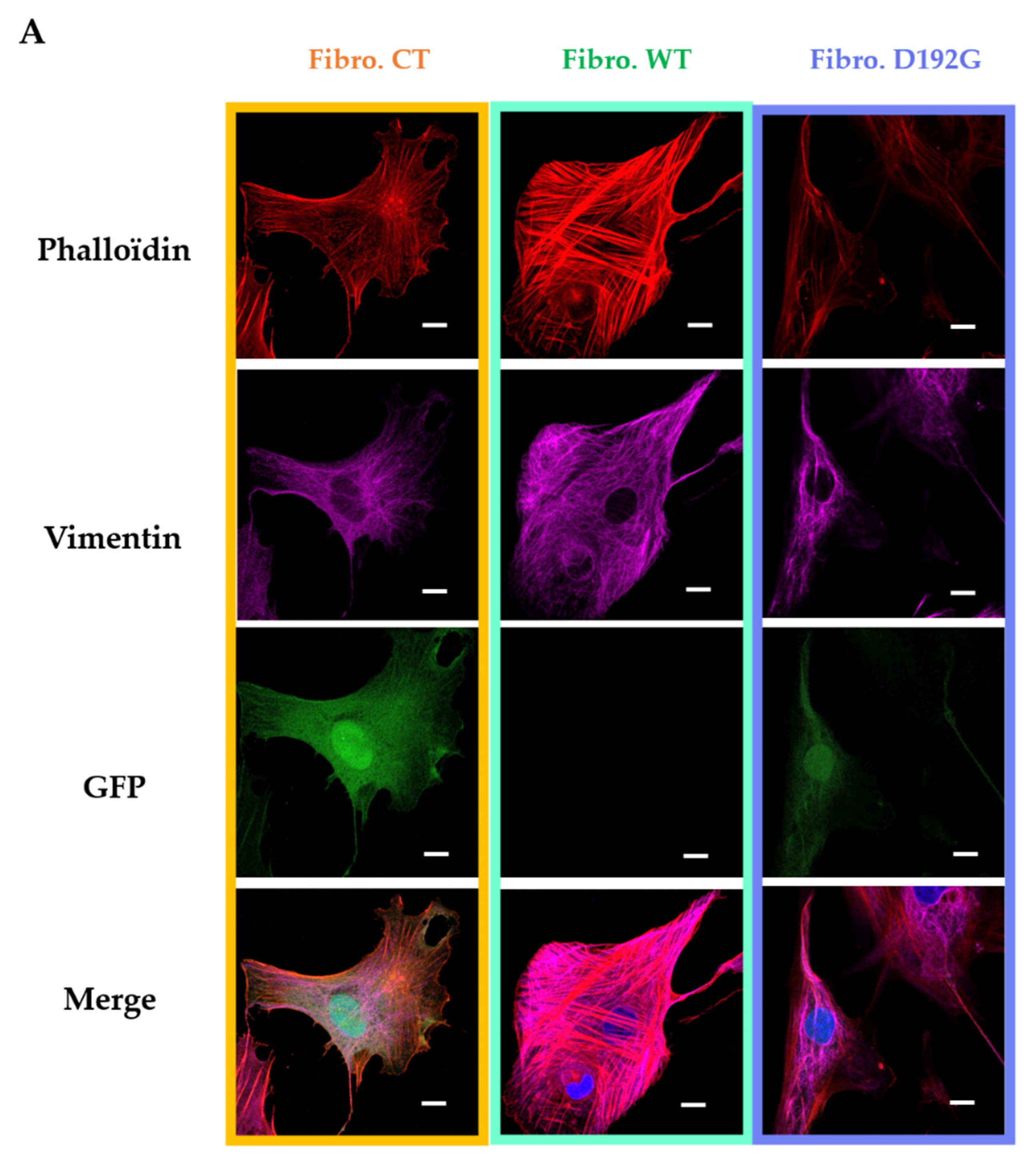
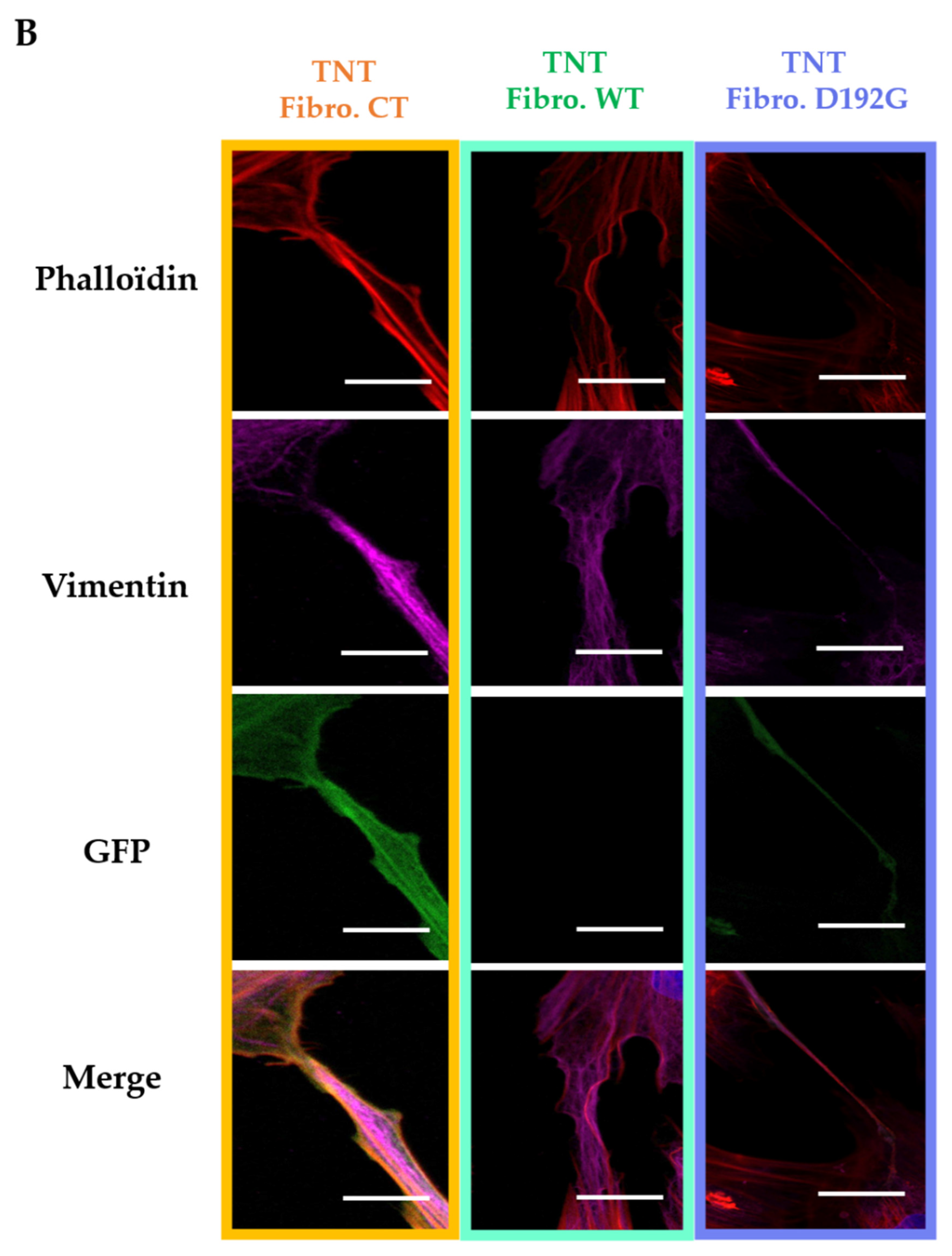
2.1. Immunofluorescence Showed Actin Disorganization and Brittle TNTs in Fibro-MT
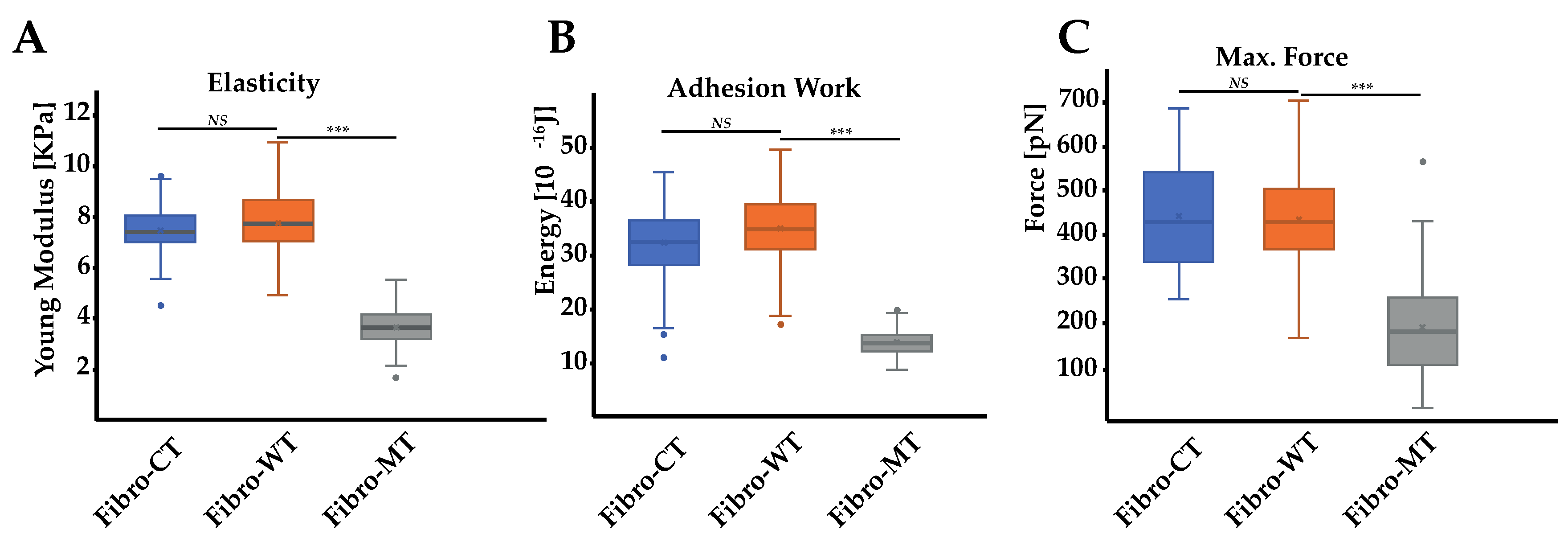
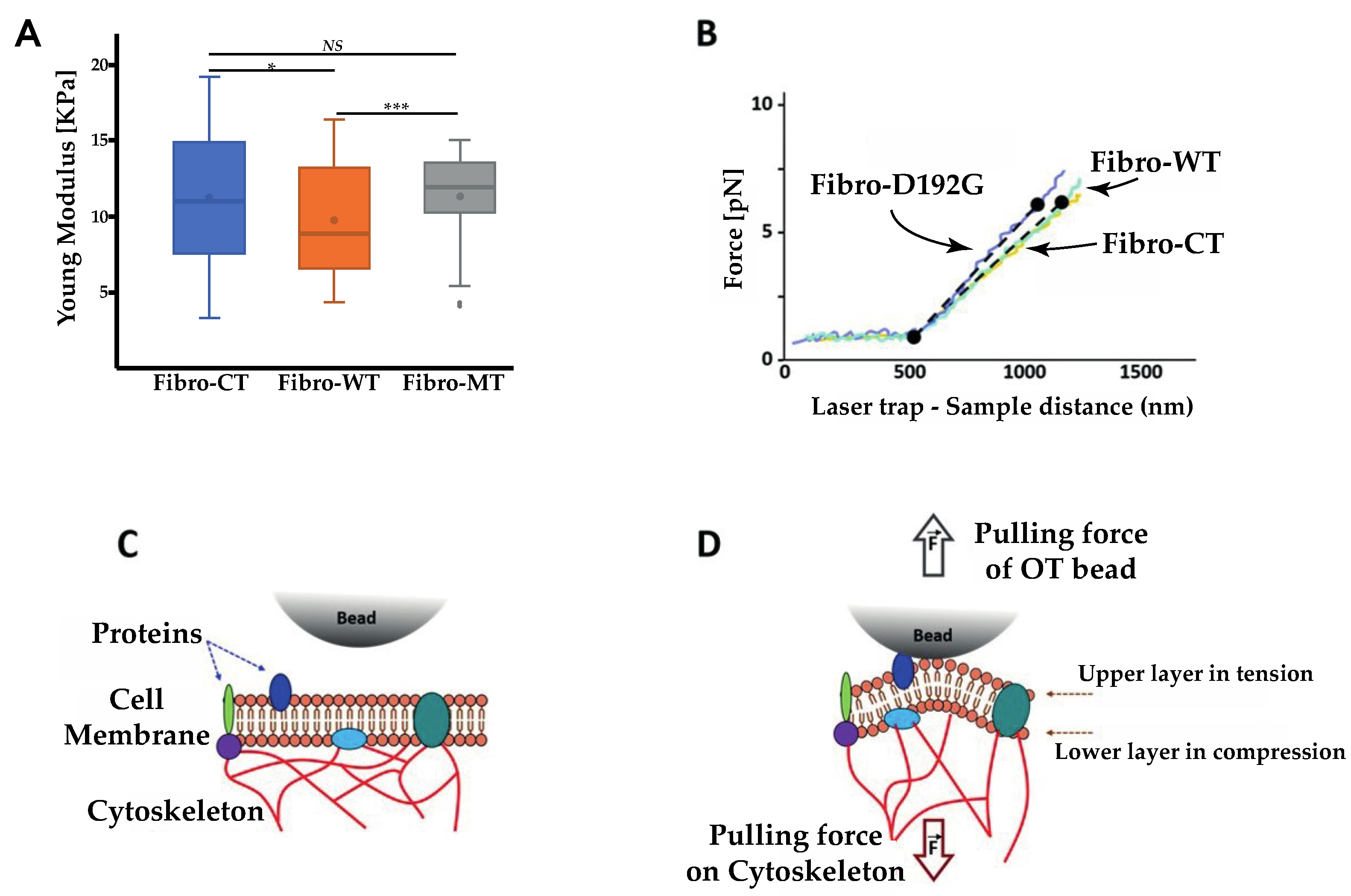
2.2. AFM Tests Highlighted Biomechanical Changes in Fibro-MT
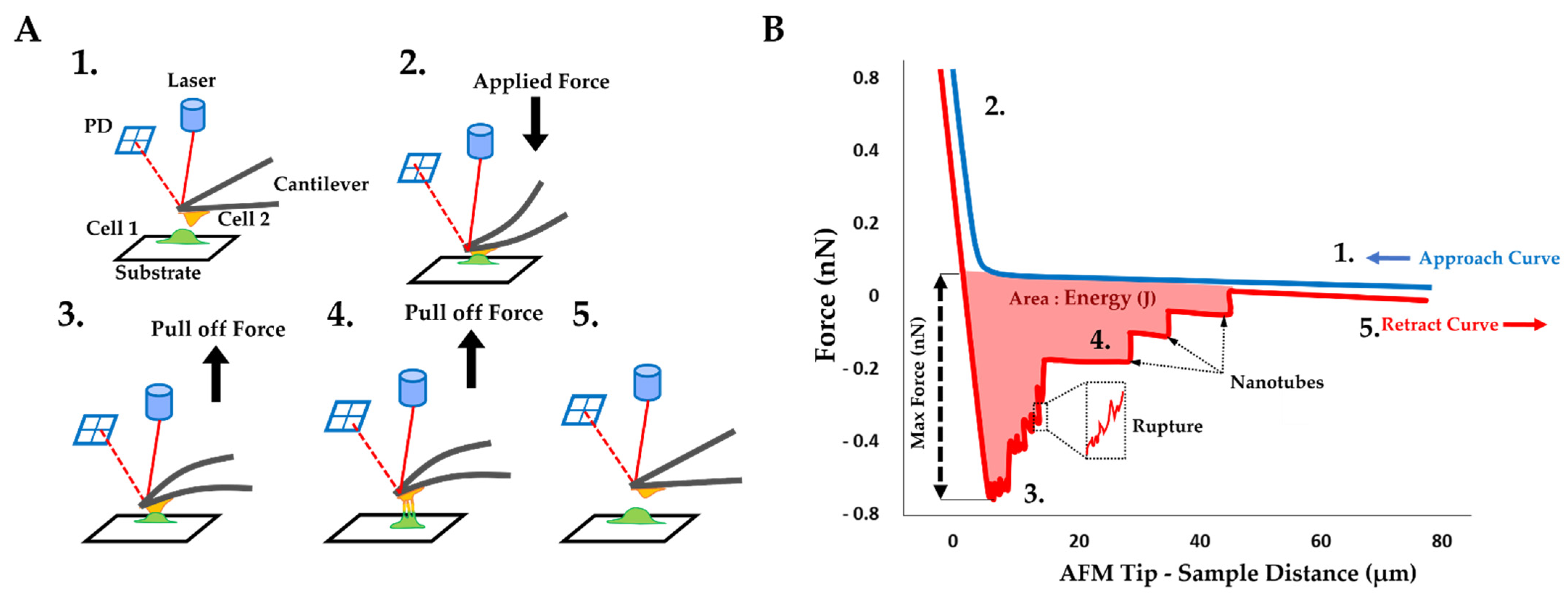
2.3. AFM on Cell–Cell Adhesion Confirmed Changes in Fibro-MT
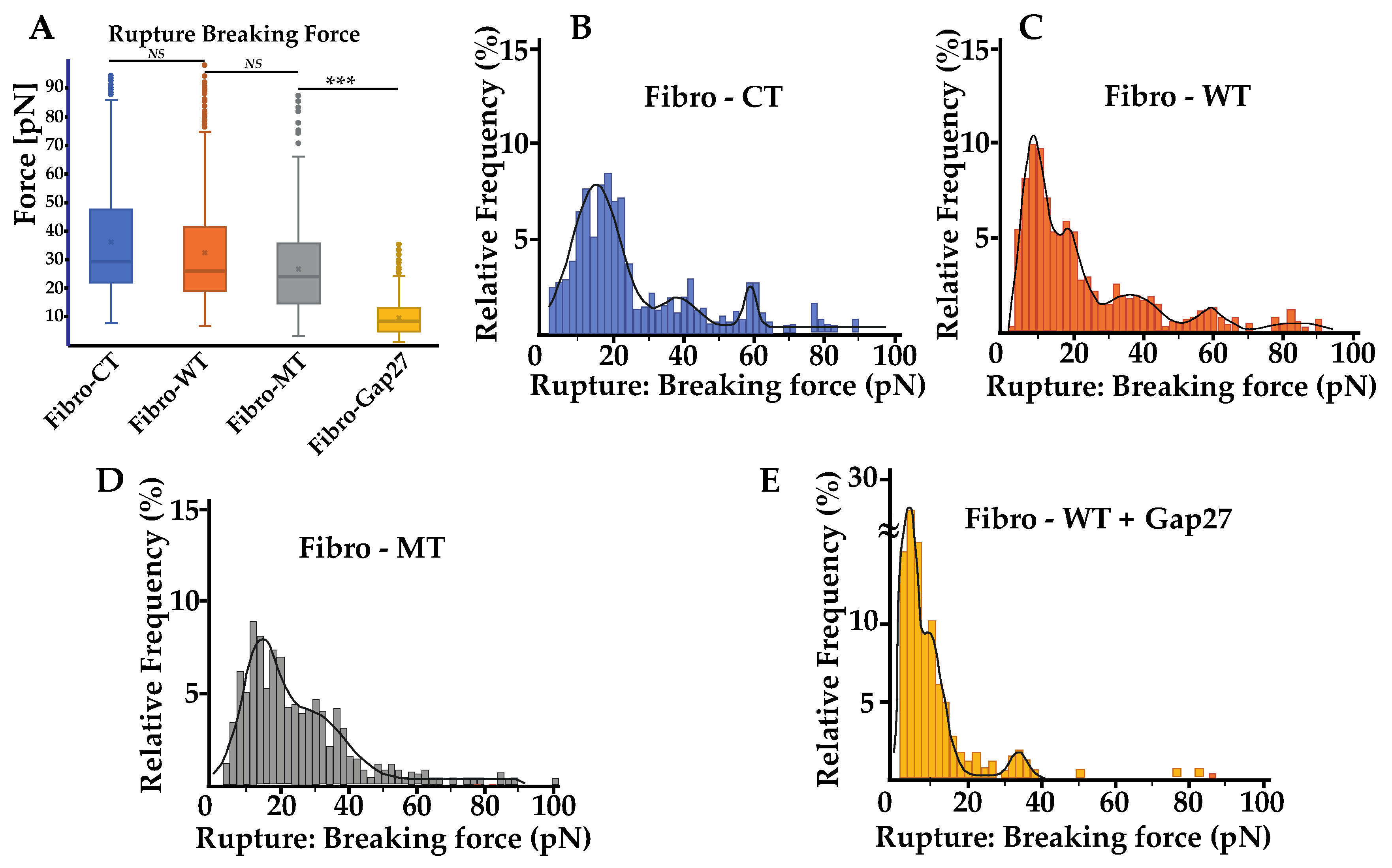
2.4. Force Rupture Distribution and Nanotube Patterns Are Different in Fibro-MT
2.5. Cx43 Is Correlated to the Biomechanical Changes in Fibro-MT
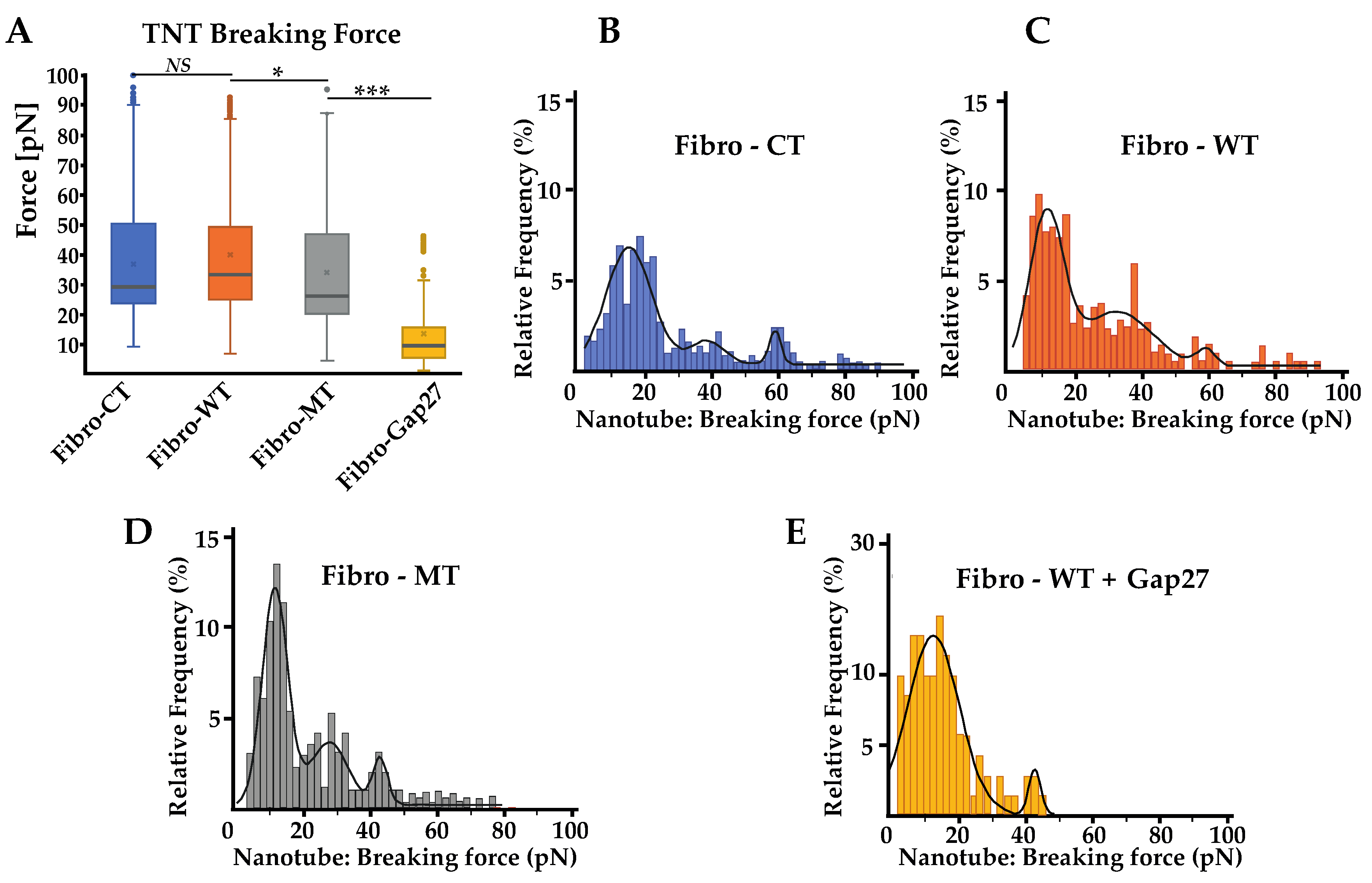
2.6. In Fibro-MT, a Decrease of the Highest Value of the TNT’s Breaking Force Was Found
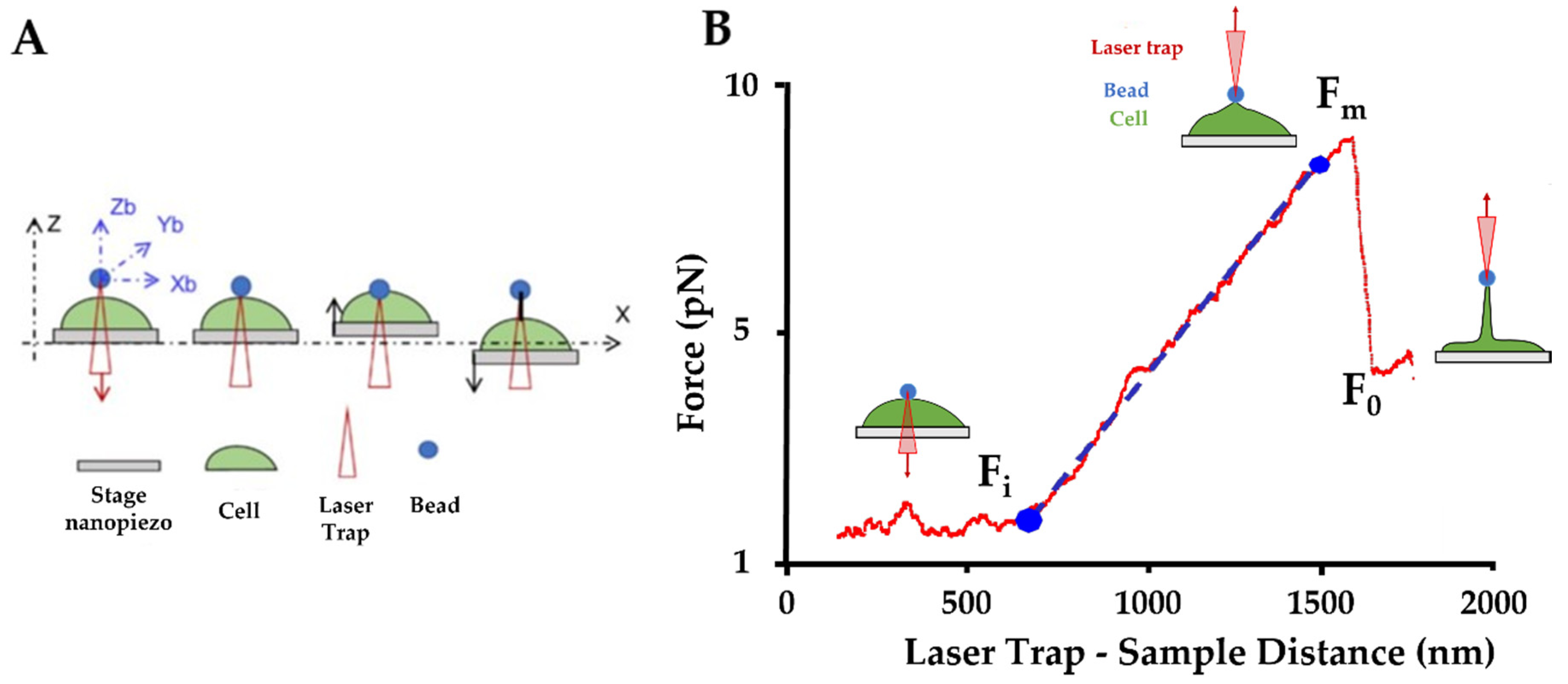
2.7. Optical Tweezers
3. Discussion
3.1. Lamin A/C D192G Gene Mutation Effect on the LINC Complex
3.2. Impact of LMNA D192G Mutation on TNT Mechanical Properties
3.3. Extrusion of Single Nanotube by OT
3.4. Impact on Adhesion Protein in Mutated Condition
3.5. Impacts of the LMNA D192G Mutation in NRVFs on the Heart in ACM
3.6. Study Limitations
4. Materials and Methods
4.1. Isolation and Culture of Ventricular Fibroblasts from Neonatal Rat (NRVFs)
4.2. Isolation Adenoviral Constructs and Infection
4.3. Immunofluorescence
4.4. Single Cell Force Spectroscopy by AFM
4.5. Cell–Cell Adhesion Experiments by AFM
4.5.1. Cell Preparation
4.5.2. Data Acquisition
4.5.3. Data Analysis
4.5.4. Synthetic Connexin 43 Mimetic Peptide (Gap 27) for Cx43 Blocking
4.6. Optical Tweezers
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Towbin, J.A.; McKenna, W.J.; Abrams, D.; Ackerman, M.J.; Calkins, H.; Darrieux, F.; Daubert, J.P.; de Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019, 16, e301–e372. [Google Scholar] [CrossRef] [Green Version]
- Pilichou, K.; Thiene, G.; Bauce, B.; Rigato, I.; Lazzarini, E.; Migliore, F.; Marra, M.P.; Rizzo, S.; Zorzi, A.; Daliento, L.; et al. Arrhythmogenic cardiomyopathy. Orphanet J. Rare Dis. 2016, 11, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.N.; Sbaizero, O.; Taylor, M.R.G.; Mestroni, L. Lamin A/C Cardiomyopathy: Implications for Treatment. Curr. Cardiol. Rep. 2019, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- Saffitz, J.E. Molecular mechanisms in the pathogenesis of arrhythmogenic cardiomyopathy. Cardiovasc. Pathol. 