Classification and Treatment of Diseases in the Age of Genome Medicine Based on Pathway Pathology
Abstract
:1. Introduction
1.1. Traditional and Emerging Ways of Classifying Diseases
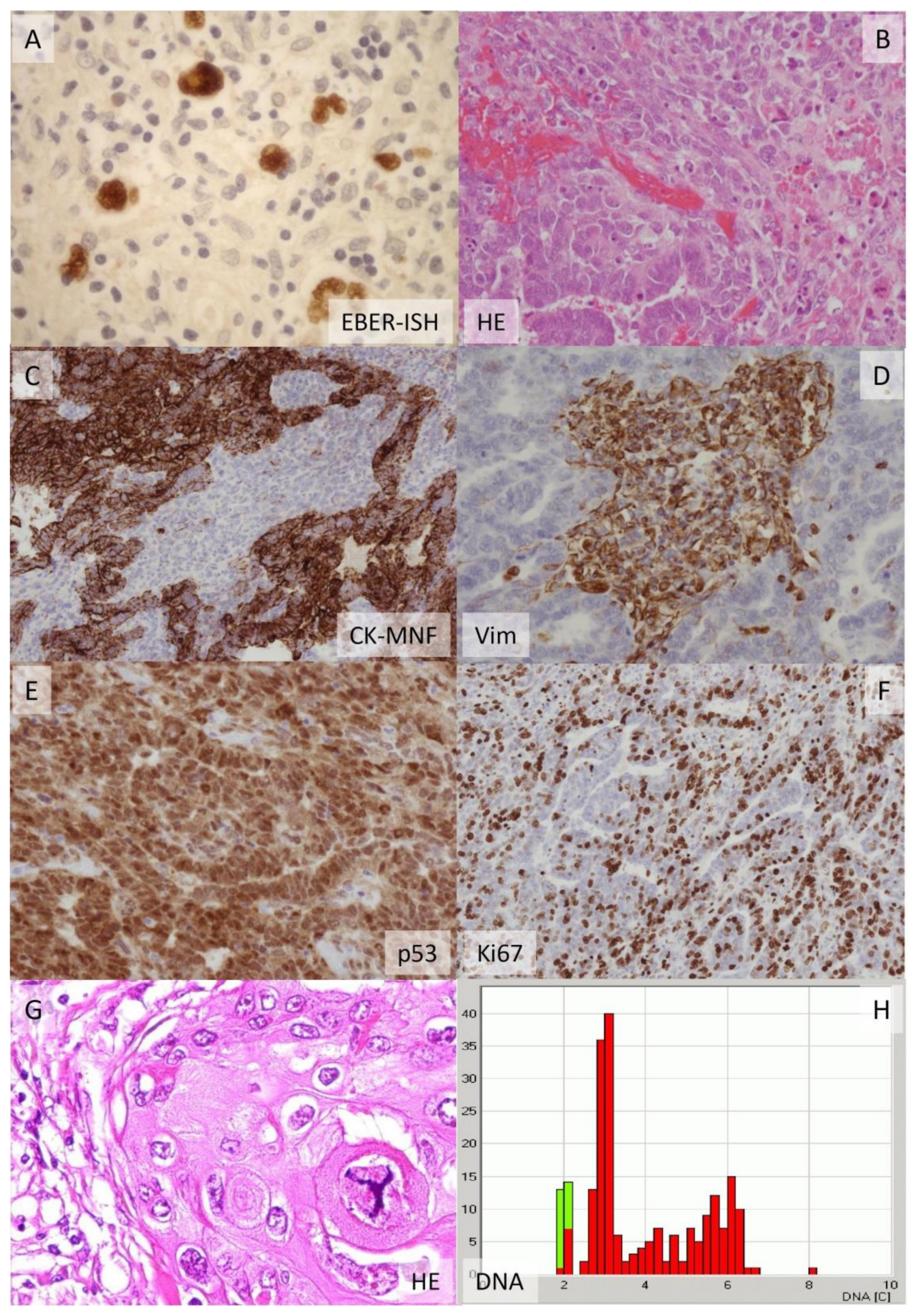
1.2. Big Data in Disease Classification, Predictive Pathology and Precision Medicine
