Is “Delayed Onset Muscle Soreness” a False Friend? The Potential Implication of the Fascial Connective Tissue in Post-Exercise Discomfort
Abstract
:1. Introduction
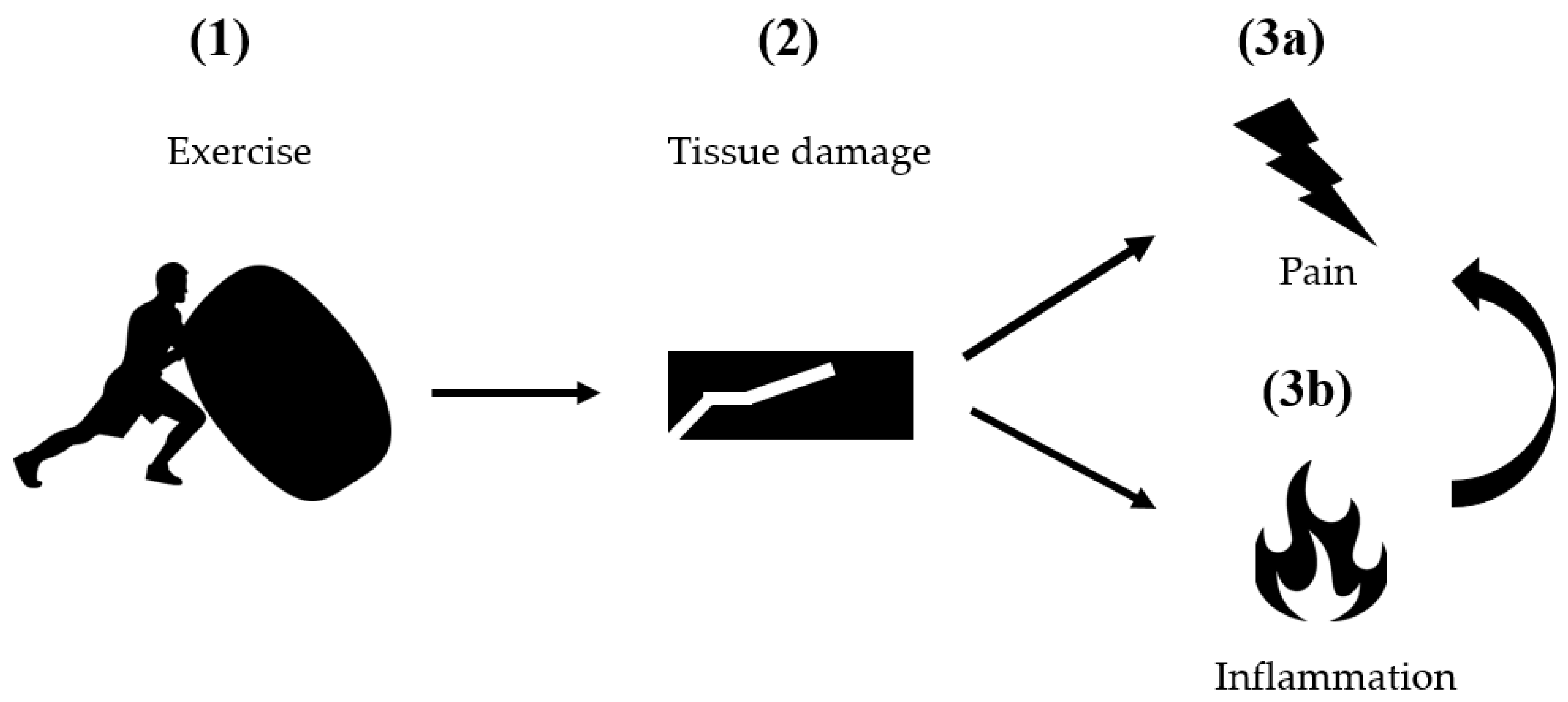
2. Classical Pathogenetic Models
3. Possible Involvement of the Connective Tissue
3.1. Structural Damage: Anatomy of Fascia
3.2. DOMS-Specific Evidence
3.3. Sensory Contribution: Physiology of Fascia
3.4. DOMS-Related Evidence
4. Practical Implications and Perspectives for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, K.; Hume, P.A.; Maxwell, L. Delayed onset muscle soreness. Treatment strategies and performance factors. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef]
- Hody, S.; Croisier, J.-L.; Bury, T.; Rogister, B.; Leprince, P. Eccentric Muscle Contractions: Risks and Benefits. Front. Physiol. 2019, 10, 536. [Google Scholar] [CrossRef]
- Eston, R.G.; Mickleborough, J.; Baltzopoulos, V. Eccentric activation and muscle damage: Biomechanical and physiological consid-erations during downhill running. Br. J. Sports Med. 1995, 29, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.L.; Brunetz, M.H.; Chenier, T.C.; McCammon, M.R.; Houmard, J.A.; Franklin, M.E.; Israle, R.G. The effects of static and ballistic stretching on delayed onset muscle sorness and creatine kinase. Res. Q. Exerc. Sport. 1993, 64, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Jamurtas, A.Z.; Fatouros, I.G.; Bruckenmeyer, P.J.; Kokkinidis, E. Effects of plyometric exercise on muscle sorness and plasma creatine kinase levels and ist comparison with eccentric and concentric exercise. J. Strength Cond. Res. 2000, 14, 68–74. [Google Scholar]
- Zügel, M.; Maganaris, C.N.; Wilke, J.; Jurkat-Rott, K.; Klingler, W.; Wearing, S.C.; Findley, T.; Barbe, M.; Steinacker, J.M.; Vleeming, A.; et al. Fascial tissue research in sports medicine: From molecules to tissue adaptation, injury and diagnostics: Consensus statement. Br. J. Sports Med. 2018, 52, 1497. [Google Scholar] [CrossRef] [Green Version]
- Pollak, K.A.; Swenson, J.D.; Vanhaitsma, T.A.; Hughen, R.W.; Jo, D.; White, A.T.; Light, K.C.; Schweinhardt, P.; Amann, M.; Light, A.R. Exogenously applied muscle metabolites synergis-tically evoke sensations of muscle fatigue and pain in human subjects. Exp. Physiol. 2014, 99, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.S.; Whitley, P.E.; Sluka, K.A. Effect of Intramuscular Protons, Lactate, and ATP on Muscle Hyperalgesia in Rats. PLoS ONE 2015, 10, e0138576. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.A.; König, C.; Zimmermann, K.; Barry, C.M.; Wiklendt, L.; Brookes, S.J.H. Effects of Lactate on One Class of Group III (CT3) Muscle Afferents. Front. Cell. Neurosci. 2020, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Darques, J.L.; Decherchi, P.; Jammes, Y. Mechanisms of fatigue-induced activation of group IV muscle afferents: The roles played by lactic acid and inflammatory mediators. Neurosci. Lett. 1998, 257, 109–112. [Google Scholar] [CrossRef]