2017, 28, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vimalanathan, A.K.; Ehler, E.; Gehmlich, K. Genetics of and pathogenic mechanisms in arrhythmogenic right ventricular cardiomyopathy. Biophys. Rev. 2018, 10, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Qiu, Y.; Zhang, H.M.; Yang, D. Intercalated discs: Cellular adhesion and signaling in heart health and diseases. Heart Fail. Rev. 2019, 24, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Lanzicher, T.; Martinelli, V.; Puzzi, L.; Del Favero, G.; Codan, B.; Long, C.S.; Mestroni, L.; Taylor, M.R.G.; Sbaizero, O. The Cardiomyopathy Lamin A/C D192G Mutation Disrupts Whole-Cell Biomechanics in Cardiomyocytes as Measured by Atomic Force Microscopy Loading-Unloading Curve Analysis. Sci. Rep. 2015, 5, 13388. [Google Scholar] [CrossRef] [Green Version]
- Lanzicher, T.; Martinelli, V.; Long, C.; Del Favero, G.; Puzzi, L.; Borelli, M.; Mestroni, L.; Taylor, M.R.G.; Sbaizero, O. AFM single-cell force spectroscopy links altered nuclear and cytoskeletal mechanics to defective cell adhesion in cardiac myocytes with a nuclear lamin mutation. Nucleus 2015, 6, 394–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loosdregt, I.A.E.W.; Kamps, M.A.F.M.; Oomens, C.W.J.; Loerakker, S.; Broers, J.L.V.; Bouten, C.C. Lmna knockout mouse embryonic fibroblasts are less contractile than their wild-type counterparts. Integr. Biol. 2017, 9, 709–721. [Google Scholar] [CrossRef] [Green Version]
- Borin, D.; Peña, B.; Chen, S.N.; Long, C.S.; Taylor, M.R.; Mestroni, L.; Sbaizero, O. Altered microtubule structure, hemichannel localization and beating activity in cardiomyocytes expressing pathologic nuclear lamin A/C. Heliyon 2020, 6, e03175. [Google Scholar] [CrossRef]
- Khatau, S.B.; Bloom, R.J.; Bajpai, S.; Razafsky, D.; Zhang, S.; Giri, A.; Wu, P.-H.; Marchand, J.; Celedon, A.; Hale, C.; et al. The distinct roles of the nucleus and nucleus-cytoskeleton connections in three-dimensional cell migration. Sci. Rep. 2012, 2, 488. [Google Scholar] [CrossRef] [Green Version]
- Houben, F.; Ramaekers, F.; Snoeckx, L.; Broers, J. Role of nuclear lamina-cytoskeleton interactions in the maintenance of cellular strength. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 675–686. [Google Scholar] [CrossRef] [Green Version]
- Sbaizero, O.; Lanzicher, T.; Martinelli, V.; Long, C.; Slavov, D.; Del Favero, G.; Taylor, M.; Mestroni, L. Altered Nuclear and Cytoskeletal Mechanics and Defective Cell Adhesion in Cardiac Myocytes Carrying the Cardiomyopathy LMNA D192G Mutation. Circulation 2014, 130 (Suppl. S2), A17200. [Google Scholar] [CrossRef]
- Gerdes, H.-H.; Bukoreshtliev, N.V.; Barroso, J.F. Tunneling nanotubes: A new route for the exchange of components between animal cells. FEBS Lett. 2007, 581, 2194–2201. [Google Scholar] [CrossRef] [Green Version]
- Koyanagi, M.; Brandes, R.; Haendeler, J.; Zeiher, A.M.; Dimmeler, S. Cell-to-Cell Connection of Endothelial Progenitor Cells with Cardiac Myocytes by Nanotubes. Circ. Res. 2005, 96, 1039–1041. [Google Scholar] [CrossRef] [Green Version]