1.3. International Classification of Diseases: ICD, ICD-O and TNM
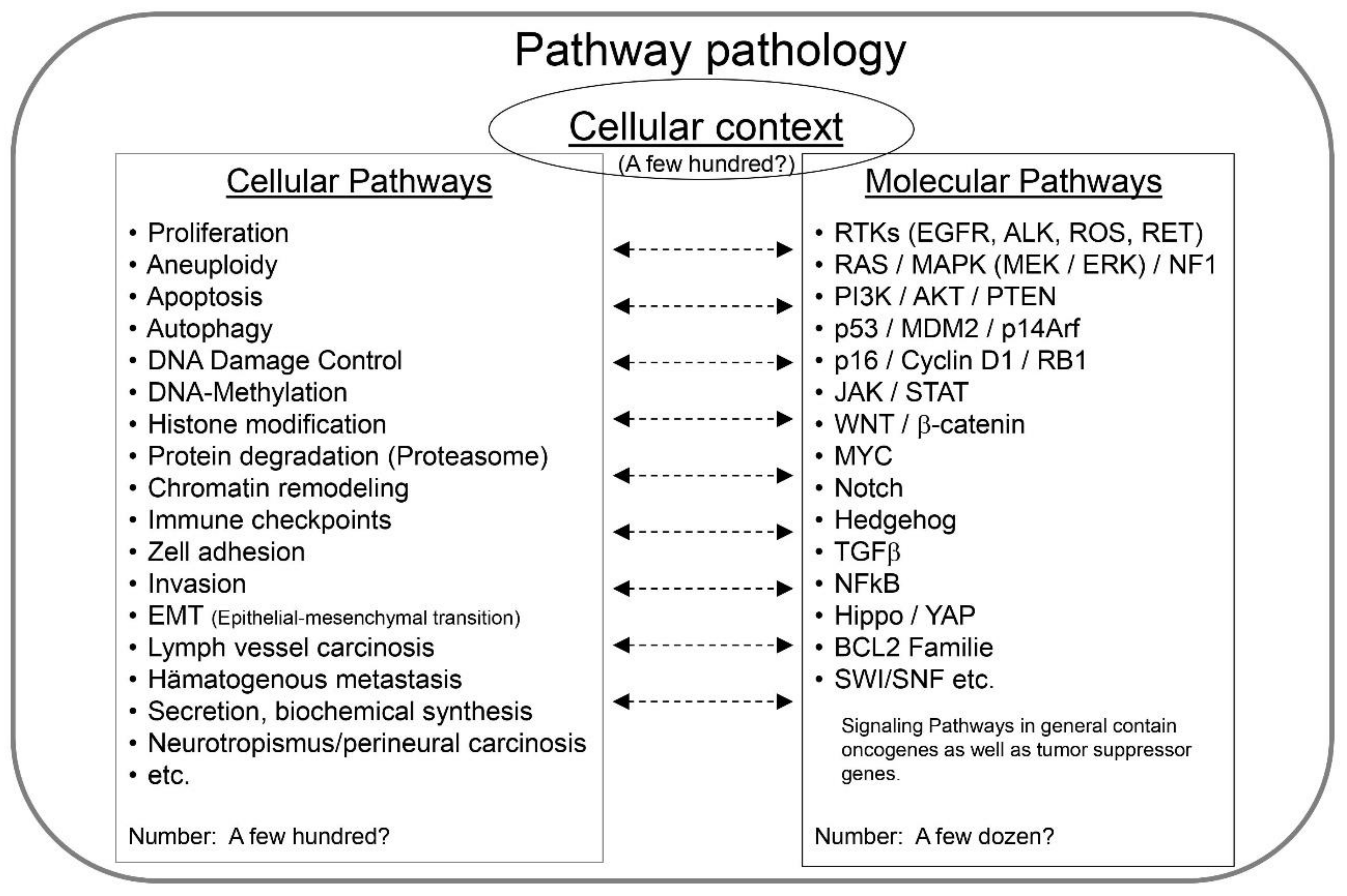
2. Pathway Pathology—The Key Principles
- (1)
- The cellular context of the disease;
- (2)
- The genes and proteins being affected, i.e., the molecular pathways;
- (3)
- The cellular mechanisms being altered, i.e., the cellular pathways (Figure 2).
