The Multi-Elemental Composition of the Aqueous Humor of Patients Undergoing Cataract Surgery, Suffering from Coexisting Diabetes, Hypertension, or Diabetic Retinopathy
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Analysis
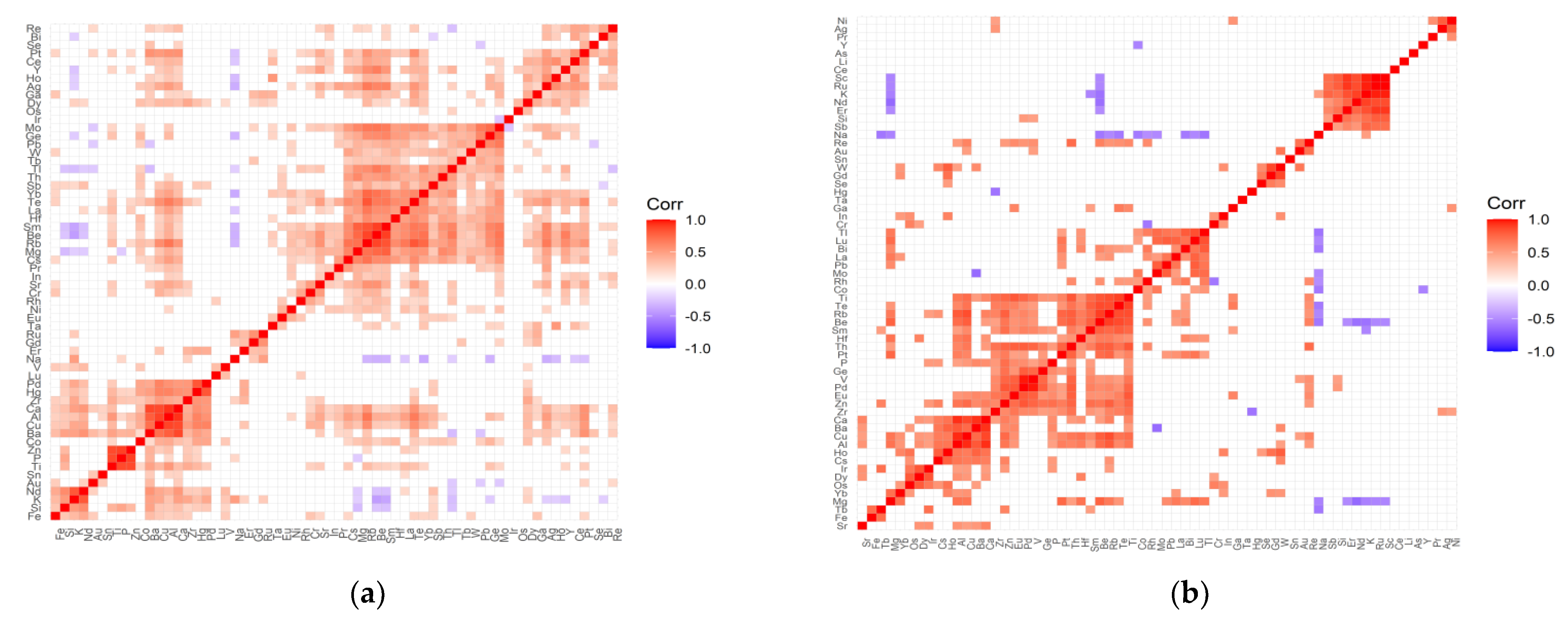
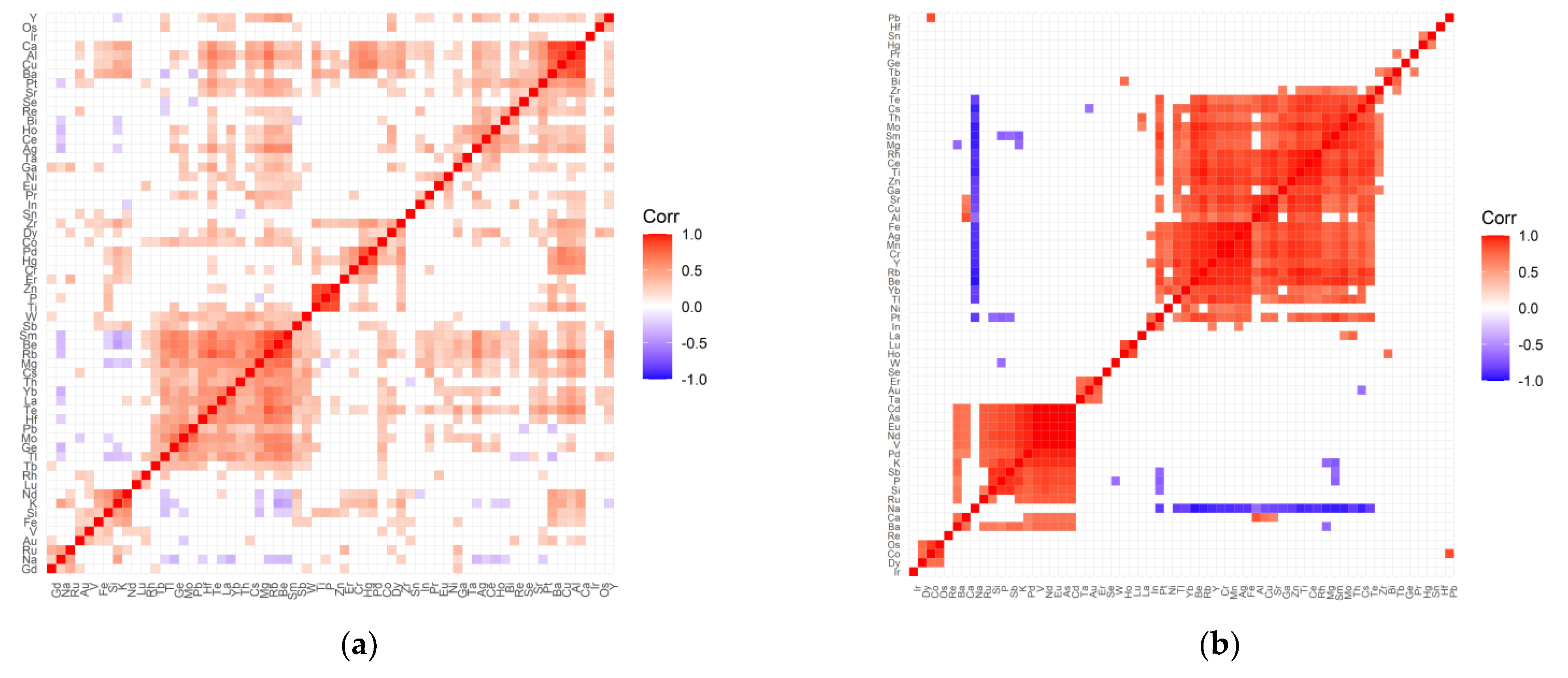
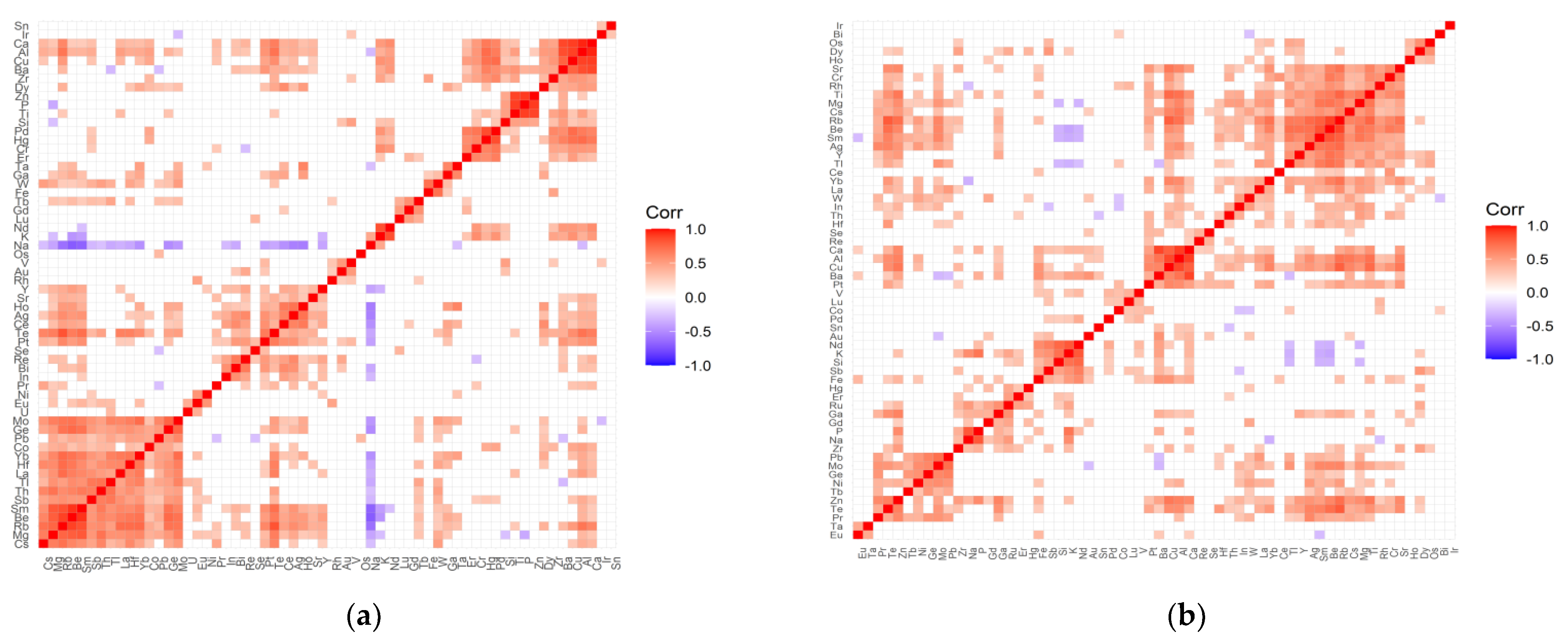
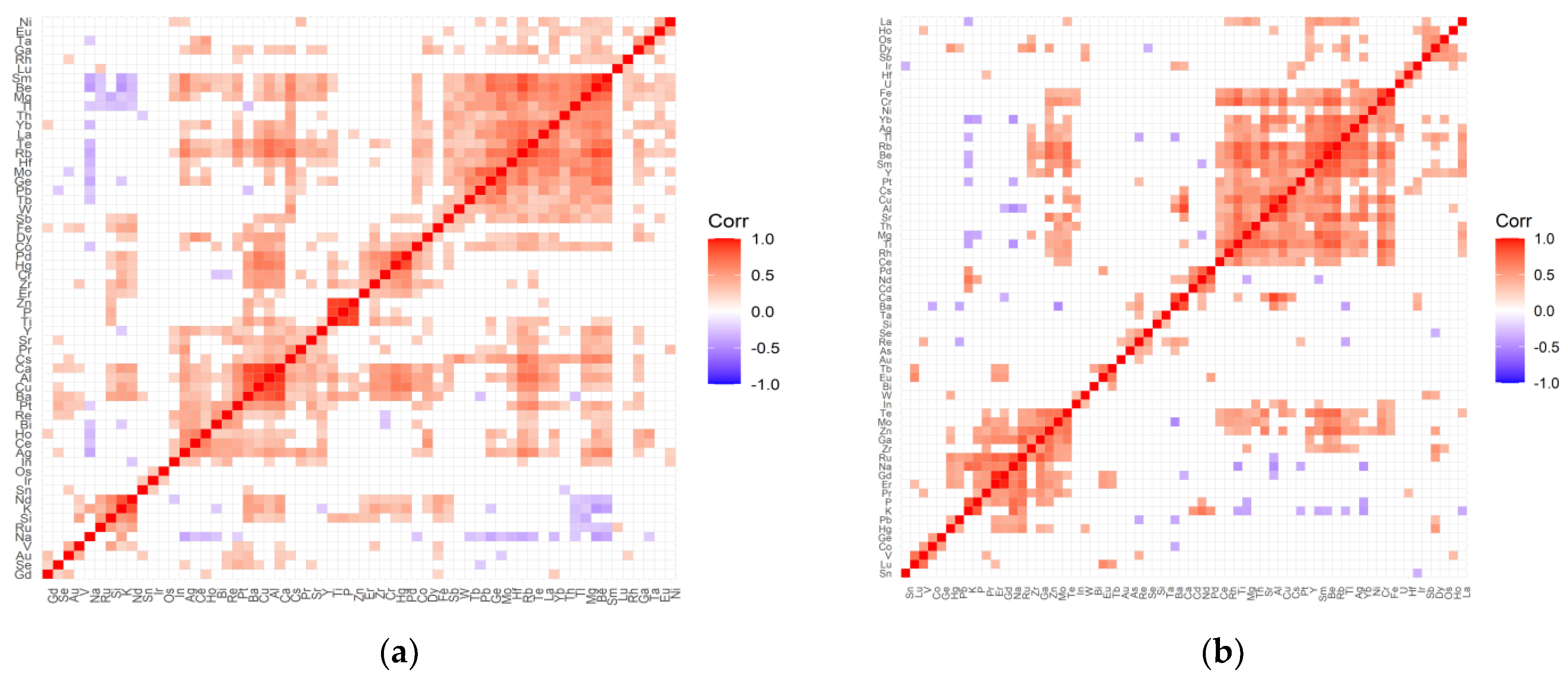
2.2. The Inter-Elemental Correlations
2.3. The Statistically Significant Inter-Correlations
3. Materials and Methods
3.1. Subjects
3.2. Sample Preparation Procedure
3.3. ICP-OES Measurements
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Blindness and Vision Impairment. Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 4 June 2020).