- Watkins, S.; Salter, R.D. Functional Connectivity between Immune Cells Mediated by Tunneling Nanotubules. Immunity 2005, 23, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Eugenin, E.; Gaskill, P.; Berman, J. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: A potential mechanism for intercellular HIV trafficking. Cell. Immunol. 2009, 254, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Vidulescu, C.; Clejan, S.; O’Connor, K.C. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J. Cell. Mol. Med. 2004, 8, 388–396. [Google Scholar] [CrossRef]
- Gurke, S.; Barroso, J.F.V.; Gerdes, H.-H. The art of cellular communication: Tunneling nanotubes bridge the divide. Histochem. Cell Biol. 2008, 129, 539–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherer, N.M.; Mothes, W. Cytonemes and tunneling nanotubules in cell–cell communication and viral pathogenesis. Trends Cell Biol. 2008, 18, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Gerdes, H.-H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015, 22, 1181–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquier, J.; Guerrouahen, B.S.; Al Thawadi, H.; Ghiabi, P.; Maleki, M.; Abu-Kaoud, N.; Jacob, A.; Mirshahi, M.; Galas, L.; Rafii, S.; et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J. Transl. Med. 2013, 11, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, E.; Fujisawa, S.; Barlas, A.; Romin, Y.; Manova-Todorova, K.; Moore, M.A.; Subramanian, S. Tunneling Nanotubes. Commun. Integr. Biol. 2012, 5, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Li, X. Gap junction protein connexin43 and tunneling nanotubes in human trabecular meshwork cells. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 212–219. [Google Scholar] [PubMed]
- Ribeiro-Rodrigues, T.M.; Martins-Marques, T.; Morel, S.; Kwak, B.R.; Girão, H. Role of connexin 43 in different forms of intercellular communication—Gap junctions, extracellular vesicles and tunnelling nanotubes. J. Cell Sci. 2017, 130, 3619–3630. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Shi, X.; Zhang, X.; Dang, S.; Ma, X.; Liu, F.; Xu, M.; Lv, Z.; Han, D.; Fang, X.; et al. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc. Res. 2011, 92, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furtado, M.B.; Nim, H.T.; Boyd, S.; Rosenthal, N. View from the heart: Cardiac fibroblasts in development, scarring and regeneration. Development 2016, 143, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Ivey, M.; Tallquist, M.D. Defining the Cardiac Fibroblast. Circ. J. 2016, 80, 2269–2276. [Google Scholar] [CrossRef] [Green Version]