2.1. Cellular Context
2.2. Molecular Pathways
2.3. Cellular Pathways
3. Pathway Pathology in Research and Clinical Practice
3.1. Immortality and Cellular Senescence Represent Key Cellular Pathways
3.2. The Interplay between Molecular and Cellular Pathways in Specific Cells Predicts Biological Behavior
3.3. Analytical Tools in Pathway Pathology—Whole-Genome Analysis
3.4. Single Genes in Pathway Pathology
3.4.1. The Case of TP53
3.4.2. S100A14
3.4.3. GABARAP
3.5. Impact of Cellular Pathology and Histopathology on Pathway Pathology
3.6. Pathway Pathology and Treatment
3.7. Pathway Pathology of Anti-Cancer Drugs
3.8. Pathway Pathology—Is It Really New?

4. Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mattson, M.P. Superior pattern processing is the essence of the evolved human brain. Front. Neurosci. 2014, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Aisenberg, A.C. Historical review of lymphomas. Br. J. Haematol. 2000, 109, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.S.; Harris, N.L.; Stein, H.; Isaacson, P.G. Classification of lymphoid neoplasms: The microscope as a tool for disease discovery. Blood 2008, 112, 4384–4399. [Google Scholar] [CrossRef] [Green Version]
- Etzel, B.-M.; Gerth, M.; Chen, Y.; Wünsche, E.; Facklam, T.; Beck, J.F.; Guntinas-Lichius, O.; Petersen, I. Mutation analysis of tumor necrosis factor alpha-induced protein 3 gene in Hodgkin lymphoma. Pathol.-Res. Pr. 2017, 213, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Nagel, D.; Vincendeau, M.; Eitelhuber, A.C.; Krappmann, D. Mechanisms and consequences of constitutive NF-κB activation in B-cell lymphoid malignancies. Oncogene 2014, 33, 5655–5665. [Google Scholar] [CrossRef] [Green Version]
- Petersen, I.; Kotb, W.F.A.; Friedrich, K.-H.; Schlüns, K.; Böcking, A.; Dietel, M. Core classification of lung cancer: Correlating nuclear size and mitoses with ploidy and clinicopathological parameters. Lung Cancer 2009, 65, 312–318. [Google Scholar] [CrossRef]
- Bockmühl, U.; Schwendel, A.; Dietel, M.; Petersen, I. Distinct patterns of chromosomal alterations in high- and low-grade head and neck squamous cell carcinomas. Cancer Res. 1996, 56, 5325–5329. [Google Scholar]
- Petersen, I.; Petersen, S. Towards a Genetic-Based Classification of Human Lung Cancer. Anal. Cell. Pathol. 2001, 22, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Ried, T.; Petersen, I.; Holtgreve-Grez, H.; Speicher, M.; Schröck, E.; Du Manoir, S.; Cremer, T. Mapping of multiple DNA gains and losses in primary small cell lung carcinomas by comparative genomic hybridization. Cancer Res. 1994, 54, 1801–1806. [Google Scholar]
- Garber, M.E.; Troyanskaya, O.G.; Schluens, K.; Petersen, S.; Thaesler, Z.; Pacyna-Gengelbach, M.; van de Rijn, M.; Rosen, G.D.; Perou, C.; Whyte, R.I.; et al. Diversity of gene expression in adenocarcinoma of the lung. Proc. Natl. Acad. Sci. USA 2001, 98, 13784–13789. [Google Scholar] [CrossRef] [Green Version]
- Perou, C.; Jeffrey, S.; van de Rijn, M.; Rees, C.A.; Eisen, M.; Ross, D.T.; Pergamenschikov, A.; Williams, C.F.; Zhu, S.X.; Lee, J.C.F.; et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc. Natl. Acad. Sci. USA 1999, 96, 9212–9217. [Google Scholar] [CrossRef] [Green Version]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Koelsche, C.; Schrimpf, D.; Stichel, D.; Sill, M.; Sahm, F.; Reuss, D.E.; Blattner, M.; Worst, B.; Heilig, C.E.; Beck, K.; et al. Sarcoma classification by DNA methylation profiling. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Petersen, I. Entitäten der Weichteilsarkome. Trauma Berufskrankh. 2017, 20, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Dietel, M.; Jöhrens, K.; Laffert, M.V.; Hummel, M.; Bläker, H.; Pfitzner, B.M.; Lehmann, A.; Denkert, C.; Darb-Esfahani, S.; Lenze, D.; et al. A 2015 update on predictive molecular pathology and its role in targeted cancer therapy: A review focussing on clinical relevance. Cancer Gene Ther. 2015, 22, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Clamp, M.; Fry, B.; Kamal, M.; Xie, X.; Cuff, J.; Lin, M.F.; Kellis, M.; Lindblad-Toh, K.; Lander, E.S. Distinguishing protein-coding and noncoding genes in the human genome. Proc. Natl. Acad. Sci. USA 2007, 104, 19428–19433. [Google Scholar] [CrossRef] [Green Version]
- Kottke, J. How Many Words do Shakespeare Know. 2010. Available online: https://kottke.org/10/04/how-many-words-did-shakespeare-know (accessed on 28 August 2021).
