ABC Transporters in T Cell-Mediated Physiological and Pathological Immune Responses
Abstract
:1. Introduction: The ABC Transporter Family
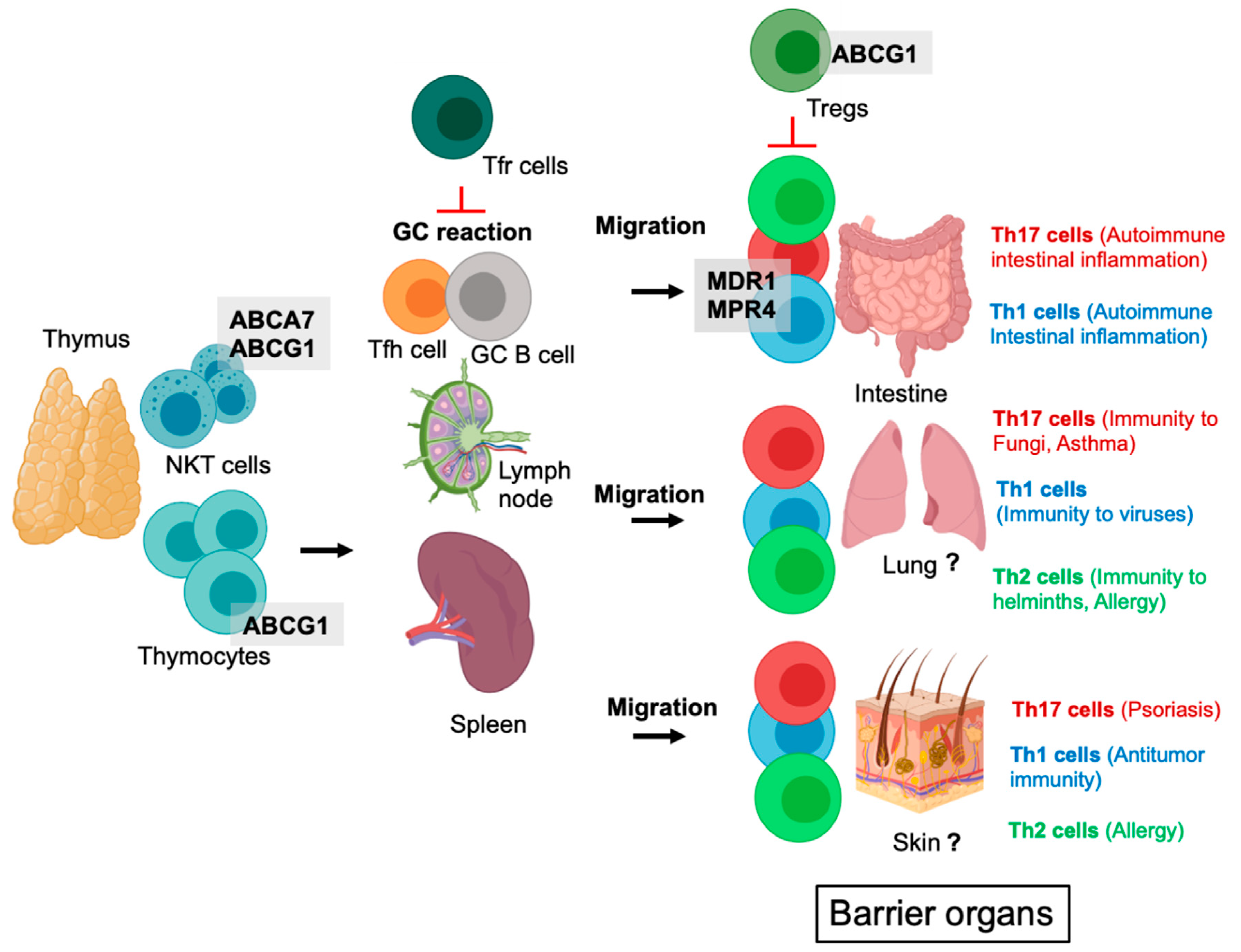
2. ABC Transporters in the Development of T Cell Subsets (T Cells, Natural Killer T Cells, Tregs)
3. ABC Transporters in CD8+ T Cells and CD8+ T Cell-Mediated Immunity
4. ABC Transporters in CD4+ T Helper Cell Populations
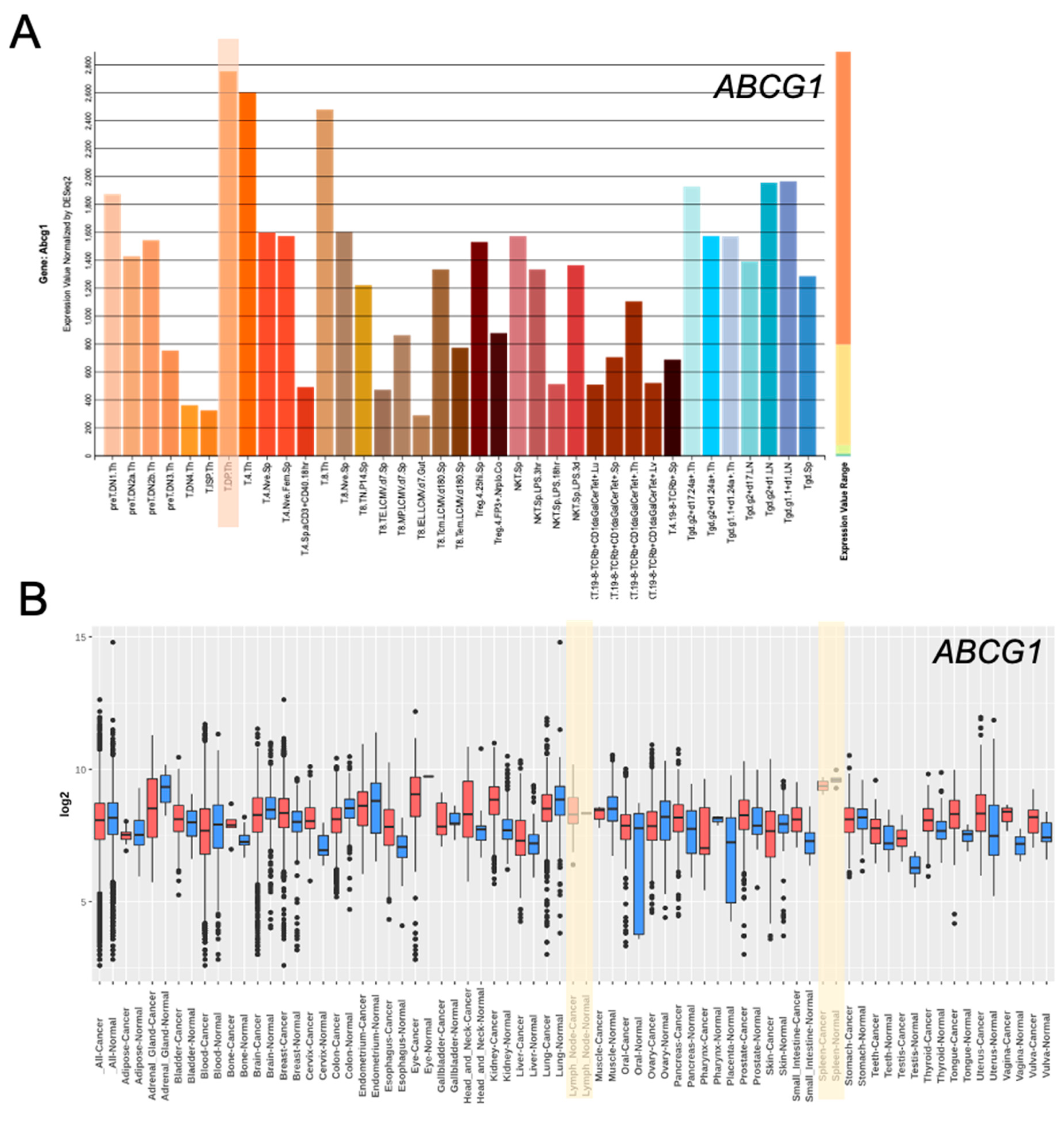
5. ABC Transporters in Tregs
6. ABC Transporters in Immune Cells during Cancer
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, C.; Tampe, R. Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [Google Scholar] [CrossRef]
- Locher, K.P. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 2016, 23, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Do, T.H.T.; Martinoia, E.; Lee, Y. Functions of ABC transporters in plant growth and development. Curr. Opin. Plant. Biol. 2018, 41, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.M.; George, A.M. Multidrug resistance in parasites: ABC transporters, P-glycoproteins and molecular modelling. Int. J. Parasitol. 2005, 35, 555–566. [Google Scholar] [CrossRef]
- Orelle, C.; Mathieu, K.; Jault, J.M. Multidrug ABC transporters in bacteria. Res. Microbiol. 2019, 170, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.M.; Whitfield, C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr Res. 2013, 378, 35–44. [Google Scholar] [CrossRef]
- Liu, X. ABC Family Transporters. Adv. Exp. Med. Biol. 2019, 1141, 13–100. [Google Scholar]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef]
- Eggensperger, S.; Tampe, R. The transporter associated with antigen processing: A key player in adaptive immunity. Biol. Chem. 2015, 396, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.Y.; Terada, T. Molecular mechanisms for biliary phospholipid and drug efflux mediated by ABCB4 and bile salts. Biomed. Res. Int. 2014, 2014, 954781. [Google Scholar] [CrossRef] [Green Version]
- Nicolaou, M.; Andress, E.J.; Zolnerciks, J.K.; Dixon, P.H.; Williamson, C.; Linton, K.J. Canalicular ABC transporters and liver disease. J. Pathol. 2012, 226, 300–315. [Google Scholar] [CrossRef]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Amawi, H.; Sim, H.M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-Mediated Multidrug-Resistant Cancer. Adv. Exp. Med. Biol. 2019, 1141, 549–580. [Google Scholar]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Beck-García, K.; Zorzin, C.; Schamel, W.W.; Davis, M.M. Inhibition of T cell receptor signaling by cholesterol sulfate, a naturally occurring derivative of membrane cholesterol. Nat. Immunol. 2016, 17, 844–850. [Google Scholar] [CrossRef]