- Pellman, J.; Zhang, J.; Sheikh, F. Myocyte-fibroblast communication in cardiac fibrosis and arrhythmias: Mechanisms and model systems. J. Mol. Cell. Cardiol. 2016, 94, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Souders, C.A.; Bowers, S.L.; Baudino, T.A. Cardiac Fibroblast. Circ. Res. 2009, 105, 1164–1176. [Google Scholar] [CrossRef]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenes. Tissue Repair 2012, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Sylvius, N.; Bilinska, Z.; Veinot, J.P.; Fidzianska, A.; Bolongo, P.M.; Poon, S.; McKeown, P.; Davies, R.A.; Chan, K.-L.; Tang, A.S.L.; et al. In vivo and in vitro examination of the functional significances of novel lamin gene mutations in heart failure patients. J. Med. Genet. 2005, 42, 639–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilińska, Z.T.; Sylvius, N.; Grzybowski, J.; Fidziańska, A.; Michalak, E.; Walczak, E.; Walski, M.; Bieganowska, W.; Szymaniak, E.; Kusmierczyk, B.; et al. Original article Dilated cardiomyopathy caused by LMNA mutations. Clinical and morphological studies. Kardiol. Pol. Pol. Heart J. 2006, 64, 8. [Google Scholar]
- Binnig, G.G.; Quate, C.F. atomic force microscope. At. Force Microsc. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolyakov, G.; Thiébot, B.; Campillo, C.; Labdi, S.; Severac, C.; Pelta, J.; Dague, É. Elasticity, Adhesion, and Tether Extrusion on Breast Cancer Cells Provide a Signature of Their Invasive Potential. ACS Appl. Mater. Interfaces 2016, 8, 27426–27431. [Google Scholar] [CrossRef] [Green Version]
- Puech, P.-H.; Poole, K.; Knebel, D.; Muller, D.J. A new technical approach to quantify cell–cell adhesion forces by AFM. Ultramicroscopy 2006, 106, 637–644. [Google Scholar] [CrossRef]
- Formosa, C.; Lachaize, V.; Gales, C.; Rols, M.P.; Martin-Yken, H.; François, J.M.; Duval, R.; Dague, E. Mapping HA-tagged protein at the surface of living cells by atomic force microscopy. J. Mol. Recognit. 2014, 28, 1–9. [Google Scholar] [CrossRef]
- Manso, A.M.; Kang, S.-M.; Ross, R.S. Integrins, Focal Adhesions and Cardiac Fibroblasts. J. Investig. Med. 2009, 57, 856–860. [Google Scholar] [CrossRef]
- Sun, Z.; Martinez-Lemus, L.A.; Trache, A.; Trzeciakowski, J.P.; Davis, G.E.; Pohl, U.; Meininger, G.A. Mechanical properties of the interaction between fibronectin and α5β1-integrin on vascular smooth muscle cells studied using atomic force microscopy. Am. J. Physiol. Circ. Physiol. 2005, 289, H2526–H2535. [Google Scholar] [CrossRef] [Green Version]
- Horton, M.; Charras, G.; Lehenkari, P. Analysis of ligand–receptor interactions in cells by atomic force microscopy. J. Recept. Signal Transduct. 2002, 22, 169–190. [Google Scholar] [CrossRef]
- Chang, E.; Heo, K.-S.; Woo, C.-H.; Lee, H.; Le, N.-T.; Thomas, T.N.; Fujiwara, K.; Abe, J.-I. MK2 SUMOylation regulates actin filament remodeling and subsequent migration in endothelial cells by inhibiting MK2 kinase and HSP27 phosphorylation. Blood 2011, 117, 2527–2537. [Google Scholar] [CrossRef] [Green Version]
