Fc Receptor Variants and Disease: A Crucial Factor to Consider in the Antibody Therapeutics in Clinic
Abstract
:1. Introduction
2. Immune Disorders and Fc Receptor Genetic Variants
2.1. FcγRIIb Genetic Variants: Insufficient Inhibitory Function of FcγRIIb Cause Autoimmune Diseases
2.2. FcεRI Genetic Variants: Hyperactive FcεRI Results in Allergic Diseases
2.3. FcαRI Genetic Variants: Upregulated FcαRI Leads to Autoimmune Diseases
3. Therapeutic Efficacy of Antibodies and Fc Receptor Genetic Variants
3.1. FcγRIIa Genetic Variants: High-Affinity FcγRIIa for IgG2 Induces ADCP and Confers Low Susceptibility to Infection and Vaccine Effect
3.2. FcγRIIIa Genetic Variants: High-Affinity FcγRIIIa for IgG Is Associated with Low Susceptibility to Cancer and Results in a Higher Response of NK Cell-Mediated ADCC of Therapeutic Antibodies
3.3. FcγRIIIb Genetic Variants: High-Affinity FcγRIIIb for IgG1 and IgG3 Induces Neutrophil-Mediated Phagocytosis and Contributes to Low Susceptibility to Infections
3.4. FcRn Genetic Variants: High-Expression of FcRn Increases IgG Half-Life
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cellular cytotoxicity |
| ADCP | Antibody-dependent cellular phagocytosis |
| AIA | Aspirin-intolerant asthma |
| AICU | Aspirin-intolerant chronic urticarial |
| APC | Antigen presenting cell |
| BCR | B-cell receptor |
| CDC | Complement-mediated cytotoxicity |
| CGD | Chronic granulomatous disease |
| CLL | Chronic lymphocytic leukemia |
| CTLA-4 | Cytotoxic T lymphocyte associated protein-4 |
| CVID | Common variable immunodeficiency disorder |
| DC | Dendritic cell |
| EC | Extracellular |
| EMA | European Medicines Agency |
| Fab | Fragment antigen-binding |
| Fc | Fragment crystallizable |
| FcRs | Fc receptors |
| FcRn | Neonatal Fc receptor |
| FDA | Food and Drug Administration |
| gMG | Generalized myasthenia gravis |
| GN | Glomerulonephritis |
| IC | Immune complex |
| Ig | Immunoglobulin |
| IgAN | IgA nephropathy |
| IGH | Immunoglobulin heavy chain |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| ITIM | Immunoreceptor tyrosine-based inhibitory motif |
| IVIg | Intravenous immunoglobulin |
| mAbs | Monoclonal antibodies |
| MHC | Major histocompatibility complex |
| NK | Natural killer |
| PBMC | Peripheral blood mononuclear cell |
| pIgR | Polymeric immunoglobulin receptor |
| PMNs | Polymorphonuclear neutrophils |
| RA | Rheumatoid arthritis |
| SLE | Systemic lupus erythematosus |
| SNP | Single nucleotide polymorphism |
| TCR | T-cell receptor |
| TSS | Transcription start site |
| VNTR | Variable number of tandem repeats |
References
- Urquhart, L. Top companies and drugs by sales in 2020. Nat. Rev. Drug Discov. 2021, 19, 228–229. [Google Scholar] [CrossRef]
- Urquhart, L. Top product forecasts for 2020. Nat. Rev. Drug Discov. 2020, 19, 86. [Google Scholar] [CrossRef]
- Antibody Society. Available online: https://www.antibodysociety.org/resources/approved-antibodies/ (accessed on 25 May 2021).
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef]
- Adler, M.J.; Dimitrov, D.S. Therapeutic Antibodies Against Cancer. Hematol. Clin. N. Am. 2012, 26, 447–481. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.H.; Jung, S.T. Reprogramming the Constant Region of Immunoglobulin G Subclasses for Enhanced Therapeutic Potency against Cancer. Biomolecules 2020, 10, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Ng, S.M.; Hassouna, E.; Warrington, A.; Oh, S.-H.; Rodriguez, M. Human-derived natural antibodies: Biomarkers and potential therapeutics. Future Neurol. 2015, 10, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Zhang, X.; Yang, Y.; Hotez, P.J.; Du, L. Neutralizing antibodies for the treatment of COVID-19. Nat. Biomed. Eng. 2020, 4, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.R.; Wilkie, B.N.; Winter, A.J. Natural and Immune Antibodies for Vibrio fetus in Serum and Secretions of Cattle. Infect. Immun. 1972, 5, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Roche, A.M.; Richard, A.L.; Rahkola, J.; Janoff, E.N.; Weiser, J.N. Antibody blocks acquisition of bacterial colonization through agglutination. Mucosal Immunol. 2014, 8, 176–185. [Google Scholar] [CrossRef]
- Mavilio, D.; Hosmalin, A.; Scott-Algara, D. Natural killer cells and human immunodeficiency virus. In Natural Killer Cells; Elsevier: Amsterdam, The Netherlands, 2010; pp. 481–497. [Google Scholar]
- Román, V.R.G.; Murray, J.C.; Weiner, L.M. Antibody-dependent cellular cytotoxicity (ADCC). In Antibody Fc; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–27. [Google Scholar]
- Jochems, C.; Hodge, J.W.; Fantini, M.; Fujii, R.; Ii, Y.M.M.; Greiner, J.W.; Padget, M.R.; Tritsch, S.R.; Tsang, K.Y.; Campbell, K.S.; et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 2016, 7, 86359–86373. [Google Scholar] [CrossRef] [Green Version]
- Tay, M.Z.; Wiehe, K.; Pollara, J. Antibody-Dependent Cellular Phagocytosis in Antiviral Immune Responses. Front. Immunol. 2019, 10, 332. [Google Scholar] [CrossRef]