- du Plessis, L.; Škunca, N.; Dessimoz, C. The what, where, how and why of gene ontology—A primer for bioinformaticians. Briefings Bioinform. 2011, 12, 723–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancellotta, V.; Guinot, J.L.; Fionda, B.; Rembielak, A.; Di Stefani, A.; Gentileschi, S.; Federico, F.; Rossi, E.; Guix, B.; Chyrek, A.J.; et al. SKIN-COBRA (Consortium for Brachytherapy data Analysis) ontology: The first step towards interdisciplinary standardized data collection for personalized oncology in skin cancer. J. Contemp. Brachyther. 2020, 12, 105–110. [Google Scholar] [CrossRef]
- Tagliaferri, L.; Kovács, G.; Autorino, R.; Budrukkar, A.; Guinot, J.L.; Hildebrand, G.; Johansson, B.; Monge, R.M.; Meyer, J.E.; Niehoff, P.; et al. ENT COBRA (Consortium for Brachytherapy Data Analysis): Interdisciplinary standardized data collection system for head and neck patients treated with interventional radiotherapy (brachytherapy). J. Contemp. Brachyther. 2016, 8, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Aymé, S.; Bellet, B.; Rath, A. Rare diseases in ICD11: Making rare diseases visible in health information systems through appropriate coding. Orphanet J. Rare Dis. 2015, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D. Rudolf Virchow, the founder of cellular pathology. Rom. J. Morphol. Embryol. 2019, 60, 1381–1382. [Google Scholar] [PubMed]
- Dawood, S.; Austin, L.; Cristofanilli, M. Cancer stem cells: Implications for cancer therapy. Oncology 2014, 28, 1101–1107. [Google Scholar] [PubMed]
- Yamanaka, S. A Fresh Look at iPS Cells. Cell 2009, 137, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayflick, L.; Stinebring, W.R. Intracellular growth of Pleuropneumonia-like organisms. Anatomincal. Record. 1955, 121, 477–478. [Google Scholar]
- Hayflick, L.; Stinebring, W.R. Intracellular Growth of Pleuropneumonialike Organisms (PPLO) in Tissue Culture and in OVO*. Ann. N. Y. Acad. Sci. 1960, 79, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Chanock, R.M. Mycoplasma Species of Man. Bacteriol. Rev. 1965, 29, 185–221. [Google Scholar] [CrossRef]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Ben-David, U.; Amon, A. Context is everything: Aneuploidy in cancer. Nat. Rev. Genet. 2020, 21, 44–62. [Google Scholar] [CrossRef]
- Poulin, E.J.; Bera, A.K.; Lu, J.; Lin, Y.-J.; Strasser, S.D.; Paulo, J.A.; Huang, T.Q.; Morales, C.; Yan, W.; Cook, J.; et al. Tissue-Specific Oncogenic Activity of KRASA146T. Cancer Discov. 2019, 9, 738–755. [Google Scholar] [CrossRef] [Green Version]
- Hirata, E.; Sahai, E. Tumor Microenvironment and Differential Responses to Therapy. Cold Spring Harb. Perspect. Med. 2017, 7, a026781. [Google Scholar] [CrossRef] [Green Version]
- Hayflick, L.; Norton, T.W.; Plotkin, S.A.; Koprowski, H. Preparation of Polio vaccines in a human fetal diploid cell strain. Am. J. Hygiene 1962, 75, 240–258. [Google Scholar]
- Olshansky, S.J.; Hayflick, L. The Role of the WI-38 Cell Strain in Saving Lives and Reducing Morbidity. AIMS Public Health 2017, 4, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Galindo, C.; Allen, C.E. Langerhans cell histiocytosis. Blood 2020, 135, 1319–1331. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, F.; Liu, L. Alternative Lengthening of Telomeres (ALT) in Tumors and Pluripotent Stem Cells. Genes 2019, 10, 1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Espín, D.; Cañamero, M.; Maraver, A.; López, G.G.; Contreras, J.; Murillo-Cuesta, S.; Rodríguez-Baeza, A.; Varela-Nieto, I.; Ruberte, J.; Collado, M.; et al. Programmed Cell Senescence during Mammalian Embryonic Development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Storer, M.; Mas, A.; Robert-Moreno, À.; Pecoraro, M.; Ortells, M.C.; Di Giacomo, V.; Yosef, R.; Pilpel, N.; Krizhanovsky, V.; Sharpe, J.; et al. Senescence Is a Developmental Mechanism that Contributes to Embryonic Growth and Patterning. Cell 2013, 155, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras Provokes Premature Cell Senescence Associated with Accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.; Serrano, M. Senescence in tumours: Evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, T.O.; Poulin, N.M.; Ladanyi, M. Synovial Sarcoma: Recent Discoveries as a Roadmap to New Avenues for Therapy. Cancer Discov. 