- De Leeuw, R.; Gruenbaum, Y.; Medalia, O. Nuclear Lamins: Thin Filaments with Major Functions. Trends Cell Biol. 2018, 28, 34–45. [Google Scholar] [CrossRef]
- Hah, J.; Kim, D.-H. Deciphering Nuclear Mechanobiology in Laminopathy. Cells 2019, 8, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisp, M.; Liu, Q.; Roux, K.; Rattner, J.B.; Shanahan, C.; Burke, B.; Stahl, P.D.; Hodzic, D. Coupling of the nucleus and cytoplasm: Role of the LINC complex. J. Cell Biol. 2005, 172, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Östlund, C.; Folker, E.S.; Choi, J.C.; Gomes, E.; Gundersen, G.G.; Worman, H.J. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J. Cell Sci. 2009, 122, 4099–4108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosa, B.A.; Kutay, U.; Schwartz, T.U. Structural insights into LINC complexes. Curr. Opin. Struct. Biol. 2013, 23, 285–291. [Google Scholar] [CrossRef]
- Sullivan, T.; Escalante-Alcalde, D.; Bhatt, H.; Anver, M.; Bhat, N.; Nagashima, K.; Stewart, C.L.; Burke, B. Loss of a-Type Lamin Expression Compromises Nuclear Envelope Integrity Leading to Muscular Dystrophy. J. Cell Biol. 1999, 147, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Munck, M.; Swaminathan, K.; Kapinos, L.E.; Noegel, A.A.; Neumann, S. Mutations in LMNA Modulate the Lamin A–Nesprin-2 Interaction and Cause LINC Complex Alterations. PLoS ONE 2013, 8, e71850. [Google Scholar] [CrossRef] [Green Version]
- Bouzid, T.; Kim, E.; Riehl, B.D.; Esfahani, A.M.; Rosenbohm, J.; Yang, R.; Duan, B.; Lim, J.Y. The LINC complex, mechanotransduction, and mesenchymal stem cell function and fate. J. Biol. Eng. 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Derényi, I.; Julicher, F.; Prost, J. Formation and Interaction of Membrane Tubes. Phys. Rev. Lett. 2002, 88, 238101. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Graham, J.S.; Hegedus, B.; Marga, F.; Zhang, Y.; Forgacs, G.; Grandbois, M. Multiple Membrane Tethers Probed by Atomic Force Microscopy. Biophys. J. 2005, 89, 4320–4329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saif, T. Mechanical response of single living cells under controlled stretch and indentation using functionalized micro force sensors. In Proceedings of the 2006 International Conference on Microtechnologies in Medicine and Biology, Okinawa, Japan, 9–12 May 2006; p. 3. [Google Scholar]
- Basoli, F.; Giannitelli, S.M.; Gori, M.; Mozetic, P.; Bonfanti, A.; Trombetta, M.; Rainer, A. Biomechanical Characterization at the Cell Scale: Present and Prospects. Front. Physiol. 2018, 9, 1449. [Google Scholar] [CrossRef]