- Mense, S. Functional anatomy of muscle: Muscle nociceptors and afferent fibers. In Muscle Pain: Understanding the Mechanisms; Mense, S., Gerwin, R.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–48. [Google Scholar]
- Jankowski, M.P.; Rau, K.K.; Ekmann, K.M.; Anderson, C.E.; Koerber, H.R. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J. Neurophysiol. 2013, 109, 2374–2381. [Google Scholar] [CrossRef]
- Amann, M.; Wan, H.-Y.; Thurston, T.S.; Georgescu, V.P.; Weavil, J.C. On the Influence of Group III/IV Muscle Afferent Feedback on Endurance Exercise Performance. Exerc. Sport Sci. Rev. 2020, 48, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.J.; Castracane, V.D.; Hollander, D.B.; Tryniecki, J.L.; Bamman, M.M.; O’Neal, S.; Hebert, E.P.; Kraemer, R.R. Hormonal Responses from Concentric and Eccentric Muscle Contractions. Med. Sci. Sports Exerc. 2003, 35, 937–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwane, J.A.; Watrous, B.G.; Johnson, S.R.; Armstrong, R.B. Is Lactic Acid Related to Delayed-Onset Muscle Soreness? Physician Sportsmed. 1983, 11, 124–131. [Google Scholar] [CrossRef]
- Hough, T. Ergographic studies in muscular soreness. Am. J. Physiol. 1902, 7, 76–92. [Google Scholar] [CrossRef] [Green Version]
- Fridén, J.; Kjörell, U.; Thornell, L.-E. Delayed Muscle Soreness and Cytoskeletal Alterations: An Immunocytological Study in Man. Int. J. Sports Med. 1984, 5, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Fridén, J.; Sjostrom, M.; Ba, E. A morphological study of delayed muscle soreness. Experientia 1981, 37, 506–507. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L. Biomechanical response of skeletal muscle to eccentric contractions. J. Sport Health Sci. 2018, 7, 294–309. [Google Scholar] [CrossRef]
- Lauritzen, F.; Paulsen, G.; Raastad, T.; Bergersen, L.H.; Owe, S.G. Gross ultrastructural changes and necrotic fiber segments in elbow flexor muscles after maximal voluntary eccentric action in humans. J. Appl. Physiol. 2009, 107, 1923–1934. [Google Scholar] [CrossRef]
- Prince, F.P.; Hikida, R.S.; Hagerman, F.C.; Staron, R.S.; Allen, W.H. A morphometric analysis of human muscle fibers with relation to fiber types and adaptations to exercise. J. Neurol. Sci. 1981, 49, 165–179. [Google Scholar] [CrossRef]
- Horowits, R. Passive force generation and titin isoforms in mammalian skeletal muscle. Biophys. J. 1992, 61, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Prado, L.G.; Makarenko, I.; Andresen, C.; Krüger, M.; Opitz, C.; Linke, W.A. Isoform Diversity of Giant Proteins in Relation to Passive and Active Contractile Properties of Rabbit Skeletal Muscles. J. Gen. Physiol. 2005, 126, 461–480. [Google Scholar] [CrossRef] [Green Version]
- Saka, T.; Akova, B.; Yazici, Z.; Sekir, U.; Gür, H.; Ozarda, Y. Difference in the magnitude of muscle damage between elbow flexors and knee extensors eccentric exercises. J. Sports Sci. Med. 2009, 8, 107–115. [Google Scholar]
- Yu, J.-G.; Carlsson, L.; Thornell, L.-E. Evidence for myofibril remodeling as opposed to myofibril damage in human muscles with DOMS: An ultrastructural and immunoelectron microscopic study. Histochem. Cell Biol. 2004, 121, 219–227. [Google Scholar] [CrossRef]
