Radiation-Induced Osteocyte Senescence Alters Bone Marrow Mesenchymal Stem Cell Differentiation Potential via Paracrine Signaling
Abstract
:1. Introduction
2. Results
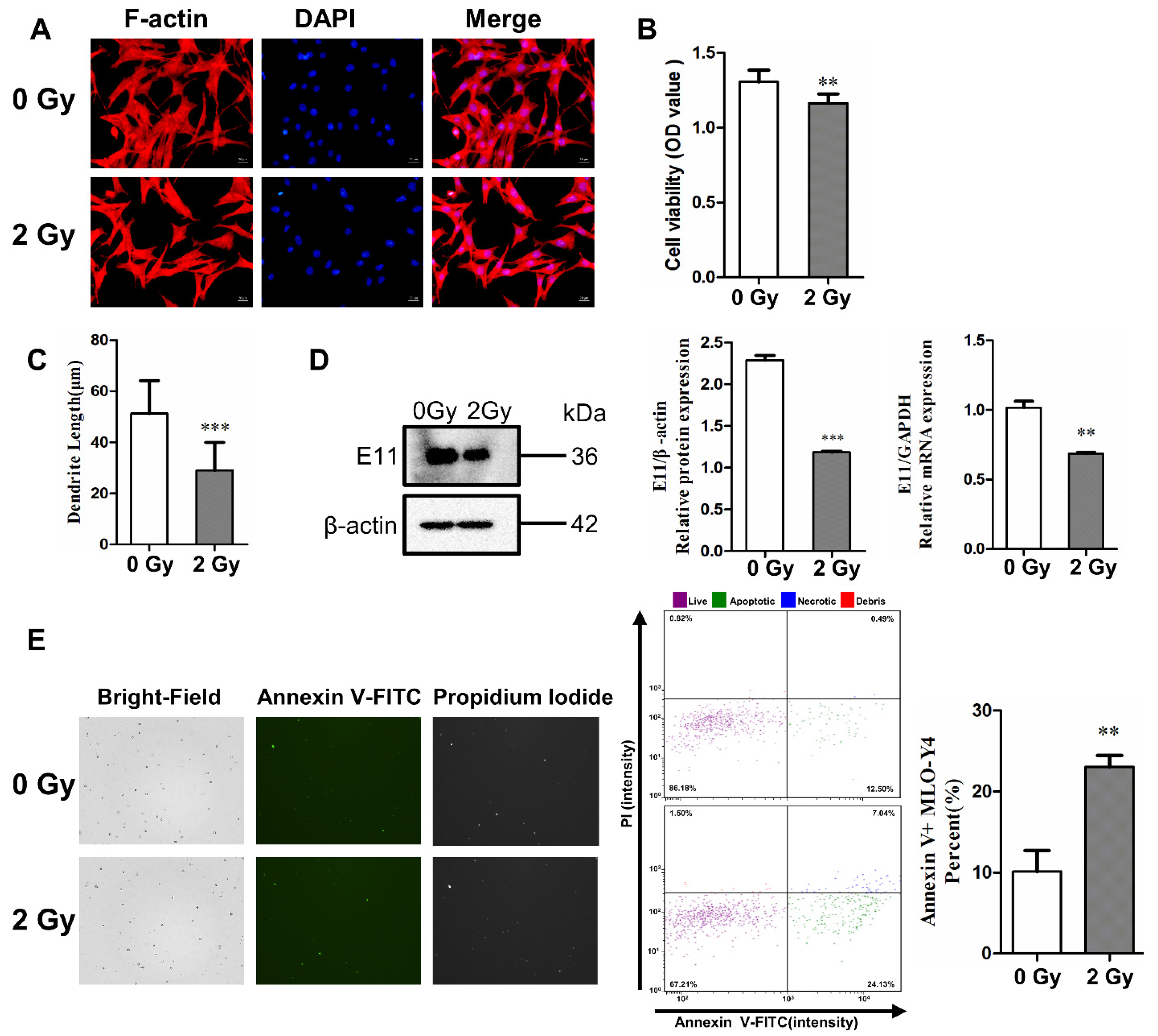
2.1. Irradiation Impairs Dendritic Morphology and Cell Viability, and Induces Cell Apoptosis
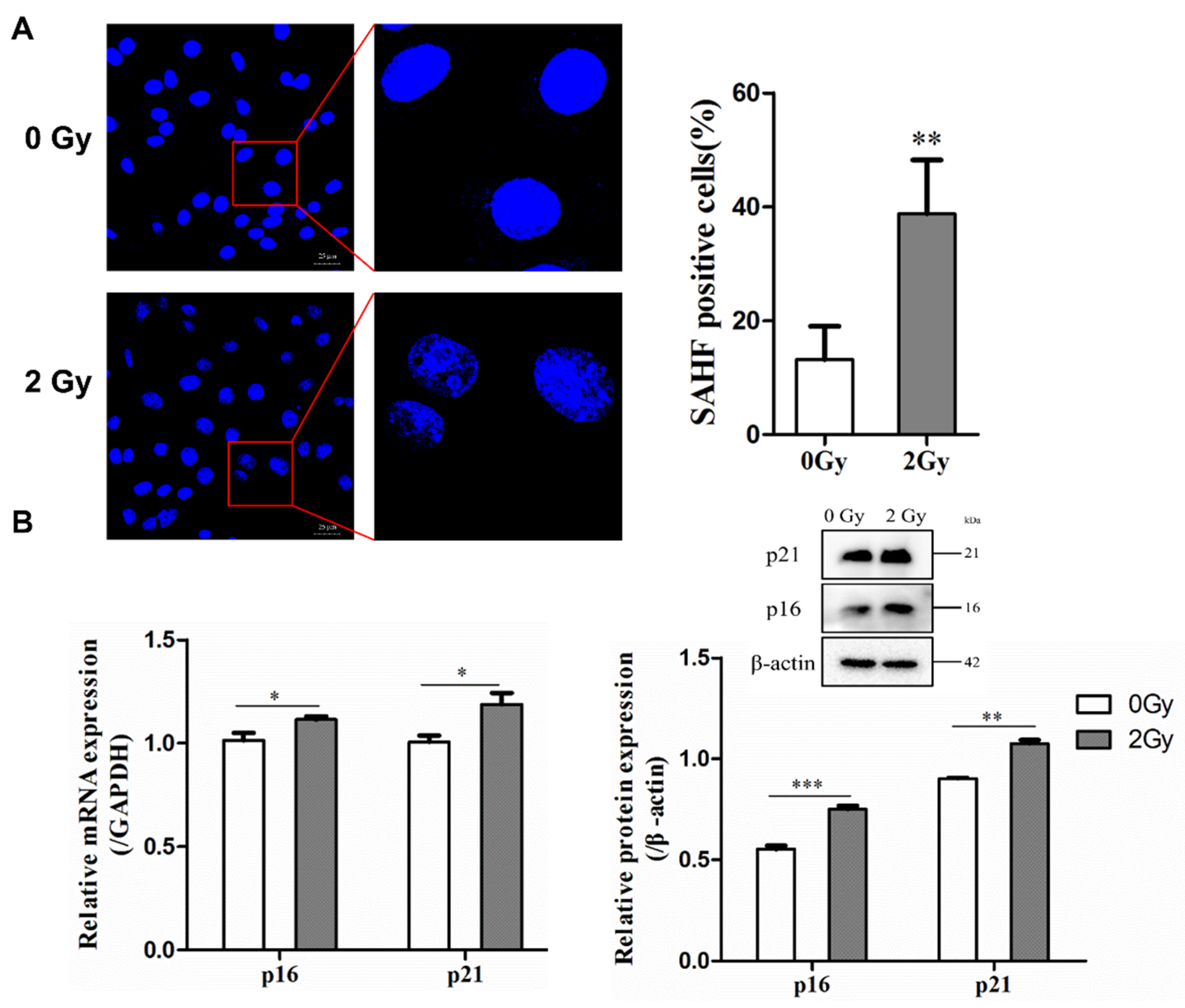
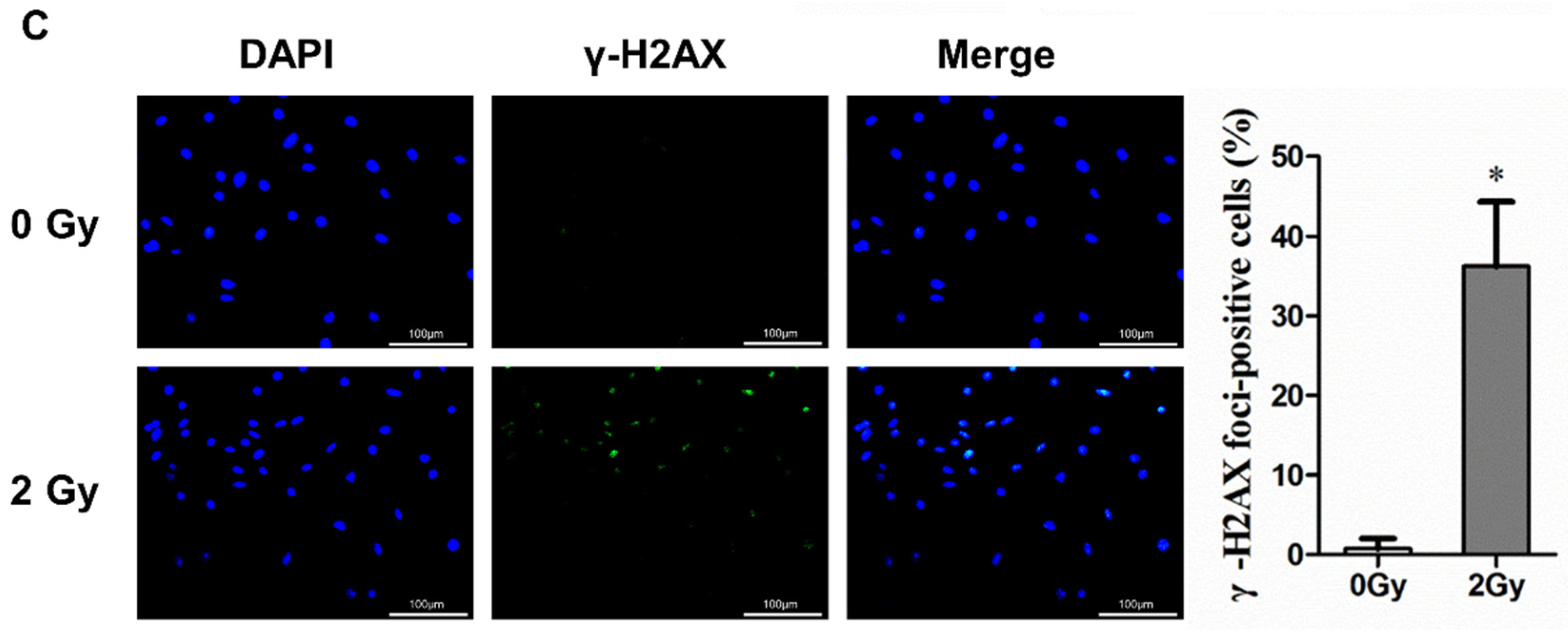
2.2. Irradiation Induces Cellular Senescence and DNA Damage
2.3. Irradiation Enhanced SASP Secretion
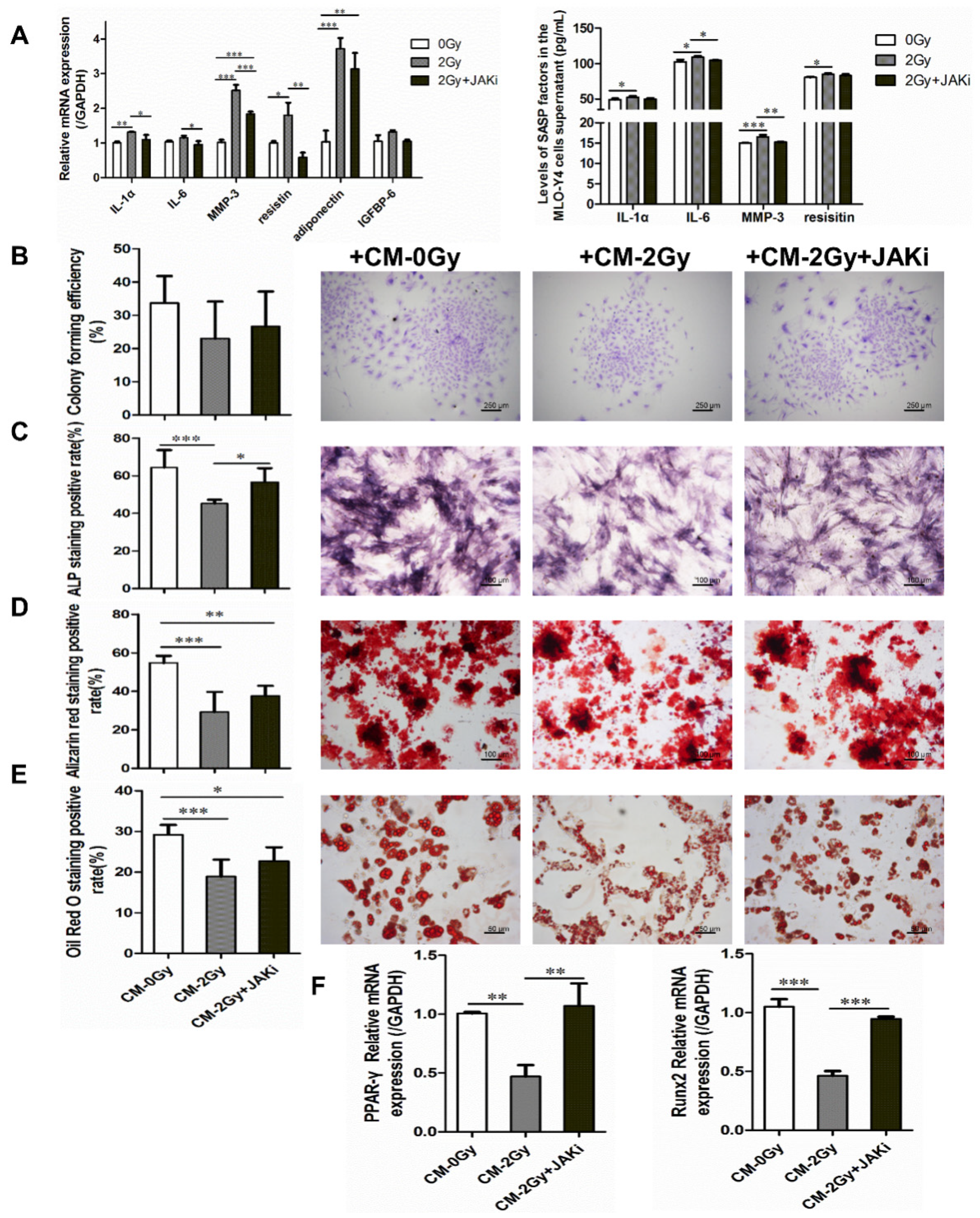
2.4. Radiation-Induced Senescent Osteocytes Perturb BMSCs via a Paracrine Pathway
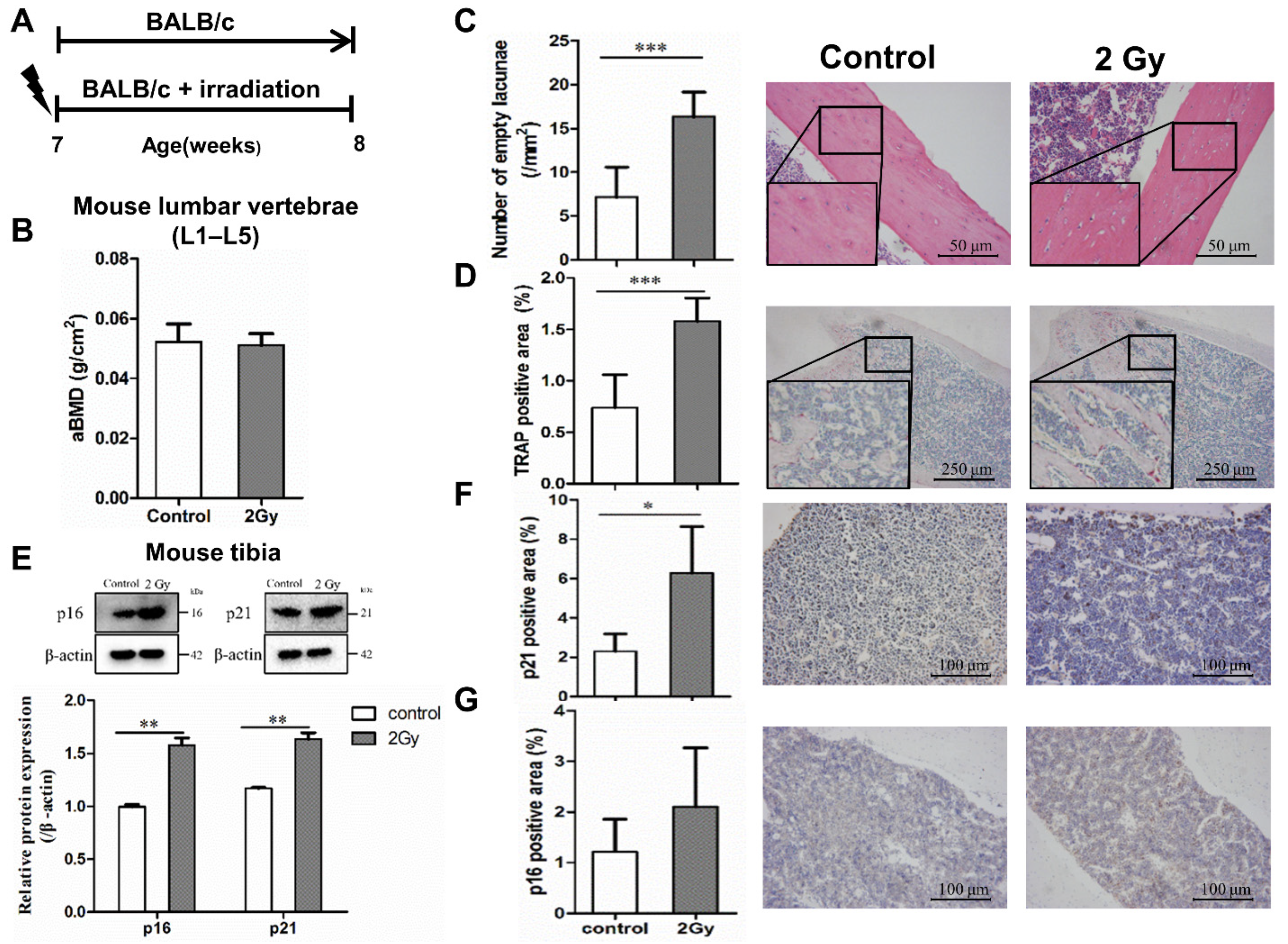
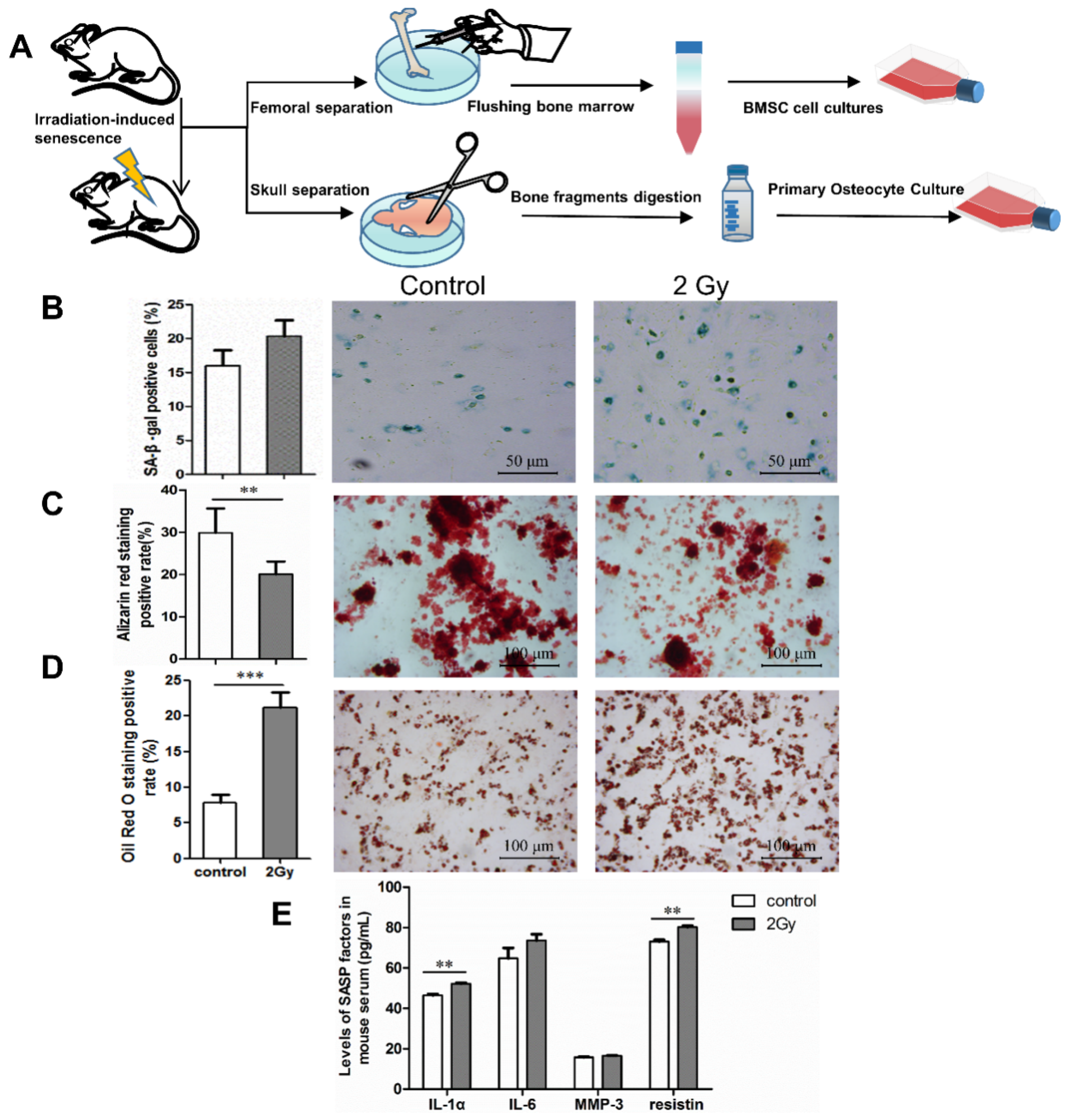
2.5. Bone Aging in Radiation-Induced Bone Damage Mice
2.6. Changes in Osteocyte and Bone Formation in Radiation-Induced Bone Damage Mice
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Irradiation
4.2. Radiation-Induced Morphological and Functional Changes in MLO-Y4
4.3. Radiation-Induced Cellular Senescence and Its Secretory Phenotype
4.4. The Effect of Radiation-Induced Senescent Osteocytes on BMSCs
4.5. Osteocyte Senescence in a Mouse Model of Radiation-Induced Bone Loss
4.6. RNA Extraction and Real-Time Quantitative PCR
4.7. Western Blot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SASP | Senescence-associated secretory phenotype |
| MCP | Membrane cofactor protein |
| EGF | Endothelial growth factor |
| HGF | Hepatocyte growth factor |
| FGF | Fibroblast growth factor |
| VEGF | Vascular endothelial growth factor |
| IGFBP | Insulin-like growth factor binding protein |
| MMP | Matrix metalloproteinase |
| ICAM | Intercellular adhesion molecule |
| IL | Interleukin |
| PPAR-γ | Peroxisome proliferator-activated receptor-γ |
References
- Sierra, F. The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb. Perspect. Med. 2016, 6, a025163. [Google Scholar] [CrossRef] [Green Version]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Chan, G.K.; Duque, G. Age-related bone loss: Old bone, new facts. Gerontology 2002, 48, 62–71. [Google Scholar] [CrossRef]