- Yang, W.; Pan, W.; Chen, S.; Trendel, N.; Jiang, S.; Xiao, F.; Xue, M.; Wu, W.; Peng, Z.; Li, X.; et al. Dynamic regulation of CD28 conformation and signaling by charged lipids and ions. Nat. Struct. Mol. Biol. 2017, 24, 1081–1092. [Google Scholar] [CrossRef]
- Zech, T.; Ejsing, C.S.; Gaus, K.; de Wet, B.; Shevchenko, A.; Simons, K.; Harder, T. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 2009, 28, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Molnár, E.; Swamy, M.; Holzer, M.; Beck-García, K.; Worch, R.; Thiele, C.; Guigas, G.; Boye, K.; Luescher, I.F.; Schwille, P.; et al. Cholesterol and sphingomyelin drive ligand-independent T-cell antigen receptor nanoclustering. J. Biol. Chem. 2012, 287, 42664–42674. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.J.; Gebre, A.K.; Parks, J.S.; Hedrick, C.C. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J. Immunol. 2010, 184, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcerm, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D.; et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 2008, 134, 97–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sag, D.; Wingender, G.; Nowyhed, H.; Wu, R.; Gebre, A.K.; Parks, J.S.; Kronenberg, M.; Hedrick, C.C. ATP-binding cassette transporter G1 intrinsically regulates invariant NKT cell development. J. Immunol. 2012, 189, 5129–5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowyhed, H.N.; Chandra, S.; Kiosses, W.; Marcovecchio, P.; Andary, F.; Zhao, M.; Fitzgerald, M.L.; Kronenberg, M.; Hedrick, C.C. ATP Binding Cassette Transporter ABCA7 Regulates NKT Cell Development and Function by Controlling CD1d Expression and Lipid Raft Content. Sci. Rep. 2017, 7, 40273. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhao, Y.; Shirai, K.; Molodtsov, A.; Kolling, F.W.; Fisher, J.L.; Zhang, P.; Yan, S.; Searles, T.G.; Bader, J.M.; et al. Resident and circulating memory T cells persist for years in melanoma patients with durable responses to immunotherapy. Nat. Cancer 2021, 2, 300–311. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Stanczak, M.A.; Villa, M.; Uhl, F.M.; Corrado, M.; Klein Geltink, R.I.; Sanin, D.E.; Apostolova, P.; Rana, N.; Edwards-Hicks, J.; et al. Fever supports CD8+ effector T cell responses by promoting mitochondrial translation. Proc. Natl. Acad. Sci. USA 2021, 118, e2023752118. [Google Scholar] [CrossRef] [PubMed]