- Kamen, L.; Myneni, S.; Langsdorf, C.; Kho, E.; Ordonia, B.; Thakurta, T.; Zheng, K.; Song, A.; Chung, S. A novel method for determining antibody-dependent cellular phagocytosis. J. Immunol. Methods 2019, 468, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Thornton, B.P.; Vĕtvicka, V.; Ross, G.D. Natural antibody and complement-mediated antigen processing and presentation by B lymphocytes. J. Immunol. 1994, 152, 1727–1737. [Google Scholar]
- Terajima, M.; Cruz, J.; Co, M.D.T.; Lee, J.-H.; Kaur, K.; Wilson, P.C.; Ennis, F.A. Complement-Dependent Lysis of Influenza A Virus-Infected Cells by Broadly Cross-Reactive Human Monoclonal Antibodies. J. Virol. 2011, 85, 13463–13467. [Google Scholar] [CrossRef] [Green Version]
- Van Erp, E.A.; Luytjes, W.; Ferwerda, G.; Van Kasteren, P.B. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front. Immunol. 2019, 10, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruhns, P.; Jönsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.M.; Lawley, T.J.; Hamburger, M.I.; Brown, E.J. Immunoglobulin G Fc Receptor-Mediated Clearance in Autoimmune Diseases. Ann. Intern. Med. 1983, 98, 206–218. [Google Scholar] [CrossRef]
- Boross, P.; Arandhara, V.L.; Martin-Ramirez, J.; Santiago-Raber, M.-L.; Carlucci, F.; Flierman, R.; Van Der Kaa, J.; Breukel, C.; Claassens, J.W.C.; Camps, M.; et al. The Inhibiting Fc Receptor for IgG, FcγRIIB, Is a Modifier of Autoimmune Susceptibility. J. Immunol. 2011, 187, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Ghumra, A.; Shi, J.; McIntosh, R.S.; Rasmussen, I.B.; Braathen, R.; Johansen, F.-E.; Sandlie, I.; Mongini, P.K.; Areschoug, T.; Lindahl, G.; et al. Structural requirements for the interaction of human IgM and IgA with the human Fcα/μ receptor. Eur. J. Immunol. 2009, 39, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Borrok, M.J.; Wu, Y.; Beyaz, N.; Yu, X.-Q.; Oganesyan, V.; Dall’Acqua, W.F.; Tsui, P. pH-dependent binding engineering reveals an FcRn affinity threshold that governs IgG recycling. J. Biol. Chem. 2015, 290, 4282–4290. [Google Scholar] [CrossRef] [Green Version]
- Kaetzel, C.S.; Robinson, J.K.; Chintalacharuvu, K.R.; Vaerman, J.P.; Lamm, M.E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: A local defense function for IgA. Proc. Natl. Acad. Sci. USA 1991, 88, 8796–8800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [Green Version]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daëron, M. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef]
- Bengtén, E.; Wilson, M.; Miller, N.; Clem, L.W.; Pilström, L.; Warr, G.W. Immunoglobulin Isotypes: Structure, Function, and Genetics. Orig. Evol. Vertebr. Immune Syst. 2000, 248, 189–219. [Google Scholar] [CrossRef]
- Stavnezer, J. Immunoglobulin class switching. Curr. Opin. Immunol. 1996, 8, 199–205. [Google Scholar] [CrossRef]
- Geha, R.S.; Rosen, F.S. The Genetic Basis of Immunoglobulin-Class Switching. N. Engl. J. Med. 1994, 330, 1008–1009. [Google Scholar] [CrossRef] [PubMed]
- Binstadt, B.A.; Geha, R.S.; Bonilla, F.A. IgG Fc receptor polymorphisms in human disease: Implications for intravenous immunoglobulin therapy. J. Allergy Clin. Immunol. 2003, 111, 697–703. [Google Scholar] [CrossRef]
- Marrack, P.; Kappler, J.W.; Kotzin, B.L. Autoimmune disease: Why and where it occurs. Nat. Med. 2001, 7, 899–905. [Google Scholar] [CrossRef]
- Ermann, J.; Fathman, C.G. Autoimmune diseases: Genes, bugs and failed regulation. Nat. Immunol. 2001, 2, 759–761. [Google Scholar] [CrossRef]
- Wallace, P.K.; Howell, A.L.; Fanger, M.W. Role of Fcγ receptors in cancer and infectious disease. J. Leukoc. Biol. 1994, 55, 816–826. [Google Scholar] [CrossRef]
- Gavin, P.G.; Song, N.; Kim, S.R.; Lipchik, C.; Johnson, N.L.; Bandos, H.; Finnigan, M.; Rastogi, P.; Fehrenbacher, L.; Mamounas, E.P. Association of polymorphisms in FCGR2A and FCGR3A with degree of trastuzumab benefit in the adjuvant treatment of ERBB2/HER2–positive breast cancer: Analysis of the NSABP B-31 trial. JAMA Oncol. 2017, 3, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Kjersem, J.B.; Skovlund, E.; Ikdahl, T.; Guren, T.; Kersten, C.; Dalsgaard, A.M.; Yilmaz, M.K.; Fokstuen, T.; Tveit, K.M.; Kure, E.H. FCGR2A and FCGR3A polymorphisms and clinical outcome in metastatic colorectal cancer patients treated with first-line 5-fluorouracil/folinic acid and oxaliplatin+/− cetuximab. BMC Cancer 2014, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.G.C.; Clatworthy, M.R. FcγRIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat. Rev. Immunol. 2010, 10, 328–343. [Google Scholar] [CrossRef] [Green Version]
- Verbrugge, A.; Meyaard, L. Signaling by ITIM-Bearing Receptors. Curr. Immunol. Rev. 2005, 1, 201–212. [Google Scholar] [CrossRef]
- Ben Mkaddem, S.; Benhamou, M.; Monteiro, R.C. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Front. Immunol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbeek, J.S.; Hirose, S.; Nishimura, H. The Complex Association of FcγRIIb with Autoimmune Susceptibility. Front. Immunol. 2019, 10, 2061. [Google Scholar] [CrossRef] [Green Version]
- Willcocks, L.C.; Carr, E.J.; Niederer, H.A.; Rayner, T.F.; Williams, T.N.; Yang, W.; Scott, J.A.G.; Urban, B.C.; Peshu, N.; Vyse, T.J. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 2010, 107, 7881–7885. [Google Scholar] [CrossRef] [Green Version]