- Farr, J.N.; Almeida, M. The spectrum of fundamental basic science discoveries contributing to organismal aging. J. Bone Miner. Res. 2018, 33, 1568–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.N.; Chang, J.; Shao, L.; Han, L.; Iyer, S.; Manolagas, S.C.; O’Brien, C.A.; Jilka, R.L.; Zhou, D.; Almeida, M. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell 2017, 16, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, R.; Suzuki, Y.; Baskoro, H.; Kanayama, K.; Sugimoto, K.; Sato, T.; Sugimoto, M. Elimination of p19ARF-expressing cells protects against pulmonary emphysema in mice. Aging Cell 2018, 17, e12827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Cristofalo, V.J.; Pignolo, R.J. Replicative senescence of human fibroblast-like cells in culture. Physiol. Rev. 1993, 73, 617–638. [Google Scholar] [CrossRef]
- Zhou, T.; Chou, J.W.; Simpson, D.A.; Zhou, Y.; Mullen, T.E.; Medeiros, M.; Bushel, P.R.; Paules, R.S.; Yang, X.; Hurban, P.; et al. Profiles of global gene expression in ionizing-radiation-damaged human diploid fibroblasts reveal synchronization behind the G1 checkpoint in a G0-like state of quiescence. Environ. Health Perspect. 2006, 114, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, R.; Stock, H. Effects of radiation on bone. Curr. Osteoporos. Rep. 2013, 11, 299–304. [Google Scholar] [CrossRef]
- Yaprak, G.; Gemici, C.; Temizkan, S.; Ozdemir, S.; Dogan, B.C.; Seseogullari, O.O. Osteoporosis development and vertebral fractures after abdominal irradiation in patients with gastric cancer. BMC Cancer 2018, 18, 972. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Yumoto, K.; Alwood, J.S.; Mojarrab, R.; Wang, A.; Almeida, E.A.; Searby, N.D.; Limoli, C.L.; Globus, R.K. Oxidative stress and gamma radiation-induced cancellous bone loss with musculoskeletal disuse. J. Appl. Physiol. 2010, 108, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Murali, B.; Ren, Q.; Luo, X.; Faget, D.V.; Cole, T.; Ricci, B.; Thotala, D.; Monahan, J.; Van Deursen, J.M.; et al. Therapy-induced senescence drives bone loss. Cancer Res. 2020, 80, 1171–1182. [Google Scholar] [CrossRef]