- Pruner, K.B.; Pepper, M. Local memory CD4 T cell niches in respiratory viral infection. J. Exp. Med. 2021, 218, e20201733. [Google Scholar] [CrossRef]
- Lu, S.X.; De Neef, E.; Thomas, J.D.; Sabio, E.; Rousseau, B.; Gigoux, M.; Knorr, D.A.; Greenbaum, B.; Elhanati, Y.; Hogg, S.J.; et al. Pharmacologic modulation of RNA splicing enhances anti-tumor immunity. Cell 2021, 184, 4032–4047. [Google Scholar] [CrossRef]
- Šustić, M.; Cokarić Brdovčak, M.; Lisnić, B.; Materljan, J.; Juranić Lisnić, V.; Rožmanić, C.; Indenbirken, D.; Hiršl, L.; Busch, D.H.; Brizić, I.; et al. Memory CD8 T Cells Generated by Cytomegalovirus Vaccine Vector Expressing NKG2D Ligand Have Effector-Like Phenotype and Distinct Functional Features. Front. Immunol. 2021, 12, 681380. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Sun, A.; Cao, W.; Eliason, A.; Mendez, K.M.; Getzler, A.J.; Tsuda, S.; Diao, H.; Mukori, C.; Bruno, N.E.; et al. Physiological expression and function of the MDR1 transporter in cytotoxic T lymphocytes. J. Exp. Med. 2020, 217, e20191388. [Google Scholar] [CrossRef] [PubMed]
- Boddupalli, C.S.; Nair, S.; Gray, S.M.; Nowyhed, H.N.; Verma, R.; Gibson, J.A.; Abraham, C.; Narayan, D.; Vasquez, J.; Hedrick, C.C.; et al. ABC transporters and NR4A1 identify a quiescent subset of tissue-resident memory T cells. J. Clin. Investig. 2016, 126, 3905–3916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, U.; Kahlfuss, S.; Yang, J.; Ivanova, E.; Koralov, S.B.; Feske, S. Calcium Signaling Controls Pathogenic Th17 Cell-Mediated Inflammation by Regulating Mitochondrial Function. Cell Metab. 2019, 29, 1104–1118.e6. [Google Scholar] [CrossRef]
- Kahlfuss, S.; Kaufmann, U.; Concepcion, A.R.; Noyer, L.; Raphael, D.; Vaeth, M.; Yang, J.; Pancholi, P.; Maus, M.; Muller, J.; et al. STIM1-mediated calcium influx controls antifungal immunity and the metabolic function of non-pathogenic Th17 cells. EMBO Mol. Med. 2020, 12, e11592. [Google Scholar] [CrossRef] [PubMed]
- Peck, A.; Mellins, E.D. Precarious balance: Th17 cells in host defense. Infect. Immun. 2010, 78, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, A.; Robles, R.J.; Mukherjee, S.; Zhang, H.; Feldbrügge, L.; Csizmadia, E.; Wu, Y.; Enjyoji, K.; Moss, A.C.; Otterbein, L.E.; et al. HIF-1alpha-induced xenobiotic transporters promote Th17 responses in Crohn’s disease. J. Autoimmun. 2018, 94, 122–133. [Google Scholar] [CrossRef]