- Beebe, D.C.; Holekamp, N.M.; Shui, Y.B. Oxidative damage and the prevention of age-related cataracts. Ophthalmic. Res. 2010, 44, 155–165. [Google Scholar] [CrossRef]
- Shinohara, T.; Singh, D.P.; Chylack, L.T. Review: Age-related cataract: Immunity and lens epithelium-derived growth factor (LEDGF). J. Ocul. Pharmacol. Ther. 2000, 16, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Andjelić, S.; Hawlina, M. Cataractogenesis. Pregl. Ćlanek 2012, 81, 122–132. [Google Scholar]
- Gum, G.G.; Gelatt, K.N.; Esson, D.W. Physiology of the eye. In Veterinary Ophthalmology, 4th ed.; Gelatt, K.N., Ed.; Blackwell Pub.: Ames, IA, USA, 2007. [Google Scholar]
- Biros, D.J. Immunology and the Eye. In Veterinary Ophthalmology, 4th ed.; Gelatt, K.N., Ed.; Blackwell Pub.: Ames, IA, USA, 2007. [Google Scholar]
- Chowdhury, U.R.; Madden, B.J.; Charlesworth, M.C.; Fautsch, M.P. Proteome analysis of human aqueous humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4921–4931. [Google Scholar] [CrossRef] [PubMed]
- Miric, D.J.; Kisic, B.M.; Zoric, L.D.; Miric, B.M.; Mirkovic, M.; Mitic, R. Influence of cataract maturity on aqueous humor lipid peroxidation markers and antioxidant enzymes. Eye 2014, 28, 72–77. [Google Scholar] [CrossRef]
- Umapathy, A.; Donaldson, P.; Lim, J. Antioxidant delivery pathways in the anterior eye. Biomed. Res. Int. 2013, 2013, 207250. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.S.; Chung, H.J.; Sontakke, S.B.; Chung, I.Y.; Ju, S.; Seo, S.W.; Yoo, J.M.; Kim, S.J. Ascorbic acid concentrations in aqueous humor after systemic vitamin C supplementation in patients with cataract: Pilot study. BMC Ophthalmol. 2017, 17, 121. [Google Scholar] [CrossRef]
- Ji, Y.; Rong, X.; Lu, Y. Metabolic characterization of human aqueous humor in the cataract progression after pars plana vitrectomy. BMC Ophthalmol. 2018, 18, 63. [Google Scholar] [CrossRef]
- Cho, M.C.; Kim, R.B.; Ahn, J.Y.; Yoo, W.S.; Kim, S.J. Aqueous humor and serum 25-Hydroxyvitamin D levels in patients with cataracts. BMC Ophthalmol. 2020, 20, 6. [Google Scholar] [CrossRef]
- Flieger, J.; Święch-Zubilewicz, A.; Śniegocki, T.; Dolar-Szczasny, J.; Pizoń, M. Determination of Tryptophan and Its Major Metabolites in Fluid from the Anterior Chamber of the Eye in Diabetic Patients with Cataract by Liquid Chromotography Mass Spectrometry (LC-MS/MS). Molecules 2018, 23, 3012. [Google Scholar] [CrossRef]
- El-Sayyad, H.I.H.; Bakr, E.H.M.; El-Ghawet, H.A.; El-Desoky, T.M.G.E. Overview of Congenital, Senile and Metabolic Cataract. J. Ocul. Biol. 2015, 3, 12. [Google Scholar]
- Tamiya, S.; Dean, W.L.; Paterson, C.A.; Delamere, N.A. Regional distribution of Na,K-ATPase activity in porcine lens epithelium. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4395–4399. [Google Scholar] [CrossRef]
- Delamere, N.A.; Tamiya, S. Expression, regulation and function of Na,K-ATPase in the lens. Prog. Retin. Eye Res. 2004, 23, 593–615. [Google Scholar] [CrossRef]
- Zeimer, R.; Weinreb, A.; Loewinger, E.; Kalman, Z.H.; Belkin, M. Detection and analysis of metals in the eye by x-ray spectrometry. Med. Phys. 1974, 1, 251–256. [Google Scholar] [CrossRef]
- Erie, J.C.; Butz, J.A.; Good, J.A.; Erie, E.A.; Burritt, M.F.; Cameron, J.D. Heavy metal concentrations in human eyes. Am. J. Ophthalmol. 2005, 139, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Schmeling, M.; Gaynes, B.I.; Tidow-Kebritchi, S. Heavy metal analysis in lens and aqueous humor of cataract patients by total reflection X-ray fluorescence spectrometry. Powder Diffr. 2014, 29, 155–158. [Google Scholar] [CrossRef][Green Version]
- Dolar-Szczasny, J.; Święch, A.; Flieger, J.; Tatarczak-Michalewska, M.; Niedzielski, P.; Proch, J.; Majerek, D.; Kawka, J.; Mackiewicz, J. Levels of Trace Elements in the Aqueous Humor of Cataract Patients Measured by the Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2019, 24, 4127. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Cumurcu, T.; Özugurlu, F.; Özyurt, H.; Sahinoglu, S.; Mendil, D.; Hasdemir, E. Levels of Iron, Zinc, and Copper in Aqueous Humor, Lens, and Serum in Nondiabetic and Diabetic Patients: Their Relation to Cataract. Biol. Trace Elem. Res. 2005, 108, 33–42. [Google Scholar] [CrossRef]
- Stillman, M.J.; Presta, A. Characterizing metal ion interactions with biological molecules—the spectroscopy of metallothionein. In Molecular Biology and Toxicology of Metals; Zalups, R.Z., Koropatnick, J., Eds.; Taylor & Francis: New York, NY, USA, 2000; pp. 276–299. [Google Scholar]