- Le Boulch, M.; Ahmed, E.K.; Rogowska-Wrzesinska, A.; Baraibar, M.A.; Friguet, B. Proteome oxidative carbonylation during oxidative stress-induced premature senescence of WI-38 human fibroblasts. Mech. Ageing Dev. 2018, 170, 59–71. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Sharpless, N.E. Senescence in health and disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, C.; Wu, J.; Quan, W.; Cheng, C.; Zhou, J.; Chen, L.; Xiang, L.; Li, F.; Zhang, K.; et al. In vitro expansion affects the response of human bone marrow stromal cells to irradiation. Stem Cell Res. Ther. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, A.; Lagnado, A.B.; Farr, J.N.; Monroe, D.G.; Park, S.; Hachfeld, C.; Tchkonia, T.; Kirkland, J.L.; Khosla, S.; Passos, J.F.; et al. Targeted reduction of senescent cell burden alleviates focal radiotherapy-related bone loss. J. Bone Miner. Res. 2020, 35, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Law, S.F.; Chandra, A. Bone aging, cellular senescence, and osteoporosis. JBMR Plus 2021, 5, e10488. [Google Scholar] [CrossRef]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in human mesenchymal stem cells: Functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.; Marie, P.J. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell 2011, 10, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, P.; Xu, S.; Li, Y.; Dekker, J.D.; Li, B.; Fan, Y.; Zhang, Z.; Hong, Y.; Yang, G.; et al. FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging. J. Clin. Investig. 2017, 127, 1241–1253. [Google Scholar] [CrossRef]
- Lanzillotti, C.; De Mattei, M.; Mazziotta, C.; Taraballi, F.; Rotondo, J.C.; Tognon, M.; Martini, F. Long Non-coding RNAs and MicroRNAs interplay in osteogenic differentiation of mesenchymal stem cells. Front. Cell Dev. Biol. 2021, 9, 646032. [Google Scholar] [CrossRef]