- Nussenzveig, H.M. Cell membrane biophysics with optical tweezers. Eur. Biophys. J. 2018, 47, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, N.; Kumar, P.S.; Radhakrishnan, R. Mesoscale computational studies of membrane bilayer remodeling by curvature-inducing proteins. Phys. Rep. 2014, 543, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Derényi, I.; Koster, G.; van Duijn, M.; Czövek, A.; Dogterom, M.; Prost, J.; van Duijn, M. Membrane Nanotubes. Control. Nanoscale Motion 2007, 711, 141–159. [Google Scholar] [CrossRef]
- Tian, F.; Yue, T.; Dong, W.; Yi, X.; Zhang, X. Size-dependent formation of membrane nanotubes: Continuum modeling and molecular dynamics simulations. Phys. Chem. Chem. Phys. 2018, 20, 3474–3483. [Google Scholar] [CrossRef] [PubMed]
- Figard, L.; Sokac, A.M. A membrane reservoir at the cell surface. BioArchitecture 2014, 4, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Kapustina, M.; Elston, T.C.; Jacobson, K. Compression and dilation of the membrane-cortex layer generates rapid changes in cell shape. J. Cell Biol. 2013, 200, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, C.; Echard, A.; Niedergang, F. Phagocytosis and Cytokinesis: Do Cells Use Common Tools to Cut and to Eat? Highlights on Common Themes and Differences. Traffic 2013, 14, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Jordan, K.; Simek, J.; Shao, Q.; Jedeszko, C.; Walton, P.; Laird, D.W. Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J. Cell Sci. 2005, 118, 4451–4462. [Google Scholar] [CrossRef] [Green Version]
- Camelliti, P.; Borg, T.K.; Kohl, P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005, 65, 40–51. [Google Scholar] [CrossRef]
- Lombardi, R.; Chen, S.N.; Ruggiero, A.; Gurha, P.; Czernuszewicz, G.Z.; Willerson, J.T.; Marian, A.J. Cardiac Fibro-Adipocyte Progenitors Express Desmosome Proteins and Preferentially Differentiate to Adipocytes Upon Deletion of the Desmoplakin Gene. Circ. Res. 2016, 119, 41–54. [Google Scholar] [CrossRef]
- Maione, A.S.; Pilato, C.A.; Casella, M.; Gasperetti, A.; Stadiotti, I.; Pompilio, G.; Sommariva, E. Fibrosis in Arrhythmogenic Cardiomyopathy: The Phantom Thread in the Fibro-Adipose Tissue. Front. Physiol. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maione, A.; Stadiotti, I.; Pilato, C.; Perrucci, G.; Saverio, V.; Catto, V.; Vettor, G.; Casella, M.; Guarino, A.; Polvani, G.; et al. Excess TGF-β1 Drives Cardiac Mesenchymal Stromal Cells to a Pro-Fibrotic Commitment in Arrhythmogenic Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 2673. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.D.; Ambler, S.K.; Mitchell, M.D.; Long, C. THE CARDIAC FIBROBLAST: Therapeutic Target in Myocardial Remodeling and Failure. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 657–687. [Google Scholar] [CrossRef] [PubMed]