- Eichenbaum, J.W.; Zheng, W. Distribution of lead and transthyretin in human eyes. J. Toxicol. Clin. Toxicol. 2000, 38, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Ono, Y.; Kuromiya, A.; Toyosawa, K.; Ueda, Y.; Bril, V. Long-Term Treatment with Ranirestat (AS-3201), a Potent Aldose Reductase Inhibitor, Suppresses Diabetic Neuropathy and Cataract Formation in Rats. J. Pharmacol. Sci. 2008, 107, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Devamanoharan, P.S.; Henein, M.; Ali, A.H.; Varma, S.D. Diabetes-induced biochemical changes in rat lens: Attenuation of cataractogenesis by pyruvate. Diabetes Obes. Metab. 2000, 2, 165–174. [Google Scholar] [CrossRef]
- Kayiklik, A.; Alyamac Sukgen, E. Biochemical analysis of aqueous humor in diabetic and non-diabetic patients with cataracts. Ophthalmol. J. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Stopa, P.; Rejdak, R.; Michalke, B.; Chaudhri, A.; Schlotzer-Schrehardt, U.; Kruse, F.E.; Zrenner, E.; Junemann, A.G. Levels of Aqueous Humour Trace Elements in Patients with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4109. [Google Scholar]
- Hohberger, B.; Chaudhri, M.A.; Michalke, B.; Lucio, M.; Nowomiejska, K.; Schlötzer-Schrehardt, U.; Grieb, P.; Rejdak, R.; Jünemann, A.G.M. Levels of aqueous humor trace elements in patients with open-angle glaucoma. J. Trace Elem. Med. Biol. 2018, 45, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zeng, J.; Zhuo, M.; Xu, W.; Sun, L.; Wang, J.; Liu, X. Involvement of caspase-3 and p38 mitogen-activated protein kinase in cobalt chloride-induced apoptosis in PC12 cells. J. Neurosci. Res. 2002, 67, 837–843. [Google Scholar] [CrossRef]
- Ortega, R.; Bresson, C.; Fraysse, A.; Sandre, C.; Deve’s, G.; Gombert, C.; Tabarant, M.; Bleuet, P.; Seznec, H.; Simionovici, A.; et al. Cobalt distribution in keratinocyte cells indicates nuclear and perinuclear accumulation and interaction with magnesium and zinc homeostasis. Toxicol. Lett. 2009, 188, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Choi, S.K. Analysis of aqueous humor calcium and phosphate from cataract eyes with and without diabetes mellitus. Korean J. Ophthalmol. 2007, 21, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Kao, J.P.Y.; Matteson, D.R. Cellular Physiology and Neurophysiology, 2nd ed.; Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Argüello, J.M.; Raimunda, D.; González-Guerrero, M. Metal Transport across Biomembranes: Emerging Models for a Distinct Chemistry. J. Biol. Chem. 2012, 287, 13510–13517. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, D. Binding and transport of metal ions at the dimer interface of the Escherichia coli metal transporter YiiP. J. Biol. Chem. 2006, 281, 23492–23502. [Google Scholar] [CrossRef] [PubMed]
- De Feo, C.J.; Aller, S.G.; Siluvai, G.S.; Blackburn, N.J.; Unger, V.M. Three-dimensional structure of the human copper transporter hCTR1. Proc. Natl. Acad. Sci. USA 2009, 106, 4237–4242. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Argüello, J.M. Mechanism of Cu-transporting ATPases: Soluble Cu chaperones directly transfer Cu to transmembrane transport sites. Proc. Natl. Acad. Sci. USA 2008, 105, 5992–5997. [Google Scholar] [CrossRef]
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and functional aspects of metal sites in biology. Chem. Rev. 1996, 96, 2239–2314. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Palmgren, M.G.; Nissen, P. P-type ATPases. Annu. Rev. Biophys. 2011, 40, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Rensing, C.; Rosen, B.P.; Mitra, B. The ATP hydrolytic activity of purified ZntA, a Pb(II)/Cd(II)/Zn(II)-translocating ATPase from Escherichia coli. J. Biol. Chem. 2000, 275, 3873–3978. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Cheung, W.D.; Argüello, J.M. Characterization of a thermophilic P-type Ag/Cu-ATPase from the extremophile Archaeoglobus fulgidus. J. Biol. Chem. 2002, 277, 7201–7208. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lim, D.; Park, K.H.; Chae, J.B.; Jang, H.; Lee, J.; Chung, H. Quantitative proteomic analysis of aqueous humor from patients with drusen and reticular pseudodrusen in age-related macular degeneration. BMC Ophthalmol. 2018, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Fresquez, M.R.; Pappas, R.S.; Watson, C.H. Establishment of the toxic metal reference range in tobacco from US cigarettes. J. Anal. Toxicol. 2013, 37, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Karovic, O.; Tonazzini, I.; Rebola, N.; Edström, E.; Lövdahl, C.; Fredholm, B.B.; Daré, E. Toxic effects of cobalt in primary cultures of mouse astrocytes. Similarities with hypoxia and role of HIF-1alpha. Biochem. Pharmacol. 2007, 73, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y. Cobalt Inhibits the Interaction between Hypoxia-inducible Factor-alpha and von Hippel-Lindau Protein by Direct Binding to Hypoxiainducible Factor-alpha. J. Biol. Chem. 2003, 278, 15911–15916. [Google Scholar] [CrossRef]