- Yaswen, P.; Campisi, J. Oncogene-induced senescence pathways weave an intricate tapestry. Cell 2007, 128, 233–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Hemmer, R.M.; Sedivy, J.M. Role of p14ARF in replicative and induced senescence of human fibroblasts. Mol. Cell. Biol. 2001, 21, 6748–6757. [Google Scholar] [CrossRef] [Green Version]
- Lafargue, A.; Degorre, C.; Corre, I.; Alves-Guerra, M.C.; Gaugler, M.H.; Vallette, F.; Pecqueur, C.; Paris, F. Ionizing radiation induces long-term senescence in endothelial cells through mitochondrial respiratory complex II dysfunction and superoxide generation. Free. Radic. Biol. Med. 2017, 108, 750–759. [Google Scholar]
- Papaconstantinou, J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells 2019, 8, 1383. [Google Scholar] [CrossRef] [Green Version]
- Teo, Y.V.; Rattanavirotkul, N.; Olova, N.; Salzano, A.; Quintanilla, A.; Tarrats, N.; Kiourtis, C.; Müller, M.; Green, A.R.; Adams, P.D.; et al. Notch signaling mediates secondary senescence. Cell Rep. 2019, 27, 997–1007.e5. [Google Scholar]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Jilka, R.L.; O’Brien, C.A. The role of osteocytes in age-related bone loss. Curr. Osteoporos. Rep. 2016, 14, 16–25. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Bai, J.; Wang, J.; Zhai, J.; Tong, L.; Zhu, G. Irradiation-induced osteocyte damage promotes HMGB1-mediated osteoclastogenesis in vitro. J. Cell. Physiol. 2019, 234, 17314–17325. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Mehrzadi, S.; Reiter, R.J.; Khatami, N.; Yousefi, B. Melatonin: A pleiotropic molecule that modulates DNA damage response and repair pathways. J. Pineal Res. 2017, 63, e12416. [Google Scholar] [CrossRef]
- Sak, A.; Stuschke, M. Use of γH2AX and other biomarkers of double-strand breaks during radiotherapy. Semin. Radiat. Oncol. 2010, 20, 223–231. [Google Scholar] [CrossRef]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef]