- Baricza, E.; Tamási, V.; Marton, N.; Buzás, E.I.; Nagy, G. The emerging role of aryl hydrocarbon receptor in the activation and differentiation of Th17 cells. Cell Mol. Life Sci. 2016, 73, 95–117. [Google Scholar] [CrossRef]
- De Lima, K.A.; Donate, P.B.; Talbot, J.; Davoli-Ferreira, M.; Peres, R.S.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q. TGFbeta1 signaling sustains aryl hydrocarbon receptor (AHR) expression and restrains the pathogenic potential of TH17 cells by an AHR-independent mechanism. Cell Death Dis. 2018, 9, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.; Kayama, H.; Chen, M.L.; Delmas, A.; Sun, A.; Kim, S.Y.; Rangarajan, E.S.; McKevitt, K.; Beck, A.P.; Jackson, C.B.; et al. The Xenobiotic Transporter Mdr1 Enforces T Cell Homeostasis in the Presence of Intestinal Bile Acids. Immunity 2017, 47, 1182–1196.e10. [Google Scholar] [CrossRef] [Green Version]
- Prechtl, S.; Roellinghoff, M.; Scheper, R.; Cole, S.P.; Deeley, R.G.; Lohoff, M. The multidrug resistance protein 1: A functionally important activation marker for murine Th1 cells. J. Immunol. 2000, 164, 754–761. [Google Scholar] [CrossRef] [Green Version]
- Kleemann, P.; Casper, B.; Huber, M.; Schuetz, J.D.; Lohoff, M. Multidrug-resistance-associated protein 1 (Mrp1) is probably not required for murine Th cell activation. Int. Immunol. 2006, 18, 1603–1606. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.Y.; Gaddis, D.E.; Wu, R.; McSkimming, C.; Haynes, L.D.; Taylor, A.M.; McNamara, C.A.; Sorci-Thomas, M.; Hedrick, C.C. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J. Clin. Investig. 2016, 126, 3236–3246. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Luo, T.; Xi, D.; Guo, K.; Hu, L.; Zhao, J.; Chen, S.; Guo, Z. Silencing ZAP70 prevents HSP65-induced reverse cholesterol transport and NF-kappaB activation in T cells. Biomed. Pharmacother. 2018, 102, 271–277. [Google Scholar] [CrossRef]
- Liu, J.; Guo, K.; Hu, L.; Luo, T.; Ma, Y.; Zhang, Y.; Lai, W.; Guo, Z. ZAP70 deficiency promotes reverse cholesterol transport through MAPK/ERK pathway in Jurkat cell. Mol. Immunol. 2019, 107, 21–28. [Google Scholar] [CrossRef]