- Laurini, E.; Martinelli, V.; Lanzicher, T.; Puzzi, L.; Borin, D.; Chen, S.N.; Long, C.S.; Lee, P.; Mestroni, L.; Taylor, M.R.G.; et al. Biomechanical defects and rescue of cardiomyocytes expressing pathologic nuclear lamins. Cardiovasc. Res. 2018, 114, 846–857. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, V.; Cellot, G.; Fabbro, A.; Bosi, S.; Mestroni, L.; Ballerini, L. Improving cardiac myocytes performance by carbon nanotubes platforms. Front. Physiol. 2013, 4, 239. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, V.; Cellot, G.; Toma, F.M.; Long, C.; Caldwell, J.H.; Zentilin, L.; Giacca, M.; Turco, A.; Prato, M.; Ballerini, L.; et al. Carbon Nanotubes Promote Growth and Spontaneous Electrical Activity in Cultured Cardiac Myocytes. Nano Lett. 2012, 12, 1831–1838. [Google Scholar] [CrossRef]
- Golden, H.B.; Gollapudi, D.; Gerilechaogetu, F.; Li, J.; Cristales, R.J.; Peng, X.; Dostal, D.E. Isolation of Cardiac Myocytes and Fibroblasts from Neonatal Rat Pups. In Advanced Structural Safety Studies; Peng, X., Entonyak, M., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 205–214. [Google Scholar] [CrossRef]
- Kass-Eisler, A.; Falck-Pedersen, E.; Alvira, M.; Rivera, J.; Buttrick, P.M.; Wittenberg, B.A.; Cipriani, L.; Leinwand, L. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1993, 90, 11498–11502. [Google Scholar] [CrossRef] [Green Version]
- Hajjar, R.J.; Kang, J.X.; Gwathmey, J.K.; Rosenzweig, A. Physiological Effects of Adenoviral Gene Transfer of Sarcoplasmic Reticulum Calcium ATPase in Isolated Rat Myocytes. Circulation 1997, 95, 423–429. [Google Scholar] [CrossRef]
- Emerson, R.; Camesano, T. On the importance of precise calibration techniques for an atomic force microscope. Ultramicroscopy 2006, 106, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Hertz, H. Ueber die Berührung fester elastischer Körper. J. Reine Angew. Math 1882, 92, 156–171. [Google Scholar] [CrossRef]
- Dague, E.; Genet, G.; Lachaize, V.; Frugier, C.; Fauconnier, J.; Mias, C.; Payré, B.; Chopinet, L.; Alsteens, D.; Kasas, S.; et al. Atomic force and electron microscopic-based study of sarcolemmal surface of living cardiomyocytes unveils unexpected mitochondrial shift in heart failure. J. Mol. Cell. Cardiol. 2014, 74, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Lachaize, V.; Formosa-Dague, C.; Smolyakov, G.; Guilbeau-Frugier, C.; Galés, C.; Dague, E. Atomic Force Microscopy: An innovative technology to explore cardiomyocyte cell surface in cardiac physio/pathophysiology. Lett. Appl. NanoBioScience 2015, 4, 321–324. [Google Scholar]