- Farr, J.N.; Fraser, D.G.; Wang, H.; Jaehn, K.; Ogrodnik, M.B.; Weivoda, M.M.; Drake, M.T.; Tchkonia, T.; LeBrasseur, N.K.; Kirkland, J.L.; et al. Identification of senescent cells in the bone microenvironment. J. Bone Miner. Res. 2016, 31, 1920–1929. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; Van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.Y.; Chang, K.W.; Liu, C.J.; Tseng, Y.H.; Lu, H.H.; Lee, S.Y.; Lin, S.C. Ripe areca nut extract induces G1 phase arrests and senescence-associated phenotypes in normal human oral keratinocyte. Carcinogenesis 2006, 27, 1273–1284. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, D.; Lebedeva, I.V.; Emdad, L.; Kang, D.C.; Baldwin, A.S., Jr.; Fisher, P.B. Human polynucleotide phosphorylase (hPNPaseold-35): A potential link between aging and inflammation. Cancer Res. 2004, 64, 7473–7478. [Google Scholar] [CrossRef] [Green Version]
- Rodier, F.; Coppé, J.P.; Patil, C.K.; Hoeijmakers, W.A.; Muñoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef]
- Kim, K.S.; Seu, Y.B.; Baek, S.H.; Kim, M.J.; Kim, K.J.; Kim, J.H.; Kim, J.R. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol. Biol. Cell 2007, 18, 4543–4552. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Morales, A.M.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.A.; Corina, A.; Quintana-Navarro, G.; Cardelo, M.P.; Yubero-Serrano, E.; Malagon, M.M.; Delgado-Lista, J.; Ordovas, J.M.; et al. Biological senescence risk score. A practical tool to predict biological senescence status. Eur. J. Clin. Investig. 2020, 50, e13305. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; Van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.; Kieu, T.; Robbins, P.D. The Ercc1-/Δ mouse model of accelerated senescence and aging for identification and testing of novel senotherapeutic interventions. Aging 2020, 12, 24481–24483. [Google Scholar] [CrossRef]