- Fukuyama, H.; Nimmerjahn, F.; Ravetch, J.V. The inhibitory Fcγ receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat. Immunol. 2004, 6, 99–106. [Google Scholar] [CrossRef]
- McGaha, T.L.; Karlsson, M.C.; Ravetch, J.V. FcγRIIB deficiency leads to autoimmunity and a defective response to apoptosis in Mrl-MpJ mice. J. Immunol. 2008, 180, 5670–5679. [Google Scholar] [CrossRef] [Green Version]
- Pendergraft, W.F.; Badhwar, A.K.; Preston, G.A. Autoantigen complementarity and its contributions to hallmarks of autoimmune disease. J. Theor. Biol. 2015, 375, 88–94. [Google Scholar] [CrossRef]
- Bournazos, S.; Woof, J.M.; Hart, S.P.; Dransfield, I. Functional and clinical consequences of Fc receptor polymorphic and copy number variants. Clin. Exp. Immunol. 2009, 157, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wu, J.; Edberg, J.C.; Li, X.; Ferguson, P.; Cooper, G.S.; Langefeld, C.D.; Kimberly, R.P. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcγRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J. Immunol. 2004, 172, 7186–7191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blank, M.C.; Stefanescu, R.N.; Masuda, E.; Marti, F.; King, P.D.; Redecha, P.B.; Wurzburger, R.J.; Peterson, M.G.; Tanaka, S.; Pricop, L. Decreased transcription of the human FCGR2B gene mediated by the -343 G/C promoter polymorphism and association with systemic lupus erythematosus. Qual. Life Res. 2005, 117, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Mackay, M.; Stanevsky, A.; Wang, T.; Aranow, C.; Li, M.; Koenig, S.; Ravetch, J.V.; Diamond, B. Selective dysregulation of the FcγIIB receptor on memory B cells in SLE. J. Exp. Med. 2006, 203, 2157–2164. [Google Scholar] [CrossRef] [Green Version]
- Carreño, L.J.; Pacheco, R.; Gutiérrez, M.A.; Jacobelli, S.; Kalergis, A.M. Disease activity in systemic lupus erythematosus is associated with an altered expression of low-affinity Fcγ receptors and costimulatory molecules on dendritic cells. Immunology 2009, 128, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.C.; Malbec, O.; Arock, M.; Cambier, J.C.; Fridman, W.H.; Daëron, M. Selective in vivo recruitment of the phosphatidylinositol phosphatase SHIP by phosphorylated FcγRIIB during negative regulation of IgE-dependent mouse mast cell activation. Immunol. Lett. 1996, 54, 83–91. [Google Scholar] [CrossRef]
- Malbec, O.; Fridman, W.H.; Daëron, M. Negative Regulation of Hematopoietic Cell Activation and Proliferation by FcγRIIB. Immunoreceptor Tyrosine-Based Inhib. Motifs 1999, 244, 13–27. [Google Scholar] [CrossRef]
- Kepley, C.L.; Cambier, J.C.; Morel, P.D.; Lujan, D.; Ortega, E.; Wilson, B.S.; Oliver, J.M. Negative regulation of FcϵRI signaling by FcγRII costimulation in human blood basophils. J. Allergy Clin. Immunol. 2000, 106, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Daëron, M.; Malbec, O.; Latour, S.; Arock, M.; Fridman, W.H. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J. Clin. Investig. 1995, 95, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Tam, S.W.; Demissie, S.; Thomas, D.; Daëron, M. A bispecific antibody against human IgE and human FcγRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy 2004, 59, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.C.; Kepley, C.L.; Saxon, A.; Zhang, K. Modifications to an Fcγ-Fcɛ fusion protein alter its effectiveness in the inhibition of FcɛRI-mediated functions. J. Allergy Clin. Immunol. 2007, 120, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Cemerski, S.; Chu, S.Y.; Moore, G.L.; Muchhal, U.S.; Desjarlais, J.R.; Szymkowski, D.E. Suppression of mast cell degranulation through a dual-targeting tandem IgE–IgG Fc domain biologic engineered to bind with high affinity to FcγRIIb. Immunol. Lett. 2012, 143, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Horton, H.M.; Pong, E.; Leung, I.W.; Chen, H.; Nguyen, D.-H.; Bautista, C.; Muchhal, U.S.; Bernett, M.J.; Moore, G.L.; et al. Reduction of total IgE by targeted coengagement of IgE B-cell receptor and FcγRIIb with Fc-engineered antibody. J. Allergy Clin. Immunol. 2012, 129, 1102–1115. [Google Scholar] [CrossRef]
- Horton, H.M.; Chu, S.Y.; Ortiz, E.C.; Pong, E.; Cemerski, S.; Leung, I.W.L.; Jacob, N.; Zalevsky, J.; DesJarlais, J.R.; Stohl, W.; et al. Antibody-Mediated Coengagement of FcγRIIb and B Cell Receptor Complex Suppresses Humoral Immunity in Systemic Lupus Erythematosus. J. Immunol. 2011, 186, 4223–4233. [Google Scholar] [CrossRef]
- Chu, S.Y.; Yeter, K.; Kotha, R.; Pong, E.; Miranda, Y.; Phung, S.; Chen, H.; Lee, S.H.; Leung, I.; Bonzon, C. Suppression of Rheumatoid Arthritis B Cells by XmAb5871, an Anti-CD19 Antibody That Coengages B Cell Antigen Receptor Complex and Fcγ Receptor IIb Inhibitory Receptor. Arthritis Rheumatol. 2014, 66, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Ra, C.; Jouvin, M.H.; Kinet, J.P. Complete structure of the mouse mast cell receptor for IgE (Fc ϵ RI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J. Biol. Chem. 1989, 264, 15323–15327. [Google Scholar] [CrossRef]