- Nosaka, K.; Newton, M.; Sacco, P. Delayed-onset muscle soreness does not reflect the magnitude of eccentric exercise-induced muscle damage. Scand. J. Med. Sci. Sports 2002, 12, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nurenberg, P.; Giddings, C.J.; Stray-Gundersen, J.; Fleckenstein, J.L.; Gonyea, W.J.; Peshock, R.M. MR imaging-guided muscle biopsy for correlation of increased signal intensity with ultrastructural change and delayed-onset muscle soreness after exercise. Radiology 1992, 184, 865–869. [Google Scholar] [CrossRef]
- Lightfoot, J.; Char, D.; McDermott, J.; Goya, C. Immediate Postexercise Massage Does Not Attenuate Delayed Onset Muscle Soreness. J. Strength Cond. Res. 1997, 11, 119–124. [Google Scholar]
- YYang, W.; Hu, P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Transl. 2018, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; O’Farrell, L.; Summan, M.; Hulderman, T.; Mishra, D.; Luster, M.I.; Kuziel, W.A.; Simeonova, P.P. Role of CC chemokines in skeletal muscle functional restoration after injury. Am. J. Physiol. Physiol. 2004, 286, C1031–C1036. [Google Scholar] [CrossRef] [Green Version]
- Mizumura, K.; Taguchi, T. Delayed onset muscle soreness: Involvement of neurotropic factors. J. Physiol. Sci. 2016, 66, 43–52. [Google Scholar] [CrossRef]
- Armstring, R.B. Mechanisms of exercise-induced delayed onset muscular soreness a brief review Mechanisms of exercise-induced delayed onset muscular soreness a brief review. Med. Sci. Sports Exerc. 1984, 16, 529–538. [Google Scholar]
- Yu, J.; Malm, C.; Thornell, L.-E. Eccentric contractions leading to DOMS do not cause loss of desmin nor fibre necrosis in human muscle. Histochem. Cell Biol. 2002, 118, 29–34. [Google Scholar] [CrossRef]
- Malm, C. Exercise-induced muscle damage and inflammation: Fact or fiction? Acta Physiol. Scand. 2008, 171, 233–239. [Google Scholar] [CrossRef]
- Close, G.L.; Ashton, T.; McArdle, A.; MacLaren, D.P.M. The emerging role of free radicals in delayed onset muscle soreness and con-traction-induced muscle injury. Comp. Biochem. Physiol. 2005, 142, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takanami, Y.; Kawai, Y. Oxidative stress and delayed-onset muscle soreness after exercise. Free Rad. Biol. Med. 2004, 37, 480–487. [Google Scholar] [CrossRef]
- Hellsten, Y.; Frandsen, U.; Orthenblad, N.; Sjødin, B.; Richter, E.A. Xanthine oxidase in human skeletal muscle following eccentric exercise: A role in inflammation. J. Physiol. 1997, 498, 239–248. [Google Scholar] [CrossRef]
- Kon, M.; Tanabe, K.; Lee, H.; Kimura, F.; Akimoto, T.; Kono, I. Eccentric muscle contractions induce greater oxidative stress than concentric contractions in skeletal muscle. Appl. Physiol. Nutr. Metab. 2007, 32, 273–281. [Google Scholar] [CrossRef]
- Close, G.L.; Ashton, T.; Cable, T.; Doran, D.; MacLaren, D.P.M. Eccentric exercise, isokinetic muscle torque and delayed onset muscle soreness: The role of reactive oxygen species. Eur. J. Appl. Physiol. 2004, 91, 615–621. [Google Scholar] [CrossRef]