- Fielder, E.; Weigand, M.; Agneessens, J.; Griffin, B.; Parker, C.; Miwa, S.; Von Zglinicki, T. Sublethal whole-body irradiation causes progressive premature frailty in mice. Mech. Ageing Dev. 2019, 180, 63–69. [Google Scholar] [CrossRef]
- Angelova, D.M.; Brown, D.R. Altered processing of β-Amyloid in SH-SY5Y cells induced by model senescent microglia. ACS Chem. Neurosci. 2018, 9, 3137–3152. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; Van de Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. D-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, W.; Chi, M.; Wan, Y.; Li, X.; Qi, M.; Zhou, Y. FOXO1 mediates advanced glycation end products induced mouse osteocyte-like MLO-Y4 cell apoptosis and dysfunctions. J. Diabetes Res. 2019, 2019, 6757428. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Wang, J.; Bai, J.; Zhai, J.; Zhu, G. Radiation induces primary osteocyte senescence phenotype and affects osteoclastogenesis in vitro. Int. J. Mol. Med. 2021, 47. [Google Scholar] [CrossRef] [PubMed]
- Gama, K.B.; Santos, D.S.; Evangelista, A.F.; Silva, D.N.; De Alcântara, A.C.; Dos Santos, R.R.; Soares, M.B.P.; Villarreal, C.F. Conditioned medium of bone marrow-derived mesenchymal stromal cells as a therapeutic approach to neuropathic pain: A preclinical evaluation. Stem Cells Int. 2018, 2018, 8179013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Wang, Y.; Wang, J.; Zhai, J.; He, F.; Zhu, G. Irradiation-induced senescence of bone marrow mesenchymal stem cells aggravates osteogenic differentiation dysfunction via paracrine signaling. Am. J. Physiol. Cell. Physiol. 2020, 318, C1005–C1017. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, X.; Ye, Z.; Zhang, Y.; Zhou, Y.; Tan, W.S. A modular approach to the engineering of a centimeter-sized bone tissue construct with human amniotic mesenchymal stem cells-laden microcarriers. Biomaterials 2011, 32, 7532–7542. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, X.; Wan, J.; Chang, L.; Hu, W.; Bing, Z.; Zhang, S.; Li, J.; He, J.; Wang, J.; et al. Radiation-induced cellular senescence results from a slippage of long-term G2 arrested cells into G1 phase. Cell Cycle 2013, 12, 1424–1432. [Google Scholar] [CrossRef] [Green Version]