- Luo, T.; Hu, J.; Xi, D.; Xiong, H.; He, W.; Liu, J.; Li, M.; Lu, H.; Zhao, J.; Lai, W.; et al. Lck Inhibits Heat Shock Protein 65-Mediated Reverse Cholesterol Transport in T Cells. J. Immunol. 2016, 197, 3861–3870. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, D.I.; Le Nours, J.; Andrews, D.M.; Uldrich, A.P.; Rossjohn, J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 2018, 48, 453–473. [Google Scholar] [CrossRef] [Green Version]
- Jackaman, C.; Tomay, F.; Duong, L.; Abdol Razak, N.B.; Pixley, F.J.; Metharom, P.; Nelson, D.J. Aging and cancer: The role of macrophages and neutrophils. Ageing Res. Rev. 2017, 36, 105–116. [Google Scholar] [CrossRef]
- Kaymak, I.; Williams, K.S.; Cantor, J.R.; Jones, R.G. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell. 2021, 39, 28–37. [Google Scholar] [CrossRef]
- Schreiber, S.; Hammers, C.M.; Kaasch, A.J.; Schraven, B.; Dudeck, A.; Kahlfuss, S. Metabolic Interdependency of Th2 Cell-Mediated Type 2 Immunity and the Tumor Microenvironment. Front. Immunol. 2021, 12, 632581. [Google Scholar] [CrossRef]
- Jacobo-Albavera, L.; Domínguez-Pérez, M.; Medina-Leyte, D.J.; González-Garrido, A.; Villarreal-Molina, T. The Role of the ATP-Binding Cassette A1 (ABCA1) in Human Disease. Int. J. Mol. Sci. 2021, 22, 1593. [Google Scholar] [CrossRef] [PubMed]
- Viaud, M.; Abdel-Wahab, O.; Gall, J.; Ivanov, S.; Guinamard, R.; Sore, S.; Merlin, J.; Ayrault, M.; Guilbaud, E.; Jacquel, A.; et al. ABCA1 Exerts Tumor-Suppressor Function in Myeloproliferative Neoplasms. Cell Rep. 2020, 30, 3397–3410.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goossens, P.; Rodriguez-Vita, J.; Etzerodt, A.; Masse, M.; Rastoin, O.; Gouirand, V.; Ulas, T.; Papantonopoulou, O.; Van Eck, M.; Auphan-Anezin, N.; et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019, 29, 1376–1389.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Wang, L.; Nelson, A.T.; Han, C.; He, S.; Henn, M.A.; Menon, K.; Chen, J.J.; Baek, A.E.; Vardanyan, A.; et al. 27-Hydroxycholesterol acts on myeloid immune cells to induce T cell dysfunction, promoting breast cancer progression. Cancer Lett. 2020, 493, 266–283. [Google Scholar] [CrossRef]
- Badmann, S.; Heublein, S.; Mayr, D.; Reischer, A.; Liao, Y.; Kolben, T.; Beyer, S.; Hester, A.; Zeder-Goess, C.; Burges, A.; et al. M2 Macrophages Infiltrating Epithelial Ovarian Cancer Express MDR1: A Feature That May Account for the Poor Prognosis. Cells 2020, 9, 1224. [Google Scholar] [CrossRef]