2015, 5, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Sauer, C.M.; Haugg, A.M.; Chteinberg, E.; Rennspiess, D.; Winnepenninckx, V.; Speel, E.-J.; Becker, J.C.; Kurz, A.K.; Hausen, A.Z. Reviewing the current evidence supporting early B-cells as the cellular origin of Merkel cell carcinoma. Crit. Rev. Oncol. 2017, 116, 99–105. [Google Scholar] [CrossRef]
- Dammert, M.A.; Brägelmann, J.; Olsen, R.R.; Böhm, S.; Monhasery, N.; Whitney, C.P.; Chalishazar, M.D.; Tumbrink, H.L.; Guthrie, M.R.; Klein, S.; et al. MYC paralog-dependent apoptotic priming orchestrates a spectrum of vulnerabilities in small cell lung cancer. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X. The Spectrum of MYC Alterations in Diffuse Large B-Cell Lymphoma. Acta Haematol. 2020, 143, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Z.; Xie, C.; Zhu, Y.; Shu, X.; Zhang, Z.; Li, N.; Chai, N.; Zhang, S.; Wu, K.; et al. PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis. J. Exp. Clin. Cancer Res. 2018, 37, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.; Riely, G.J.; Van Schil, P.E.; et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J. Thorac. Oncol. 2011, 6, 244–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinn, P.; Aulmann, S.; Wirtz, R.; Schott, S.; Marmé, F.; Varga, Z.; Lebeau, A.; Kreipe, H.; Schneeweiss, A. Multigene Assays for Classification, Prognosis, and Prediction in Breast Cancer: A Critical Review on the Background and Clinical Utility. Geburtshilfe Frauenheilkd. 2013, 73, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Moran, S.; Cardus, A.M.; Sayols, S.; Musulen, E.; Balaña, C.; Estival-Gonzalez, A.; Moutinho, C.; Heyn, H.; Diaz-Lagares, A.; de Moura, M.C.; et al. Epigenetic profiling to classify cancer of unknown primary: A multicentre, retrospective analysis. Lancet Oncol. 2016, 17, 1386–1395. [Google Scholar] [CrossRef]
- Duruisseaux, M.; Martínez-Cardús, A.; Calleja-Cervantes, M.E.; Moran, S.; de Moura, M.C.; Davalos, V.; Piñeyro, D.; Sanchez-Cespedes, M.; Girard, N.; Brevet, M.; et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: A multicentre, retrospective analysis. Lancet Respir. Med. 2018, 6, 771–781. [Google Scholar] [CrossRef]
- Petersen, I. Predictive pathology of lung cancer immunotherapy response. Lancet Respir. Med. 2018, 6, 731–733. [Google Scholar] [CrossRef]
- Kamps, R.; Brandão, R.D.; Bosch, B.J.V.D.; Paulussen, A.D.C.; Xanthoulea, S.; Blok, M.J.; Romano, A. Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int. J. Mol. Sci. 2017, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Jiang, S.; Li, R.; Li, W.; Chen, H.; Bo, X. A survey and evaluation of Web-based tools/databases for variant analysis of TCGA data. Briefings Bioinform. 2019, 20, 1524–1541. [Google Scholar] [CrossRef]
- Hainaut, P.; Pfeifer, G.P. SomaticTP53Mutations in the Era of Genome Sequencing. Cold Spring Harb. Perspect. Med. 2016, 6, a026179. [Google Scholar] [CrossRef] [Green Version]
- Petersen, I.; Ohgaki, H.; Ludeke, B.I.; Kleihues, P. p53 mutations in phenacetin-associated human urothelial carcinomas. Carcinogenesis 1993, 14, 2119–2122. [Google Scholar] [CrossRef] [Green Version]
- Koshland, D. Molecule of the year. Science 1993, 262, 1953. [Google Scholar] [CrossRef] [Green Version]
- Soussi, T. The history of p53. EMBO Rep. 2010, 11, 822–826. [Google Scholar] [CrossRef]