- De Oliveira, D.C.X.; Roncon Rosa, F.; Simoes-Ambrosio, L.; Jordao, A.A.; Deminice, R. Antioxidant vitamin supplementation prevents oxidative stress but does not enhance performance in young football athletes. Nutrition 2019, 63, 29–35. [Google Scholar] [CrossRef]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M. Topology of Superoxide Production from Different Sites in the Mitochondrial Electron Transport Chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [Green Version]
- Berthier, C.; Blaineau, S. Supramolecular organization of the subsarcolemmal cytoskeleton of adult skeletal muscle fibers. A review. Biol. Cell 1997, 89, 413–434. [Google Scholar] [CrossRef]
- Patel, T.J.; Lieber, R.L. Force transmission in skeletal muscle: From actomyosin to external tendons. Exerc. Sport Sci. Rev. 1997, 25, 321–363. [Google Scholar] [CrossRef] [PubMed]
- Passerieux, E.; Rossignol, R.; Letellier, T.; Delage, J.P. Physical continuity of the perimysium from myofibers to tendons: Involvement in lateral force transmission in skeletal muscle. J. Struct. Biol. 2007, 159, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Stecco, A.; Machhi, V.; Masiero, S.; Porzionato, A.; Tiengo, C.; Stecco, C.; Delmas, V.; De Caro, R. Pectoral and femoral fasciae: Common aspects and regional specializations. Surg. Radiol. Anat. 2008, 31, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Vleeming, A.; Wearing, S. Overuse Injury: The Result of Pathologically Altered Myofascial Force Transmission? Exerc. Sport Sci. Rev. 2019, 47, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Hespanhol, L.; Behrens, M. Is It All About the Fascia? A Systematic Review and Meta-analysis of the Prevalence of Extramuscular Connective Tissue Lesions in Muscle Strain Injury. Orthop. J. Sports Med. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Child, R.B.; Day, S.H.; Donelly, A.E. Indices of skeletal muscle damage and connective tissue breakdown following eccentric muscle contractions. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Mavropalias, G.; Calapre, L.; Morici, M.; Koeda, T.; Poon, W.C.K.; Barley, O.R.; Gray, E.; Blazevich, A.J.; Nosaka, K. Changes in plasma hydroxyproline and plasma cell-free DNA concentrations after higher- versus lower-intensity eccentric cycling. Eur. J. Appl. Physiol. 2021, 121, 1087–1097. [Google Scholar] [CrossRef]
- Raastad, T.; Owe, S.G.; Paulsen, G.; Enns, D.; Overgaard, K.; Crameri, R.; Kiil, S.; Belcastro, A.; Bergersen, L.; Hallén, J. Changes in Calpain Activity, Muscle Structure, and Function after Eccentric Exercise. Med. Sci. Sports Exerc. 2010, 42, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Crameri, R.M.; Aagard, K.; Qvortrup, H.; Langberg, J.; Olesen, J.; Kjaer, M. Myofibre damage in human skeletal muscle: Effects of electrical stimulation versus voluntary contraction. J. Physiol. 2007, 583, 365–380. [Google Scholar] [CrossRef]
- Tenberg, S.; Nosaka, K.; Wilke, J. Fascia thickness increases following eccentric exercise leading to delayed onset muscle soreness. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Yahia, L.; Rhalmi, S.; Newman, N.; Isler, M. Sensory innervation of human thoracolumbar fascia: An immunohistochemical study. Acta Orthop. 1992, 63, 195–197. [Google Scholar] [CrossRef]