- Wenger, R.H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002, 16, 1151–1162. [Google Scholar] [CrossRef]
- Olbryt, M.; Jarzab, M.; Jazowiecka-Rakus, J.; Simek, K.; Szala, S.; Sochanik, A. Gene expression profile of B 16(F10) murine melanoma cells exposed to hypoxic conditions in vitro. Gene Expr. 2006, 13, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Maliha, A.M.; Kuehn, S.; Hurst, J.; Herms, F.; Fehr, M.; Bartz-Schmidt, K.U.; Dick, H.B.; Joachim, S.C.; Schnichels, S. Diminished apoptosis in hypoxic porcine retina explant cultures through hypothermia. Sci. Rep. 2019, 9, 4898. [Google Scholar] [CrossRef] [PubMed]
- Jünemann, A.G.; Stopa, P.; Michalke, B.; Chaudhri, A.; Reulbach, U.; Huchzermeyer, C.; Schlötzer-Schrehardt, U.; Kruse, F.E.; Zrenner, E.; Rejdak, R. Levels of aqueous humor trace elements in patients with non-exsudative age-related macular degeneration: A case-control study. PLoS ONE 2013, 8, e56734. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lyu, D.; Dong, X.; He, J.; Yao, K. Hypertension and risk of cataract: A meta-analysis. PLoS ONE 2014, 9, e114012. [Google Scholar] [CrossRef]
- Mylona, I.; Dermenoudi, M.; Ziakas, N.; Tsinopoulos, I. Hypertension is the Prominent Risk Factor in Cataract Patients. Medicina 2019, 55, 430. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López Del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implant. Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T. Endocrine disruption induced by organotin compounds; organotins function as a powerful agonist for nuclear receptors rather than an aromatase inhibitor. J. Toxicol. Sci. 2008, 33, 269–276. [Google Scholar] [CrossRef]
- Gajda, T.; Jancsó, A. Organotins. formation, use, speciation, and toxicology. Met. Ions Life Sci. 2010, 7, 111–151. [Google Scholar] [CrossRef]
- Dopp, E.; von Recklinghausen, U.; Hippler, J.; Diaz-Bone, R.A.; Richard, J.; Zimmermann, U.; Rettenmeier, A.W.; Hirner, A.V. Toxicity of volatile methylated species of bismuth, arsenic, tin, and mercury in Mammalian cells in vitro. J. Toxicol. 2011, 2011, 503576. [Google Scholar] [CrossRef]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef] [PubMed]
- Cannata-Andía, J.B.; Rodríguez-García, M. Hyperphosphataemia as a cardiovascular risk factor—How to manage the problem. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 11), 16–19. [Google Scholar] [CrossRef] [PubMed]
- Werner, L.; Apple, D.J.; Kaskaloglu, M.; Pandey, S.K. Dense opacification of the optical component of a hydrophilic acrylic intraocular lens: A clinicopathological analysis of 9 explanted lenses. J. Cataract Refract. Surg. 2001, 27, 1485–1492. [Google Scholar] [CrossRef]
- Izak, A.M.; Werner, L.; Pandey, S.K.; Apple, D.J. Calcification of modern foldable hydrogel intraocular lens designs. Eye 2003, 17, 393–406. [Google Scholar] [CrossRef]
- Razali, N.; Wah, Y. Power Comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J. Stat. Modeling Anal. 2011, 2, 21–33. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Brown, M.B.; Forsythe, A.B. Robust Tests for the Equality of Variances. J. Am. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef]
- Neuhäuser, M. Wilcoxon–Mann–Whitney Test. In International Encyclopedia of Statistical Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1656–1658. [Google Scholar] [CrossRef]
- Cureton, E.E. Rank-biserial correlation. Psychometrika 1956, 21, 287–290. [Google Scholar] [CrossRef]
- Cohen, J.A. Power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Hemphill, J.F. Interpreting the magnitudes of correlation coefficients. Am. Psychol. 2003, 58, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Gignac, G.; Szodorai, E. Effect size guidelines for individual differences researchers. Personal. Individ. Differ. 2016, 102, 74–78. [Google Scholar] [CrossRef]
- Funder, D.C.; Ozer, D.J. Evaluating effect size in psychological research: Sense and nonsense. Adv. Methods Pract. Psychol. Sci. 2019, 2, 156–168. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 31 March 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Patil, I. Visualizations with statistical details: The ggstatsplot approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 13 February 2021).