- Dolgin, E. The most popular genes in the human genome. Nat. Cell Biol. 2017, 551, 427–431. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Bykov, V.N.; Wiman, K.G. Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett. 2014, 588, 2622–2627. [Google Scholar] [CrossRef] [Green Version]
- Gurpinar, E.; Vousden, K.H. Hitting cancers’ weak spots: Vulnerabilities imposed by p53 mutation. Trends Cell Biol. 2015, 25, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Vousden, K.H. Mutant p53 in Cancer: New Functions and Therapeutic Opportunities. Cancer Cell 2014, 25, 304–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrales, A.; Iwakuma, T. Targeting Oncogenic Mutant p53 for Cancer Therapy. Front. Oncol. 2015, 5, 288. [Google Scholar] [CrossRef] [Green Version]
- Brosh, R.; Rotter, V. When mutants gain new powers: News from the mutant p53 field. Nat. Rev. Cancer 2009, 9, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Kroemer, G. Cytoplasmic functions of the tumour suppressor p53. Nat. Cell Biol. 2009, 458, 1127–1130. [Google Scholar] [CrossRef]
- Mertens, F.; Antonescu, C.R.; Mitelman, F. Gene fusions in soft tissue tumors: Recurrent and overlapping pathogenetic themes. Genes Chromosom. Cancer 2016, 55, 291–310. [Google Scholar] [CrossRef] [Green Version]
- Canale, M.; Petracci, E.; Delmonte, A.; Bronte, G.; Chiadini, E.; Ludovini, V.; Dubini, A.; Papi, M.; Baglivo, S.; De Luigi, N.; et al. Concomitant TP53 Mutation Confers Worse Prognosis in EGFR-Mutated Non-Small Cell Lung Cancer Patients Treated with TKIs. J. Clin. Med. 2020, 9, 1047. [Google Scholar] [CrossRef] [Green Version]
- Schulze, S.; Petersen, I. Gender and ploidy in cancer survival. Cell. Oncol. 2011, 34, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Mehren, M.; Joensuu, H. Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2018, 36, 136–143. [Google Scholar] [CrossRef]
- Pietas, A.; Schlüns, K.; Marenholz, I.; Schafer, B.; Heizmann, C.W.; Petersen, I. Molecular Cloning and Characterization of the Human S100A14 Gene Encoding a Novel Member of the S100 Family. Genomics 2002, 79, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Brettmann, E.; Strong, C.D.G. Recent evolution of the human skin barrier. Exp. Dermatol. 2018, 27, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Klebig, C.; Seitz, S.; Arnold, W.; Deutschmann, N.; Pacyna-Gengelbach, M.; Scherneck, S.; Petersen, I. Characterization of {gamma}-aminobutyric acid type A receptor-associated protein, a novel tumor suppressor, showing reduced expression in breast cancer. Cancer Res. 2005, 65, 394–400. [Google Scholar]
- Salah, F.; Ebbinghaus, M.; Muley, V.Y.; Zhou, Z.; Al-Saadi, K.R.D.; Pacyna-Gengelbach, M.; O’Sullivan, G.A.; Betz, H.; König, R.; Wang, Z.-Q.; et al. Tumor suppression in mice lacking GABARAP, an Atg8/LC3 family member implicated in autophagy, is associated with alterations in cytokine secretion and cell death. Cell Death Dis. 2016, 7, e2205. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Dominguez, R.; Perez-Medina, M.; Lopez-Gonzalez, J.S.; Galicia-Velasco, M.; Aguilar-Cazares, D. The Double-Edge Sword of Autophagy in Cancer: From Tumor Suppression to Protumor Activity. Front. Oncol. 2020, 10, 2064. [Google Scholar] [CrossRef]
- Petersen, I.; Dietel, M.; Geilenkeuser, W.J.; Mireskandari, M.; Weichert, W.; Steiger, K.; Scheel, A.H.; Büttner, R.; Schirmacher, P.; Warth, A.; et al. EGFR immunohistochemistry as biomarker for antibody-based therapy of squamous NSCLC—Experience from the first ring trial of the German Quality Assurance Initiative for Pathology (QuIP®). Pathol.-Res. Pr. 2017, 213, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Zayed, H.; Petersen, I. Stem cell transcription factor SOX2 in synovial sarcoma and other soft tissue tumors. Pathol.-Res. Pr. 2018, 214, 1000–1007. [Google Scholar] [CrossRef] [Green Version]