- Stecco, C.; Porzionato, A.; Macchi, V.; Tiengo, C.; Parenti, A.; Aldegheri, R.; Delmas, V.; De Caro, R. Histological characteristics of the deep fascia of the upper limb. Ital. J. Anat. Embryol. 2006, 111, 105–110. [Google Scholar] [PubMed]
- Tesarz, J.; Hoheisel, U.; Wiedenhöfer, B.; Mense, S. Sensory innervation of the thoracolumbar fascia in rats and humans. Neuro-science 2011, 194, 302–308. [Google Scholar] [CrossRef]
- Taguchi, T.; Yasui, M.; Kubo, A.; Abe, M.; Kiyama, H.; Yamanaka, A.; Mizumura, K. Nociception originating from the crural fascia in rats. Pain 2013, 154, 1103–1114. [Google Scholar] [CrossRef]
- Barry, C.; Kestell, G.; Gillan, M.; Haberberger, R.V.; Gibbins, I. Sensory nerve fibers containing calcitonin gene-related peptide in gastrocnemius, latissimus dorsi and erector spinae muscles and thoracolumbar fascia in mice. Neuroscience 2015, 291, 106–117. [Google Scholar] [CrossRef]
- Mense, S. Innervation of the thoracolumbar fascia. Eur. J. Transl. Myol. 2019, 29, 8297. [Google Scholar] [CrossRef]
- Schilder, A.; Hoheisel, U.; Magerl, W.; Benrath, J.; Klein, T.; Treede, R.-D. Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain. Pain 2014, 155, 222–231. [Google Scholar] [CrossRef]
- Deising, S.; Weinkauf, B.; Blunk, J.; Obreja, O.; Schmelz, M.; Rukwied, R. NGF-evoked sensitization of muscle fascia nociceptors in humans. Pain 2012, 153, 1673–1679. [Google Scholar] [CrossRef]
- Schilder, A.; Magerl, W.; Hoheisel, U.; Klein, T.; Treede, R.-D. Electrical high-frequency stimulation of the human thoracolumbar fascia evokes long-term potentiation-like pain amplification. Pain 2016, 157, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Hoheisel, U.; Mense, S. Inflammation of the thoracolumbar fascia excites and sensitizes rat dorsal horn neurons. Eur. J. Pain 2015, 19, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.; Arendt-Nielsen, L.; Taguchi, T.; Mizumura, K.; Graven-Nielsen, T. Increased pain from muscle fascia following eccentric exercise: Animal and human findings. Exp. Brain Res. 2009, 194, 299–308. [Google Scholar] [CrossRef]
- Lau, W.Y.; Blazevich, A.J.; Newton, M.J.; Wu, S.S.X.; Nosaka, K. Changes in electrical pain threshold of fascia and muscle after initial and secondary bouts of elbow flexor eccentric exercise. Eur. J. Appl. Physiol. 2015, 115, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Agten, C.; Buck, F.M.; Dyer, L.; Flück, M.; Pfirrmann, C.; Rosskopf, A.B. Delayed-Onset Muscle Soreness: Temporal Assessment with Quantitative MRI and Shear-Wave Ultrasound Elastography. Am. J. Roentgenol. 2017, 208, 402–412. [Google Scholar] [CrossRef]
- Dupuy, O.; Douzi, W.; Theurot, D.; Bosquet, L.; Dugue, B. An Evidence-Based Approach for Choosing Post-exercise Recovery Techniques to Reduce Markers of Muscle Damage, Soreness, Fatigue, and Inflammation: A Systematic Review with Meta-Analysis. Front. Physiol. 2018, 9, 403. [Google Scholar] [CrossRef]