- Sjoberg, D.D.; Curry, M.; Hannum, M.; Larmarange, J.; Whiting, K.; Zabor, E.C.; Drill, E.; Flynn, J.; Lavery, J.; Lobaugh, S.; et al. Gtsummary: Presentation-Ready Data Summary and Analytic Result Tables. 2021. Available online: https://CRAN.R-project.org/package=gtsummary (accessed on 21 June 2021).




| Element/Parameter | AMD | Retinopathy | Hypertension | Diabetes | |
|---|---|---|---|---|---|
| Ca | C | 296 ± 167 | 296 ± 167 | 304 ± 193 | 311 ± 178 |
| S | 287 ± 132 | 276 ± 104 | 286 ± 131 | 252 ± 103 | |
| Δ | −8.08 | −20.63 | −18.10 | −59.13 | |
| p | 0.997 | 0.988 | 0.989 | 0.137 | |
| Cs | C | 87.6 ± 65.4 | 90.2 ± 67.1 | 97.6 ± 72.7 | 93.0 ± 68.8 |
| S | 103 ± 68 | 84.4 ± 50.6 | 82.7 ± 58.5 | 81.5 ± 57.4 | |
| Δ | 14.913 | −5.79 | −14.92 | −11.55 | |
| p | 0.381 | 0.893 | 0.254 | 0.378 | |
| K | C | 110 ± 82 | 108 ± 80 | 106 ± 86 | 111 ± 88 |
| S | 103 ± 55 | 116 ± 72 | 111 ± 73 | 103 ± 52 | |
| Δ | −6.63 | 8.27 | 5.37 | −8.49 | |
| p | 0.450 | 0.172 | 0.339 | 0.514 | |
| Mg | C | 16.9 ± 5.1 | 17.0 ± 5.0 | 16.9 ± 5.0 | 17.1 ± 5.4 |
| S | 17.5 ± 5.4 | 16.1 ± 6.3 | 17.0 ± 5.2 | 16.5 ± 4.3 | |
| Δ | 0.625 | −0.934 | 0.170 | −0.628 | |
| p | 0.256 | 0.195 | 0.900 | 0.750 | |
| Na | C | 2180 ± 690 | 2180 ± 660 | 2080 ± 400 | 2170 ± 610 |
| S | 2150 ± 250 | 2130 ± 400 | 2260 ± 790 | 2200 ± 730 | |
| Δ | −28.37 | −45.39 | 184.75 | 33.46 | |
| p | 0.837 | 0.717 | 0.105 | 0.924 | |
| P | C | 20.6 ± 33.7 | 19.7 ± 32.7 | 22.8 ± 43.8 | 20.5 ± 36.8 |
| S | 14.3 ± 2.7 | 19.7 ± 7.6 | 17.0 ± 12.4 | 17.8 ± 8.0 | |
| Δ | −6.29 | −0.074 | −5.85 | −2.72 | |
| p | 0.824 | 0.017 | 0.891 | 0.012 | |
| Rb | C | 14.5 ± 9.6 | 14.5 ± 8.9 | 15.4 ± 10.6 | 14.8 ± 9.6 |
| S | 15.0 ± 6.3 | 14.6 ± 11.9 | 13.8 ± 7.7 | 14.0 ± 8.0 | |
| Δ | 0.564 | 0.016 | −1.58 | −0.764 | |
| p | 0.266 | 0.616 | 0.664 | 0.983 | |
| Cluster/Element | Disease | Mean Conc. (mg L−1) | Median (mg L−1) | Conc. Range (mg L−1) | loge W(MannWhitney) | R 1 | CI95% 2 | p 3 | |
|---|---|---|---|---|---|---|---|---|---|
| I | Co | AMD | 0.031 (c) 0.091 (s) | 0.000 (c) 0.035 (s) | 0.000–0.40 0.000–0.30 | 6.24 | −0.36 | −0.63; −0.08 | 0.004 |
| Sn | Hypertension | 0.15 (c) 0.29 (s) | 0.000 (c) 0.049 (s) | 0.000–1.03 0.000–1.34 | 7.17 | −0.21 | −0.38; −1.47 | 0.031 | |
| III | Ru | Hypertension | 0.002 (c) 0.035 (s) | 0.000 (c) 0.000 (s) | 0.000–0.051 0.000–0.77 | 7.21 | −0.18 | −0.28; −0.07 | 0.006 |
| IV | Ti | Hypertension | 0.150 (c) 0.096 (s) | 0.100 (c) 0.083 (s) | 0.029–1.35 0.025–0.34 | 7.60 | 0.22 | 0.04; 0.38 | 0.045 |
| V | P | Retinopathy | 19.7 (c) 19.7 (s) | 13.9 (c) 17.7 (s) | 2.74–329 12.2–38.2 | 5.65 | −0.46 | −0.76; −0.09 | 0.017 |
| P | Diabetes | 20.5 (c) 17.8 (s) | 13.7 (c) 15.3 (s) | 2.74–329 6.66–47.7 | 6.85 | −0.30 | −0.49; −0.08 | 0.012 | |
| Disease | Group | Gender | n | % | Min–Max Age | Median Age | Mean Age ± SD |