- Blank, U.; Ra, C.; Miller, L.; White, K.; Metzger, H.; Kinet, J.-P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature 1989, 337, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Osterhoff, B.; Rappersberger, K.; Wang, B.; Koszik, F.; Ochiai, K.; Kinet, J.-P.; Stingl, G. Immunomorphologic Characterization of Fc∈RI-Bearing Cells Within the Human Dermis. J. Investig. Dermatol. 1994, 102, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Maurer, D.; Fiebiger, E.; Reininger, B.; Wolff-Winiski, B.; Jouvin, M.H.; Kilgus, O.; Kinet, J.P.; Stingl, G. Expression of functional high affinity immunoglobulin E receptors (Fc epsilon RI) on monocytes of atopic individuals. J. Exp. Med. 1994, 179, 745–750. [Google Scholar] [CrossRef]
- Kanada, S.; Nakano, N.; Potaczek, D.P.; Maeda, K.; Shimokawa, N.; Niwa, Y.; Fukai, T.; Sanak, M.; Szczeklik, A.; Yagita, H. Two different transcription factors discriminate the −315C> T polymorphism of the FcεRIα gene: Binding of Sp1 to−315C and of a high mobility group-related molecule to −315T. J. Immunol. 2008, 180, 8204–8210. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, M.; Nishiyama, C.; Nishiyama, M.; Akizawa, Y.; Mitsuishi, K.; Ito, T.; Kawada, H.; Furukawa, S.; Ra, C.; Okumura, K. A novel −66T/C polymorphism in FcεRI α-chain promoter affecting the transcription activity: Possible relationship to allergic diseases. J. Immunol. 2003, 171, 1927–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.-S.; Kim, S.-H.; Ye, Y.-M.; Yoon, H.J.; Suh, C.-H.; Nahm, D.-H.; Park, H.-S. Significant association of FcɛRIα promoter polymorphisms with aspirin-intolerant chronic urticaria. J. Allergy Clin. Immunol. 2007, 119, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R. Mast cells as orchestrators of the allergic reaction: The IgE-IgE receptor mast cell network. Curr. Opin. Allergy Clin. Immunol. 2001, 1, 3–6. [Google Scholar]
- Rosenwasser, L.J. Mechanisms of IgE Inflammation. Curr. Allergy Asthma Rep. 2011, 11, 178–183. [Google Scholar] [CrossRef]
- Palikhe, N.S.; Kim, S.-H.; Cho, B.-Y.; Ye, Y.-M.; Hur, G.-Y.; Park, H.-S. Association of three sets of high-affinity IgE receptor (FcepsilonR1) polymorphisms with aspirin-intolerant asthma. Respir. Med. 2008, 102, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Bae, J.S.; Holloway, J.; Lee, J.T.; Suh, C.H.; Nahm, D.H.; Park, H.S. A polymorphism of MS4A2 (−109T> C) encoding the β-chain of the high-affinity immunoglobulin E receptor (FcεR1β) is associated with a susceptibility to aspirin-intolerant asthma. Clin. Exp. Allergy 2006, 36, 877–883. [Google Scholar] [CrossRef]
- Hizawa, N.; Yamaguchi, E.; Jinushi, E.; Kawakami, Y. A common FCER1B gene promoter polymorphism* influences total serum IgE levels in a Japanese population. Am. J. Respir. Crit. Care Med. 2000, 161, 906–909. [Google Scholar] [CrossRef]
- Hill, M.; Cookson, W. A new variant of the β subunit of the high-affinity receptor for immunoglobulin E (FcεRI-β E237G): Associations with measures of atopy and bronchial hyper-responsiveness. Hum. Mol. Genet. 1996, 5, 959–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiaozhu, Z.; Weidong, Z.; Diwen, Q.; Andrew, S.; Cheng, T.W. The E237G polymorphism of the high-affinity IgE receptor β chain and asthma. Ann. Allergy Asthma Immunol. 2004, 93, 499–503. [Google Scholar] [CrossRef]
- Donnadieu, E.; Jouvin, M.-H.; Rana, S.; Moffatt, M.F.; Mockford, E.H.; Cookson, W.O.; Kinet, J.-P. Competing functions encoded in the allergy-associated FcϵRIβ gene. Immunity 2003, 18, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Shirakawa, T.; Mao, X.-Q.; Sasaki, S.; Enomoto, T.; Kawai, M.; Morimoto, K.; Hopkin, J. Association between atopic asthma and a coding variant of FcεRIβ in a Japanese population. Hum. Mol. Genet. 1996, 5, 1129–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.R.; James, A.L.; Faux, J.A.; Ryan, G.; Hopkin, J.M.; Le Souef, P.; Musk, A.W.; Cookson, W. Fc(epsilon)RI-(beta) polymorphism and risk of atopy in a general population sample. BMJ 1995, 311, 776–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirakawa, T.; Li, A.; Dubowitz, M.; Dekker, J.W.; Shaw, A.E.; Faux, J.A.; Ra, C.; Cookson, W.O.; Hopkin, J.M. Association between atopy and variants of the β subunit of the high–affinity immunoglobulin E receptor. Nat. Genet. 1994, 7, 125–130. [Google Scholar] [CrossRef]
- Hijazi, Z.; Haider, M.Z.; Khan, M.; Ai-Dowaisan, A.A. High frequency of IgE receptor FcεRIß variant (Leu181/Leu183) in Kuwaiti Arabs and its association with asthma. Clin. Genet. 2008, 53, 149–152. [Google Scholar] [CrossRef]
- Kinet, J.-P. THE HIGH-AFFINITY IgE RECEPTOR (FcεRI): From Physiology to Pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef]
- Sakurai, D.; Yamasaki, S.; Arase, K.; Park, S.Y.; Arase, H.; Konno, A.; Saito, T. FcεRIγ-ITAM is differentially required for mast cell function in vivo. J. Immunol. 2004, 172, 2374–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holgate, S.; Smith, N.; Massanari, M.; Jimenez, P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy 2009, 64, 1728–1736. [Google Scholar] [CrossRef]
- Cision PR Newswire. Available online: https://www.prnewswire.com/news-releases/novartis-receives-fda-approval-of-xolair-omalizumab-self-injection-with-prefilled-syringe-across-all-indications-for-appropriate-patients-301266937.html (accessed on 25 May 2021).