- Amit, M.; Na’Ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408. [Google Scholar] [CrossRef]
- Chiang, S.P.H.; Cabrera, R.M.; Segall, J.E. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 2016, 311, C1–C14. [Google Scholar] [CrossRef] [Green Version]
- Kotb, W.F.A.; Petersen, I. Morphology, DNA ploidy and HPV in lung cancer and head and neck cancer. Pathol.-Res. Pr. 2012, 208, 1–8. [Google Scholar] [CrossRef]
- Cosenza, M.R.; Krämer, A. Centrosome amplification, chromosomal instability and cancer: Mechanistic, clinical and therapeutic issues. Chromosom. Res. 2015, 24, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berard, A.; Kroeker, A.; McQueen, P.; Coombs, K.M. Methods and approaches to disease mechanisms using systems kinomics. Synth. Syst. Biotechnol. 2018, 3, 34–43. [Google Scholar] [CrossRef]
- Narayanan, S.; Cai, C.-Y.; Assaraf, Y.G.; Guo, H.-Q.; Cui, Q.; Wei, L.; Huang, J.-J.; Ashby, C.R.; Chen, Z.-S. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist. Updat. 2020, 48, 100663. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cuesta, L.; Peifer, M.; Lu, X.; Sun, R.; Ozretić, L.; Seidel, D.; Zander, T.; Leenders, F.; George, J.; Müller, C.; et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.P. Understanding Cancer Through the Lens of Epigenetic Inheritance, Allele-Specific Gene Expression, and High-Throughput Technology. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Aday, A.; Ridker, P.M. Antiinflammatory Therapy in Clinical Care: The CANTOS Trial and Beyond. Front. Cardiovasc. Med. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J.; Lorenzatti, A.; Krum, H.; Varigos, J.; Siostrzonek, P.; et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- Hindler, K.; Cleeland, C.S.; Rivera, E.; Collard, C.D. The Role of Statins in Cancer Therapy. Oncologist 2006, 11, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Y.; Yang, L.; Huang, H.; Hu, X.; Zhao, C.; Huang, H.; Ying, Y. Prognostic Significance of Statin Use in Colorectal Cancer. Medicine 2015, 94, e908. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.C.; Lee, H.S.; Lee, Y.I.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; Chung, J.B.; Bang, S. Concomitant Statin Use Has a Favorable Effect on Gemcitabine-Erlotinib Combination Chemotherapy for Advanced Pancreatic Cancer. Yonsei Med. J. 2016, 57, 1124–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy. J. Exp. Pharmacol. 2021, 2021, 303–328. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Weinstein, I.B.; Joe, A. Oncogene Addiction. Cancer Res. 2008, 68, 3077–3080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Fang, H.; Zhang, J.; Gu, Y. Targeting “undruggable” c-Myc protein by synthetic lethality. Front. Med. 2021, 15, 541–550. [Google Scholar] [CrossRef]
- Pilié, P.G.; Gay, C.M.; Byers, L.A.; O’Connor, M.J.; Yap, T.A. PARP Inhibitors: Extending Benefit Beyond BRCA-Mutant Cancers. Clin. Cancer Res. 2019, 25, 3759–3771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Mukherjee, S. The Emperor of All Maladies; Fourth Estate Ltd.: London, UK, 2011; ISBN 978-0-00-725092-9. [Google Scholar]

| ICD-11 | ICD-10 | ||||
|---|---|---|---|---|---|
| No. | Title | Codes | No. | Title | Codes |
| 1 | Certain infectious and parasitic diseases | 1A00-1G8Y | 1 | Certain infectious and parasitic diseases | A00-B99 |
| 2 | Neoplasms | 2A00-2F9Y | 2 | Neoplasms | C00-D48 |
| 3 | Diseases of blood and blood-forming organs | 3A00-3C0Z | 3 | Diseases of the blood and blood-forming organs in addition to certain disorders involving the immune system | D50-D89 |
| 4 | Disorders of the immune system | 4A00-4B4Z | 4 | Endocrine, nutritional and metabolic diseases | E00-E90 |
| 5 | Endocrine, nutritional or metabolic diseases | 5A00-5B3Z | 5 | Mental and behavioral disorders | F00-F99 |