|---|---|---|---|---|---|---|---|
| AMD | studied | Female | 11 | 68.75 | 67–88 | 76.0 | 76.18 ± 6.24 |
| Male | 5 | 31.25 | 74–89 | 81.0 | 80.80 ± 6.06 | ||
| control | Female | 63 | 63.64 | 55–94 | 76.0 | 75.46 ± 7.13 | |
| Male | 36 | 36.36 | 58–89 | 72.5 | 72.83 ± 7.50 | ||
| Retino-pathy | studied | Female | 5 | 50.00 | 69–86 | 77.0 | 76.80 ± 6.10 |
| Male | 5 | 50.00 | 67–77 | 71.0 | 72.00 ± 4.00 | ||
| control | Female | 69 | 65.71 | 55–94 | 76.0 | 75.48 ± 7.06 | |
| Male | 36 | 34.29 | 58–89 | 74.5 | 74.06 ± 8.13 | ||
| Hyper-tension | studied | Female | 42 | 68.85 | 60–94 | 76.0 | 75.81 ± 6.26 |
| Male | 19 | 31.15 | 63–89 | 74.0 | 75.21 ± 7.35 | ||
| control | Female | 32 | 59.26 | 55–91 | 77.0 | 75.25 ± 7.91 | |
| Male | 22 | 40.74 | 58–84 | 75.0 | 72.59 ± 8.02 | ||
| Dia-betes | studied | Female | 18 | 54.55 | 55–86 | 74.5 | 73.50 ± 7.48 |
| Male | 15 | 45.45 | 63–81 | 74.0 | 73.60 ± 5.68 | ||
| control | Female | 56 | 68.29 | 59–94 | 76.5 | 76.23 ± 6.74 | |
| Male | 26 | 31.71 | 58–89 | 74.5 | 73.92 ± 8.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flieger, J.; Dolar-Szczasny, J.; Rejdak, R.; Majerek, D.; Tatarczak-Michalewska, M.; Proch, J.; Blicharska, E.; Flieger, W.; Baj, J.; Niedzielski, P. The Multi-Elemental Composition of the Aqueous Humor of Patients Undergoing Cataract Surgery, Suffering from Coexisting Diabetes, Hypertension, or Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 9413. https://doi.org/10.3390/ijms22179413
Flieger J, Dolar-Szczasny J, Rejdak R, Majerek D, Tatarczak-Michalewska M, Proch J, Blicharska E, Flieger W, Baj J, Niedzielski P. The Multi-Elemental Composition of the Aqueous Humor of Patients Undergoing Cataract Surgery, Suffering from Coexisting Diabetes, Hypertension, or Diabetic Retinopathy. International Journal of Molecular Sciences. 2021; 22(17):9413. https://doi.org/10.3390/ijms22179413
Chicago/Turabian StyleFlieger, Jolanta, Joanna Dolar-Szczasny, Robert Rejdak, Dariusz Majerek, Małgorzata Tatarczak-Michalewska, Jędrzej Proch, Eliza Blicharska, Wojciech Flieger, Jacek Baj, and Przemysław Niedzielski. 2021. "The Multi-Elemental Composition of the Aqueous Humor of Patients Undergoing Cataract Surgery, Suffering from Coexisting Diabetes, Hypertension, or Diabetic Retinopathy" International Journal of Molecular Sciences 22, no. 17: 9413. https://doi.org/10.3390/ijms22179413
APA StyleFlieger, J., Dolar-Szczasny, J., Rejdak, R., Majerek, D., Tatarczak-Michalewska, M., Proch, J., Blicharska, E., Flieger, W., Baj, J., & Niedzielski, P. (2021). The Multi-Elemental Composition of the Aqueous Humor of Patients Undergoing Cataract Surgery, Suffering from Coexisting Diabetes, Hypertension, or Diabetic Retinopathy. International Journal of Molecular Sciences, 22(17), 9413. https://doi.org/10.3390/ijms22179413