- Monteiro, R.C.; Van De Winkel, J.G. IgA Fc receptors. Annu. Rev. Immunol. 2003, 21, 177–204. [Google Scholar] [CrossRef]
- Maliszewski, C.R.; March, C.J.; Schoenborn, M.A.; Gimpel, S.; Shen, L. Expression cloning of a human Fc receptor for IgA. J. Exp. Med. 1990, 172, 1665–1672. [Google Scholar] [CrossRef]
- Monteiro, R.C.; Kubagawa, H.; Cooper, M.D. Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in humans. J. Exp. Med. 1990, 171, 597–613. [Google Scholar] [CrossRef]
- Tsuge, T.; Shimokawa, T.; Horikoshi, S.; Tomino, Y.; Ra, C. Polymorphism in promoter region of Fcα receptor gene in patients with IgA nephropathy. Qual. Life Res. 2001, 108, 128–133. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, X.; Li, Z.; Wang, Z.; Li, S. Immunoglobulin A nephropathy: Current progress and future directions. Transl. Res. 2015, 166, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, X.; Nishi, S.; Narita, I.; Gejyo, F. Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int. 2004, 65, 1135–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Nishi, S.; Ueno, M.; Imai, N.; Sakatsume, M.; Narita, I.; Suzuki, Y.; Akazawa, K.; Shimada, H.; Arakawa, M.; et al. The efficacy of tonsillectomy on long-term renal survival in patients with IgA nephropathy. Kidney Int. 2003, 63, 1861–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimokawa, T.; Tsuge, T.; Okumura, K.; Ra, C. Identification and characterization of the promoter for the gene encoding the human myeloid IgA Fc receptor (FcαR, CD89). Immunogenetics 2000, 51, 945–954. [Google Scholar] [CrossRef]
- Monteiro, R.C.; Grossetête, B.; Nguyen, A.T.; Jungers, P.; Lehuen, A. Dysfunctions of Fcα Receptors by Blood Phagocytic Cells in IgA Nephropathy1. In IgA Nephropathy; Karger Publishers: Basel, The Switzerland, 1995; Volume 111, pp. 116–122. [Google Scholar]
- Kashem, A.; Endoh, M.; Yano, N.; Yamauchi, F.; Nomoto, Y.; Sakai, H.; Kurokawa, K. Glomerular FcαR expression and disease activity in IgA nephropathy. Am. J. Kidney Dis. 1997, 30, 389–396. [Google Scholar] [CrossRef]
- Launay, P.; Grossetête, B.; Arcos-Fajardo, M.; Gaudin, E.; Torres, S.P.; Beaudoin, L.; de Serre, N.P.-M.; Lehuen, A.; Monteiro, R.C. Fcα Receptor (Cd89) Mediates the Development of Immunoglobulin a (Iga) Nephropathy (Berger’s Disease) Evidence for Pathogenic Soluble Receptor–Iga Complexes in Patients and Cd89 Transgenic Mice. J. Exp. Med. 2000, 191, 1999–2010. [Google Scholar] [CrossRef] [Green Version]
- Berger, J. IgA glomerular deposits in renal disease. Transplant. Proc. 1969, 1, 939–944. [Google Scholar] [PubMed]
- Galla, J.H. IgA nephropathy. Kidney Int. 1995, 47, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Jasek, M.; Manczak, M.; Sawaryn, A.; Obojski, A.; Wiśniewski, A.; Łuszczek, W.; Kuśnierczyk, P. A novel polymorphism in the cytoplasmic region of the human immunoglobulin A Fc receptor gene. Eur. J. Immunogenet. 2004, 31, 59–62. [Google Scholar] [CrossRef]
- Wu, J.; Ji, C.; Xie, F.; Langefeld, C.D.; Qian, K.; Gibson, A.W.; Edberg, J.C.; Kimberly, R.P. FcαRI (CD89) Alleles Determine the Proinflammatory Potential of Serum IgA. J. Immunol. 2007, 178, 3973–3982. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.H.; Jung, S.T. Boosting therapeutic potency of antibodies by taming Fc domain functions. Exp. Mol. Med. 2019, 51, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-H.; Kang, T.H.; Godon, O.; Watanabe, M.; Delidakis, G.; Gillis, C.M.; Sterlin, D.; Hardy, D.; Cogné, M.; Macdonald, L.E. An engineered human Fc domain that behaves like a pH-toggle switch for ultra-long circulation persistence. Nat. Commun. 2019, 10, 1–11. [Google Scholar]