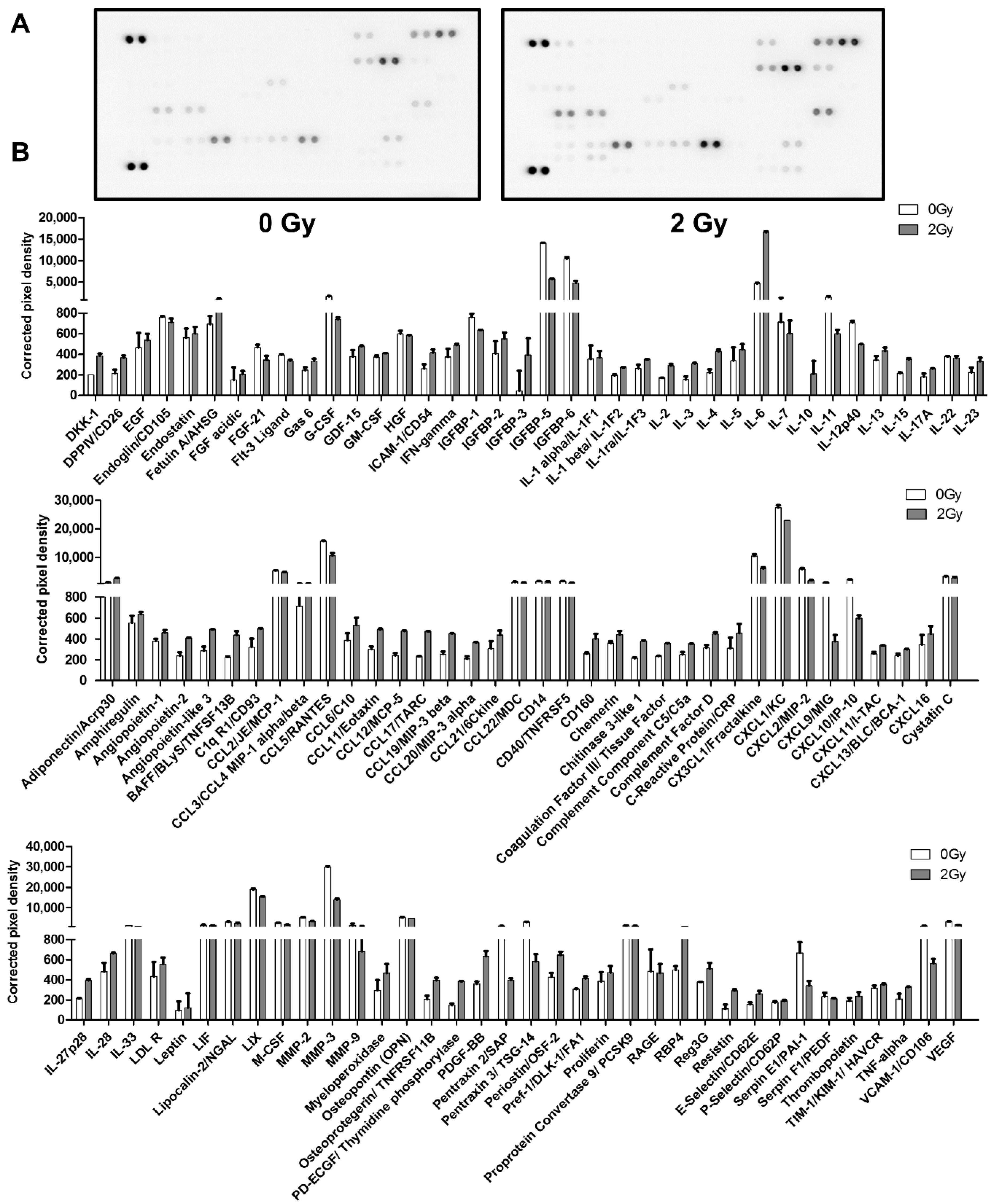
| Category | SASP Factors | Change Pattern |
|---|---|---|
| Interleukins (IL) | IL-1β, IL-1α, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, IL-15, IL-17A, IL-23, IL-27p28, IL-28 | ↑ |
| IL-7, IL-11, IL-12p40, IL-22, IL-33 | ↓ | |
| Chemokines(CXCL, CCL) | CCL3/CCL4 MIP-1 alpha/beta, CL6/C10, CCL11/eotaxin, CCL12/MCP-5, CCL17/TARC, CCL19/MIP-3 beta, CCL20/MIP-3 alpha, CCL21/6Ckine, CXCL11/I-TAC, CXCL13/BLC/BCA-1, CXCL16 | ↑ |
| CCL5/RANTES, CCL2/JE/MCP-1, CCL22/MDC, CX3CL1/fractalkine, CXCL1/KC, CXCL2/MIP-2, CXCL9/MIG, CXCL10/IP-10, LIX | ↓ | |
| Other inflammatory factor | GDF-15, GM-CSF, IFN-gamma, BAFF/BLyS/TNFSF13B, TNF-alpha, coagulation factor III/tissue factor | ↑ |
| G-CSF | ↓ | |
| Growth factors and regulators | Amphiregulin, angiopoietin-1, angiopoietin-2, angiopoietin-like 3, EGF, fetuin A/AHSG, FGF acidic, PD-ECGF/thymidine phosphorylase, PDGF-BB, proliferin, IGFBP-2, IGFBP -3 | ↑ |
| HGF, VEGF, osteopontin (OPN), FGF-21, IGFBP-1, IGFBP-5, IGFBP-6, Flt-3 ligand | ↓ | |
| Proteases and regulators | Myeloperoxidase, complement factor D, RBP4, fetuin A/AHSG | ↑ |
| M-CSF, MMP-2, MMP-3, MMP-9 proprotein convertase 9/PCSK9, serpin F1/PEDF, RAGE, cystatin C, serpin E1/PAI-1 | ↓ | |
| Soluble or shed receptors or ligands | ICAM-1/CD54, osteoprotegerin/TNFRSF11B, chemerin, chitinase 3-like 1, Gas6, LDL R, C-reactive protein/CRP | ↑ |
| LIF | ↓ | |
| Adipokines | Adiponectin/Acrp30, resistin, leptin, Pref-1/DLK-1/FA1 | ↑ |
| — | ↓ | |
| Extracellular matrix protein | CD160, complement component C5/C5a, TIM-1/KIM-1/HAVCR, periostin/OSF-2, endostatin, thrombopoietin, Reg3G, P-selectin/CD62P, Dkk-1, WISP-1/CCN4, coagulation factor III/tissue factor, C1q R1/CD93, DPPIV/CD26, E-selectin/CD62E | ↑ |