| 6 | Mental, behavioral or neurodevelopmental disorders | 6A00-6E8Z | 6 | Diseases of the nervous system | G00-G99 |
| 7 | Sleep–wake disorders | 7A00-7B2Z | 7 | Diseases of the eye and adnexa | H00-H59 |
| 8 | Diseases of the nervous system | 8A00-8E7Z | 8 | Diseases of the ear and mastoid process | H60-H95 |
| 9 | Diseases of the visual system | 9A00-9A4Z | 9 | Diseases of the circulatory system | I00-I99 |
| 10 | Diseases of the ear and mastoid process | AA00-AC0Z | 10 | Diseases of the respiratory system | J00-J99 |
| 11 | Diseases of the circulatory system | BA00-BE2Z | 11 | Diseases of the digestive system | K00-K93 |
| 12 | Diseases of the respiratory system | CA00-CB7Z | 12 | Diseases of the skin and subcutaneous tissue | L00-L99 |
| 13 | Diseases of the digestive system | DA00-DE2Z | 13 | Diseases of the musculoskeletal system and connective tissue | M00-M99 |
| 14 | Diseases of the skin | EA00-EM0Z | 14 | Diseases of the genitourinary system | N00-N99 |
| 15 | Diseases of the musculoskeletal system or connective tissue | FA00-FC0Z | 15 | Pregnancy, childbirth and the puerperium | O00-O99 |
| 16 | Diseases of the genitourinary system | GA00-GC8Z | 16 | Certain conditions originating in the perinatal period | P00-P96 |
| 17 | Conditions related to sexual health | HA00-HA8Z | 17 | Congenital malformations, deformations and chromosomal abnormalities | Q00-Q99 |
| 18 | Pregnancy, childbirth and the puerperium | JA00-JB6Z | 18 | Symptoms, signs and abnormal clinical and laboratory findings, not classified elsewhere | R00-R99 |
| 19 | Certain conditions originating in the perinatal period | KA00-KA0Z | 19 | Injury, poisoning and certain other consequences of external causes | S00-T98 |
| 20 | Developmental anomalies | LA00-LD0Z | 20 | External causes of morbidity and mortality | V01-Y98 |
| 21 | Symptoms, signs or clinical findings, not classified elsewhere | MA00-MH2Y | 21 | Factors influencing health status and contact with health services | Z00-Z99 |
| 22 | Injury, poisoning or certain other consequences of external causes | NA00-NF2Z | 22 | Codes for special purposes | U00-U99 |
| 23 | External causes of morbidity or mortality | PA00-PL2Z | |||
| 24 | Factors influencing health status or contact with health services | QA00-QF4Z | |||
| 25 | Codes for special purposes | RA00-RA26 | |||
| 26 | Supplementary chapter: traditional medicine conditions | TM1-SJ3Z | |||
| 27 | V—supplementary section for functioning assessment | VA00-VB40.Z | |||
| 28 | X—extension codes | XS8H-XD19J4 | |||
| 1 | ICD entry title |
| 2 | Classification properties |
| 3 | Textual definition |
| 4 | Terms |
| 5 | Body system/structure description |
| 6 | Temporal properties |
| 7 | Severity of subtype properties |
| 8 | Manifestation properties |
| 9 | Causal properties |
| 10 | Functioning properties |
| 11 | Specific condition properties |
| 12 | Treatment properties |
| 13 | Diagnostic criteria |
| Parameters 1–7 are essential requirements. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, I. Classification and Treatment of Diseases in the Age of Genome Medicine Based on Pathway Pathology. Int. J. Mol. Sci. 2021, 22, 9418. https://doi.org/10.3390/ijms22179418
Petersen I. Classification and Treatment of Diseases in the Age of Genome Medicine Based on Pathway Pathology. International Journal of Molecular Sciences. 2021; 22(17):9418. https://doi.org/10.3390/ijms22179418
Chicago/Turabian StylePetersen, Iver. 2021. "Classification and Treatment of Diseases in the Age of Genome Medicine Based on Pathway Pathology" International Journal of Molecular Sciences 22, no. 17: 9418. https://doi.org/10.3390/ijms22179418
APA StylePetersen, I. (2021). Classification and Treatment of Diseases in the Age of Genome Medicine Based on Pathway Pathology. International Journal of Molecular Sciences, 22(17), 9418. https://doi.org/10.3390/ijms22179418