- Ramesh, R.; Kozhaya, L.; McKevitt, K.; Djuretic, I.M.; Carlson, T.J.; Quintero, M.A.; McCauley, J.L.; Abreu, M.T.; Unutmaz, D.; Sundrud, M.S. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J. Exp. Med. 2014, 211, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Ho, W.E.; Xu, Y.J.; Xu, F.; Cheng, C.; Peh, H.Y.; Tannenbaum, S.R.; Wong, W.S.; Ong, C.N. Metabolomics reveals altered metabolic pathways in experimental asthma. Am. J. Respir Cell Mol. Biol. 2013, 48, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Pearce, E.J.; Pearce, E.L. Immunometabolism in 2017: Driving immunity: All roads lead to metabolism. Nat. Rev. Immunol. 2018, 18, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.N.; Gallart-Ayala, H.; Gómez, C.; Checa, A.; Fauland, A.; Naz, S.; Kamleh, M.A.; Djukanović, R.; Hinks, T.S.; Wheelock, C.E. Metabolomics analysis identifies different metabotypes of asthma severity. Eur. Respir. J. 2017, 49, 1601740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Zande, H.J.P.; Zawistowska-Deniziak, A.; Guigas, B. Immune Regulation of Metabolic Homeostasis by Helminths and Their Molecules. Trends Parasitol. 2019, 35, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Vaeth, M.; Kahlfuss, S.; Feske, S. CRAC Channels and Calcium Signaling in T Cell-Mediated Immunity. Trends Immunol. 2020, 41, 878–901. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, J.; Liu, M.; Xing, H.; Wang, Z.; Huang, L.; Mellor, A.L.; Wang, W.; Wu, S. Adoptive CD8(+) T cell therapy against cancer:Challenges and opportunities. Cancer Lett. 2019, 462, 23–32. [Google Scholar] [CrossRef]
- Reading, J.L.; Gálvez-Cancino, F.; Swanton, C.; Lladser, A.; Peggs, K.S.; Quezada, S.A. The function and dysfunction of memory CD8(+) T cells in tumor immunity. Immunol. Rev. 2018, 283, 194–212. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thurm, C.; Schraven, B.; Kahlfuss, S. ABC Transporters in T Cell-Mediated Physiological and Pathological Immune Responses. Int. J. Mol. Sci. 2021, 22, 9186. https://doi.org/10.3390/ijms22179186
Thurm C, Schraven B, Kahlfuss S. ABC Transporters in T Cell-Mediated Physiological and Pathological Immune Responses. International Journal of Molecular Sciences. 2021; 22(17):9186. https://doi.org/10.3390/ijms22179186
Chicago/Turabian StyleThurm, Christoph, Burkhart Schraven, and Sascha Kahlfuss. 2021. "ABC Transporters in T Cell-Mediated Physiological and Pathological Immune Responses" International Journal of Molecular Sciences 22, no. 17: 9186. https://doi.org/10.3390/ijms22179186