- Liu, R.; Oldham, R.J.; Teal, E.; Beers, S.A.; Cragg, M.S. Fc-Engineering for Modulated Effector Functions—Improving Antibodies for Cancer Treatment. Antibodies 2020, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Grevys, A.; Bern, M.; Foss, S.; Bratlie, D.B.; Moen, A.; Gunnarsen, K.S.; Aase, A.; Michaelsen, T.E.; Sandlie, I.; Andersen, J.T. Fc Engineering of Human IgG1 for Altered Binding to the Neonatal Fc Receptor Affects Fc Effector Functions. J. Immunol. 2015, 194, 5497–5508. [Google Scholar] [CrossRef] [Green Version]
- Kellner, C.; Otte, A.; Cappuzzello, E.; Klausz, K.; Peipp, M. Modulating Cytotoxic Effector Functions by Fc Engineering to Improve Cancer Therapy. Transfus. Med. Hemother. Ther. 2017, 44, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Mathieu, M.; Brezski, R.J. IgG Fc engineering to modulate antibody effector functions. Protein Cell 2017, 9, 63–73. [Google Scholar] [CrossRef]
- Sondermann, P.; Szymkowski, D.E. Harnessing Fc receptor biology in the design of therapeutic antibodies. Curr. Opin. Immunol. 2016, 40, 78–87. [Google Scholar] [CrossRef]
- Kubota, T.; Niwa, R.; Satoh, M.; Akinaga, S.; Shitara, K.; Hanai, N. Engineered therapeutic antibodies with improved effector functions. Cancer Sci. 2009, 100, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- DiLillo, D.J.; Ravetch, J.V. Differential Fc-Receptor Engagement Drives an Anti-tumor Vaccinal Effect. Cell 2015, 161, 1035–1045. [Google Scholar] [CrossRef] [Green Version]
- Bournazos, S.; Corti, D.; Virgin, H.W.; Ravetch, J.V. Fc-optimized antibodies elicit CD8 immunity to viral respiratory infection. Nature 2020, 588, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Woof, J.M.; Burton, D.R. Human antibody–Fc receptor interactions illuminated by crystal structures. Nat. Rev. Immunol. 2004, 4, 89–99. [Google Scholar] [CrossRef]
- Maxwell, K.F.; Powell, M.S.; Hulett, M.D.; Barton, P.A.; McKenzie, I.F.; Garrett, T.P.; Hogarth, P.M. Crystal structure of the human leukocyte Fc receptor, Fc gammaRIIa. Nat. Genet. 1999, 6, 437–442. [Google Scholar] [CrossRef]
- Powell, M.S.; Barnes, N.C.; Bradford, T.M.; Musgrave, I.F.; Wines, B.D.; Cambier, J.C.; Hogarth, M. Alteration of the FcγRIIa Dimer Interface Affects Receptor Signaling but Not Ligand Binding. J. Immunol. 2006, 176, 7489–7494. [Google Scholar] [CrossRef]
- Ramsland, P.A.; Farrugia, W.; Bradford, T.M.; Sardjono, C.T.; Esparon, S.; Trist, H.M.; Powell, M.S.; Tan, P.S.; Cendron, A.C.; Wines, B.D.; et al. Structural Basis for FcγRIIa Recognition of Human IgG and Formation of Inflammatory Signaling Complexes. J. Immunol. 2011, 187, 3208–3217. [Google Scholar] [CrossRef]
- Domingo, P.; Muñiz-Diaz, E.; Baraldès, M.A.; Arilla, M.; Barquet, N.; Pericas, R.; Juarez, C.; Madoz, P.; Vázquez, G. Associations between Fc gamma receptor IIA polymorphisms and the risk and prognosis of meningococcal disease. Am. J. Med. 2002, 112, 19–25. [Google Scholar] [CrossRef]
- Bazilio, A.P.; Viana, V.S.; Toledo, R.; Woronik, V.; Bonfa, E.; Monteiro, R.C. FcγRIIa polymorphism: A susceptibility factor for immune complex-mediated lupus nephritis in Brazilian patients. Nephrol. Dial. Transplant. 2004, 19, 1427–1431. [Google Scholar] [CrossRef] [Green Version]
- Clatworthy, M.R. Fcγ Receptor Polymorphisms and Susceptibility to Infection. In Antibody Fc; Elsevier: Amsterdam, The Netherlands, 2014; pp. 217–237. [Google Scholar]
- Yuan, F.F.; Sullivan, J.S. FcγRIIA polymorphism as a risk factor for invasive Streptococcus pneumoniae. Clin. Appl. Immunol. Rev. 2005, 5, 397–403. [Google Scholar] [CrossRef]