| CD14, CD40/TNFRSF5, endoglin/CD105, pentraxin 2/SAP, pentraxin 3/TSG-14, lipocalin-2/NGAL, VCAM-1/CD106, | ↓ |
| Target Genes | Primer Sequence |
|---|---|
| E11 | S 5′-CTGGCCTGAGGTCATCTTGT-3′ A 5′-TCCATCCCCACCAACAAGTG-3′ |
| p16 | S 5′-CGCAGGTTCTTGGTCACTGT-3′ A 5′-TGTTCACGAAAGCCAGAGCG-3′ |
| p21 | S 5′-CCTGGTGATGTCCGACCTG-3′ A 5′-CCATGAGCGCATCGCAATC-3′ |
| Adiponectin | S 5′-CCAGGAAGAAACCACCGGA-3′ A 5′-GAAATCAGGAAGGCTGCCAAG-3′ |
| Resistin | S 5′-CATGCCATGGGGTCCAGCATGCCACTGT-3′ A 5′-CCCAAGCTTTCAGGAAGCGACCTGCA-3′ |
| IL-6 | S 5′-ATGAACAACGATGATGCACTTG-3′ A 5′-GGTACTCCAGAAGACCAGAGG-3′ |
| IL-1α | S 5′-CTGAAGAAGAGACGGCTGAGT-3′ A 5′-CTGGTAGGTGTAAGGTGCTGAT-3′ |
| MMP-3 | S 5′-AGGGATGATGATGCTGGTATG-3′ A 5′-AACACCACACCTGGGCTTAT-3′ |
| IGFBP-6 | S 5′-GCAGCAGCTCCAGACTGA-3′ A 5′-CATTGCTTCACATACAGCTCAA-3′ |
| GAPDH | S 5′-AGGTCGGTGTGAACGGATTTG-3′ A 5′-GGGGTCGTTGATGGCAACA-3′ |
| Runx2 | S 5′-TGCCACCTCTGACTTCTGC-3′ A 5′-GTCAAGGGTCCGTAAAGTAG-3′ |
| PPAR-γ | S 5′-GGAAGACCACTGCATTCCTT-3′ A 5′-GTAATCAGCAACCATTGGGTCA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Wang, Y.; Wang, J.; Zhai, J.; Ren, L.; Zhu, G. Radiation-Induced Osteocyte Senescence Alters Bone Marrow Mesenchymal Stem Cell Differentiation Potential via Paracrine Signaling. Int. J. Mol. Sci. 2021, 22, 9323. https://doi.org/10.3390/ijms22179323
Xu L, Wang Y, Wang J, Zhai J, Ren L, Zhu G. Radiation-Induced Osteocyte Senescence Alters Bone Marrow Mesenchymal Stem Cell Differentiation Potential via Paracrine Signaling. International Journal of Molecular Sciences. 2021; 22(17):9323. https://doi.org/10.3390/ijms22179323
Chicago/Turabian StyleXu, Linshan, Yuyang Wang, Jianping Wang, Jianglong Zhai, Li Ren, and Guoying Zhu. 2021. "Radiation-Induced Osteocyte Senescence Alters Bone Marrow Mesenchymal Stem Cell Differentiation Potential via Paracrine Signaling" International Journal of Molecular Sciences 22, no. 17: 9323. https://doi.org/10.3390/ijms22179323