- Shaw, G.; Barthel, A.L.; Ross, M.L.; Wang, B.; Baar, K. Vitamin C–enriched gelatin supplementation before intermittent activity aug-ments collagen synthesis. Am. J. Clin. Nutr. 2017, 105, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, T.; Ventress, M.; Allerton, D.; Stansfield, S.; Tang, J.; Fraser, W.D.; Vanhoecke, B.; Prawitt, J.; Stevenson, E. The effects of collagen peptides on muscle damage, inflammation and bone turnover following exercise: A randomized, controlled trial. Amino Acids 2019, 51, 691–704. [Google Scholar] [CrossRef] [Green Version]
- Krause, F.; Wilke, J.; Niederer, D.; Vogt, L.; Banzer, W. Acute effects of foam rolling on passive stiffness, stretch sensation and fascial sliding: A randomized controlled trial. Hum. Mov. Sci. 2019, 67, 102514. [Google Scholar] [CrossRef]
- Pavan, P.G.; Stecco, A.; Stern, R.; Stecco, C. Painful Connections: Densification Versus Fibrosis of Fascia. Curr. Pain Headache Rep. 2014, 18, 441. [Google Scholar] [CrossRef] [PubMed]
- Wiewelhove, T.; Döweling, A.; Schneider, C.; Hottenrott, L.; Meyer, T.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. A Meta-Analysis of the Effects of Foam Rolling on Performance and Recovery. Front. Physiol. 2019, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Wilke, J.; Vogt, L.; Banzer, W. Immediate effects of self-myofascial release on latent trigger point sensitivity: A randomized, placebo-controlled trial. Biol. Sport 2018, 35, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Pearcey, G.; Bradbury-Squires, D.J.; Kawamoto, J.-E.; Drinkwater, E.; Behm, D.G.; Button, D.C. Foam Rolling for Delayed-Onset Muscle Soreness and Recovery of Dynamic Performance Measures. J. Athl. Train. 2015, 50, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naderi, A.; Rezvani, M.H.; Degens, H. Foam Rolling and Muscle and Joint Proprioception After Exercise-Induced Muscle Damage. J. Athl. Train. 2020, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilke, J.; Behringer, M. Is “Delayed Onset Muscle Soreness” a False Friend? The Potential Implication of the Fascial Connective Tissue in Post-Exercise Discomfort. Int. J. Mol. Sci. 2021, 22, 9482. https://doi.org/10.3390/ijms22179482
Wilke J, Behringer M. Is “Delayed Onset Muscle Soreness” a False Friend? The Potential Implication of the Fascial Connective Tissue in Post-Exercise Discomfort. International Journal of Molecular Sciences. 2021; 22(17):9482. https://doi.org/10.3390/ijms22179482
Chicago/Turabian StyleWilke, Jan, and Michael Behringer. 2021. "Is “Delayed Onset Muscle Soreness” a False Friend? The Potential Implication of the Fascial Connective Tissue in Post-Exercise Discomfort" International Journal of Molecular Sciences 22, no. 17: 9482. https://doi.org/10.3390/ijms22179482
APA StyleWilke, J., & Behringer, M. (2021). Is “Delayed Onset Muscle Soreness” a False Friend? The Potential Implication of the Fascial Connective Tissue in Post-Exercise Discomfort. International Journal of Molecular Sciences, 22(17), 9482. https://doi.org/10.3390/ijms22179482