- Ravetch, J.V.; Bolland, S. Igg fc receptors. Annu. Rev. Immunol. 2001, 19, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Gouel-Chéron, A.; Jönsson, F.; Bruhns, P. Contribution of human FcγRs to disease with evidence from human polymorphisms and transgenic animal studies. Front. Immunol. 2014, 5, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Taeye, S.W.; Bentlage, A.E.H.; Mebius, M.M.; Meesters, J.I.; Lissenberg-Thunnissen, S.; Falck, D.; Sénard, T.; Salehi, N.; Wuhrer, M.; Schuurman, J.; et al. FcγR Binding and ADCC Activity of Human IgG Allotypes. Front. Immunol. 2020, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Barb, A.W. Fc γ receptor compositional heterogeneity: Considerations for immunotherapy development. J. Biol. Chem. 2021, 296, 100057. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Namenuk, A.K.; Hong, K.; Meng, Y.G.; Rae, J.; Briggs, J.; Xie, D.; Lai, J.; Stadlen, A.; Li, B. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J. Biol. Chem. 2001, 276, 6591–6604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.H.; Jung, H.D.; Kim, J.G.; Lee, J.-J.; Yang, D.-H.; Park, Y.H.; Do, Y.R.; Shin, H.J.; Kim, M.K.; Hyun, M.S.; et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood 2006, 108, 2720–2725. [Google Scholar] [CrossRef] [Green Version]
- Boyerinas, B.; Jochems, C.; Fantini, M.; Heery, C.R.; Gulley, J.L.; Tsang, K.Y.; Schlom, J. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti–PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol. Res. 2015, 3, 1148–1157. [Google Scholar] [CrossRef] [Green Version]
- Calemma, R.; Ottaiano, A.; Trotta, A.M.; Nasti, G.; Romano, C.; Napolitano, M.; Galati, D.; Borrelli, P.; Zanotta, S.; Cassata, A.; et al. Fc gamma receptor IIIa polymorphisms in advanced colorectal cancer patients correlated with response to anti-EGFR antibodies and clinical outcome. J. Transl. Med. 2012, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Mahaweni, N.M.; Olieslagers, T.I.; Rivas, I.O.; Molenbroeck, S.J.J.; Groeneweg, M.; Bos, G.M.J.; Tilanus, M.G.J.; Voorter, C.E.M.; Wieten, L. A comprehensive overview of FCGR3A gene variability by full-length gene sequencing including the identification of V158F polymorphism. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Day, S.; Ramamurthy, C.; Bullock, A.J.; El-Khoueiry, A.B.; Ohanjanian, L.; Wijatyk, A.; Feliu, W.I.O.; Shapiro, I.; Ancukiewicz, M.; Chand, D.; et al. AGEN1181, a clinical stage Fc-engineered anti-CTLA-4 antibody with improved therapeutic potential for the treatment of patients with advanced malignancies. J. Clin. Oncol. 2020, 38, TPS3157. [Google Scholar] [CrossRef]
- Patel, K.R.; Roberts, J.T.; Barb, A.W. Multiple Variables at the Leukocyte Cell Surface Impact Fc γ Receptor-Dependent Mechanisms. Front. Immunol. 2019, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Van Der Pol, W.-L.; Van De Winkel, J.G.J. IgG receptor polymorphisms: Risk factors for disease. Immunogenetics 1998, 48, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Bux, J.; Stein, E.-L.; Bierling, P.; Fromont, P.; Clay, M.; Stroncek, D.; Santoso, S. Characterization of a New Alloantigen (SH) on the Human Neutrophil Fcγreceptor IIIb. Blood 1997, 89, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Ory, P.A.; Clark, M.R.; Kwoh, E.E.; Clarkson, S.B.; Goldstein, I.M. Sequences of complementary DNAs that encode the NA1 and NA2 forms of Fc receptor III on human neutrophils. J. Clin. Investig. 1989, 84, 1688–1691. [Google Scholar] [CrossRef]
- Salmon, J.E.; Millard, S.S.; Brogle, N.L.; Kimberly, R.P. Fc gamma receptor IIIb enhances Fc gamma receptor IIa function in an oxidant-dependent and allele-sensitive manner. J. Clin. Investig. 1995, 95, 2877–2885. [Google Scholar] [CrossRef] [Green Version]
- Foster, C.B.; Lehrnbecher, T.; Mol, F.; Steinberg, S.M.; Venzon, D.J.; Walsh, T.J.; Noack, D.; Rae, J.; Winkelstein, J.A.; Curnutte, J.T.; et al. Host defense molecule polymorphisms influence the risk for immune-mediated complications in chronic granulomatous disease. J. Clin. Investig. 1998, 102, 2146–2155. [Google Scholar] [CrossRef] [Green Version]
- Nasr, A.; Aljada, A.; Hamid, O.; Elsheikh, H.A.; Masuadi, E.; Al-Bawab, A.; Alenazi, T.H.; Abushouk, A.; Salah, A.M. Significant Differences in FcγRIIa, FcγRIIa and FcγRIIIb Genes Polymorphism and Anti-Malarial IgG Subclass Pattern Are Associated with Severe Malaria in Saudi Children; Research Square: Durham, NC, USA, 2021. [Google Scholar]