- Marcus, W.D.; Hochmuth, R.M. Experimental Studies of Membrane Tethers Formed from Human Neutrophils. Ann. Biomed. Eng. 2002, 30, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Pollok, S.; Pfeiffer, A.-C.; Lobmann, R.; Wright, C.S.; Moll, I.; Martin, P.E.M.; Brandner, J.M. Connexin 43 mimetic peptide Gap27 reveals potential differences in the role of Cx43 in wound repair between diabetic and non-diabetic cells. J. Cell. Mol. Med. 2011, 15, 861–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, C.S.; van Steensel, M.A.M.; Hodgins, M.B.; Martin, P.E.M. Connexin mimetic peptides improve cell migration rates of human epidermal keratinocytes and dermal fibroblasts in vitro. Wound Repair Regen. 2009, 17, 240–249. [Google Scholar] [CrossRef]
- Evans, W.H.; Boitano, S. Connexin mimetic peptides: Specific inhibitors of gap-junctional intercellular communication. Biochem. Soc. Trans. 2001, 29, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Tavano, F.; Bonin, S.; Pinato, G.; Stanta, G.; Cojoc, D. Custom-Built Optical Tweezers for Locally Probing the Viscoelastic Properties of Cancer Cells. Int. J. Optomechatron. 2011, 5, 234–248. [Google Scholar] [CrossRef] [Green Version]
- Falleroni, F.; Torre, V.; Cojoc, D. Cell Mechanotransduction with Piconewton Forces Applied by Optical Tweezers. Front. Cell. Neurosci. 2018, 12, 130. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachaize, V.; Peña, B.; Ciubotaru, C.; Cojoc, D.; Chen, S.N.; Taylor, M.R.G.; Mestroni, L.; Sbaizero, O. Compromised Biomechanical Properties, Cell–Cell Adhesion and Nanotubes Communication in Cardiac Fibroblasts Carrying the Lamin A/C D192G Mutation. Int. J. Mol. Sci. 2021, 22, 9193. https://doi.org/10.3390/ijms22179193
Lachaize V, Peña B, Ciubotaru C, Cojoc D, Chen SN, Taylor MRG, Mestroni L, Sbaizero O. Compromised Biomechanical Properties, Cell–Cell Adhesion and Nanotubes Communication in Cardiac Fibroblasts Carrying the Lamin A/C D192G Mutation. International Journal of Molecular Sciences. 2021; 22(17):9193. https://doi.org/10.3390/ijms22179193
Chicago/Turabian StyleLachaize, Veronique, Brisa Peña, Catalin Ciubotaru, Dan Cojoc, Suet Nee Chen, Matthew R. G. Taylor, Luisa Mestroni, and Orfeo Sbaizero. 2021. "Compromised Biomechanical Properties, Cell–Cell Adhesion and Nanotubes Communication in Cardiac Fibroblasts Carrying the Lamin A/C D192G Mutation" International Journal of Molecular Sciences 22, no. 17: 9193. https://doi.org/10.3390/ijms22179193
APA StyleLachaize, V., Peña, B., Ciubotaru, C., Cojoc, D., Chen, S. N., Taylor, M. R. G., Mestroni, L., & Sbaizero, O. (2021). Compromised Biomechanical Properties, Cell–Cell Adhesion and Nanotubes Communication in Cardiac Fibroblasts Carrying the Lamin A/C D192G Mutation. International Journal of Molecular Sciences, 22(17), 9193. https://doi.org/10.3390/ijms22179193