- Golay, J.T.; Da Roit, F.; Bologna, L.; Ferrara, C.; Leusen, J.H.; Rambaldi, A.; Klein, C.; Introna, M. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 2013, 122, 3482–3491. [Google Scholar] [CrossRef] [Green Version]
- Sachs, U.J.H.; Socher, I.; Braeunlich, C.G.; Kroll, H.; Bein, G.; Santoso, S. A variable number of tandem repeats polymorphism influences the transcriptional activity of the neonatal Fc receptor α-chain promoter. Immunology 2006, 119, 83–89. [Google Scholar] [CrossRef]
- Sabol, S.Z.; Hu, S.; Hamer, D. A functional polymorphism in the monoamine oxidase A gene promoter. Qual. Life Res. 1998, 103, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Passot, C.; Azzopardi, N.; Renault, S.; Baroukh, N.; Arnoult, C.; Ohresser, M.; Boisdron-Celle, M.; Gamelin, E.; Watier, H.; Paintaud, G. Influence of FCGRT gene polymorphisms on pharmacokinetics of therapeutic antibodies. mAbs 2013, 5, 614–619. [Google Scholar] [CrossRef] [Green Version]
- Gouilleux-Gruart, V.; Chapel, H.; Chevret, S.; Lucas, M.; Malphettes, M.; Fieschi, C.; Patel, S.; Boutboul, D.; Marson, M.-N.; Gérard, L.; et al. Efficiency of immunoglobulin G replacement therapy in common variable immunodeficiency: Correlations with clinical phenotype and polymorphism of the neonatal Fc receptor. Clin. Exp. Immunol. 2012, 171, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zalevsky, J.; Chamberlain, A.K.; Horton, H.M.; Karki, S.; Leung, I.W.L.; Sproule, T.J.; Lazar, G.A.; Roopenian, D.C.; Desjarlais, J.R. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 2010, 28, 157–159. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kimberly, R.P. Targeting the Fc receptor in autoimmune disease. Expert Opin. Ther. Targets 2014, 18, 335–350. [Google Scholar] [CrossRef] [Green Version]
- Kiessling, P.; Lledo-Garcia, R.; Watanabe, S.; Langdon, G.; Tran, D.; Bari, M.; Christodoulou, L.; Jones, E.; Price, G.; Smith, B.; et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study. Sci. Transl. Med. 2017, 9, eaan1208. [Google Scholar] [CrossRef] [PubMed]
- Bril, V.; Benatar, M.; Andersen, H.; Vissing, J.; Brock, M.; Greve, B.; Kiessling, P.; Woltering, F.; Griffin, L.; Van den Bergh, P. Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: A phase 2 randomized control trial. Neurology 2021, 96, e853–e865. [Google Scholar]
- Yap, D.Y.; Hai, J.; Lee, P.C.; Zhou, X.; Lee, M.; Zhang, Y.; Wang, M.; Chen, X. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of HBM9161, a novel FcRn inhibitor, in a Phase 1 Study for Healthy Chinese Volunteers. Clin. Transl. Sci. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Mellor, J.D.; Brown, M.P.; Irving, H.R.; Zalcberg, J.R.; Dobrovic, A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J. Hematol. Oncol. 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaifu, T.; Nakamura, A. Polymorphisms of immunoglobulin receptors and the effects on clinical outcome in cancer immunotherapy and other immune diseases: A general review. Int. Immunol. 2017, 29, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Van Sorge, N.; Van Der Pol, W.L.; Van de Winkel, J. FcγR polymorphisms: Implications for function, disease susceptibility and immunotherapy. Tissue Antigens 2003, 61, 189–202. [Google Scholar] [CrossRef]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2017, 18, 46–61. [Google Scholar] [CrossRef]
- Gyawali, B.; Kesselheim, A.S. FDA approval standards for anticancer agents—Lessons from two recent approvals in breast cancer. Nat. Rev. Clin. Oncol. 2021, 18, 397–398. [Google Scholar] [CrossRef]
- Markham, A. Margetuximab: First Approval. Drugs 2021, 81, 599–604. [Google Scholar] [CrossRef] [PubMed]
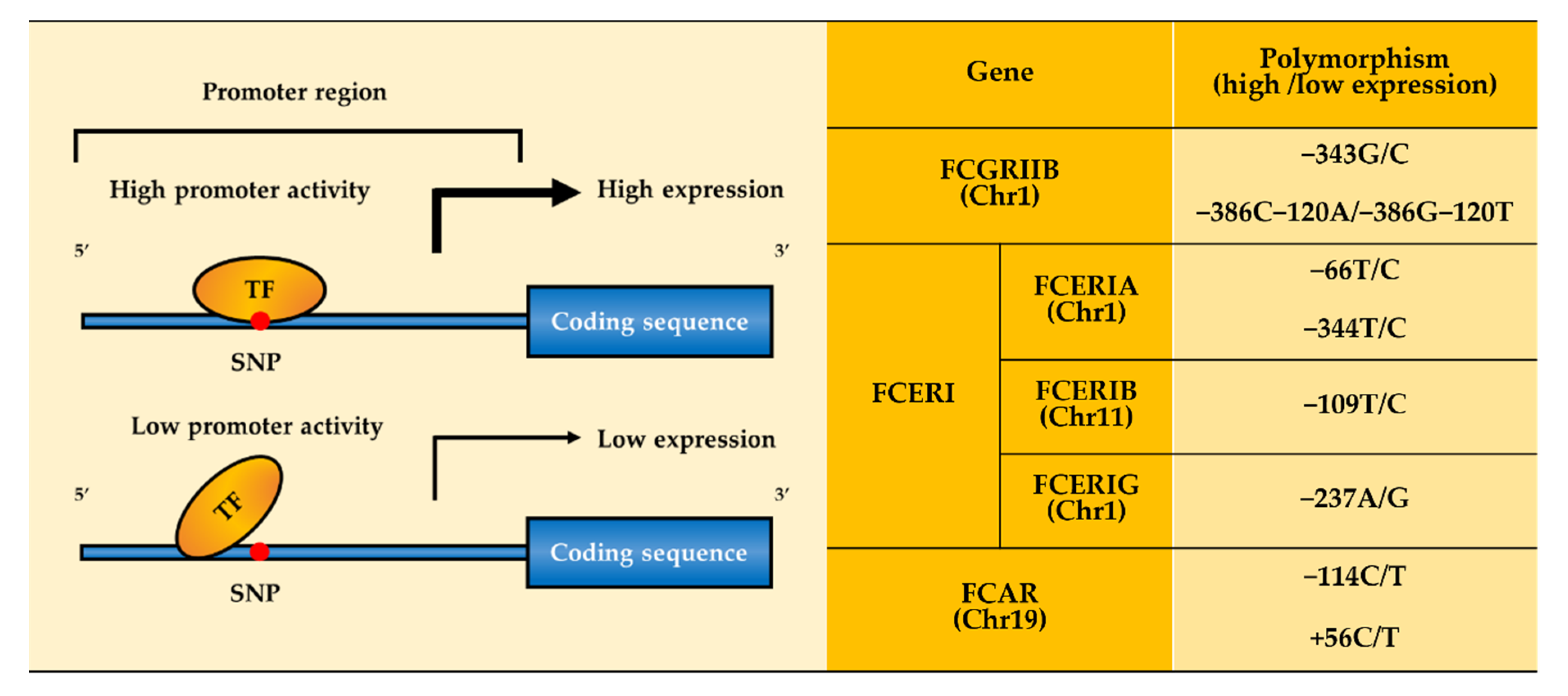
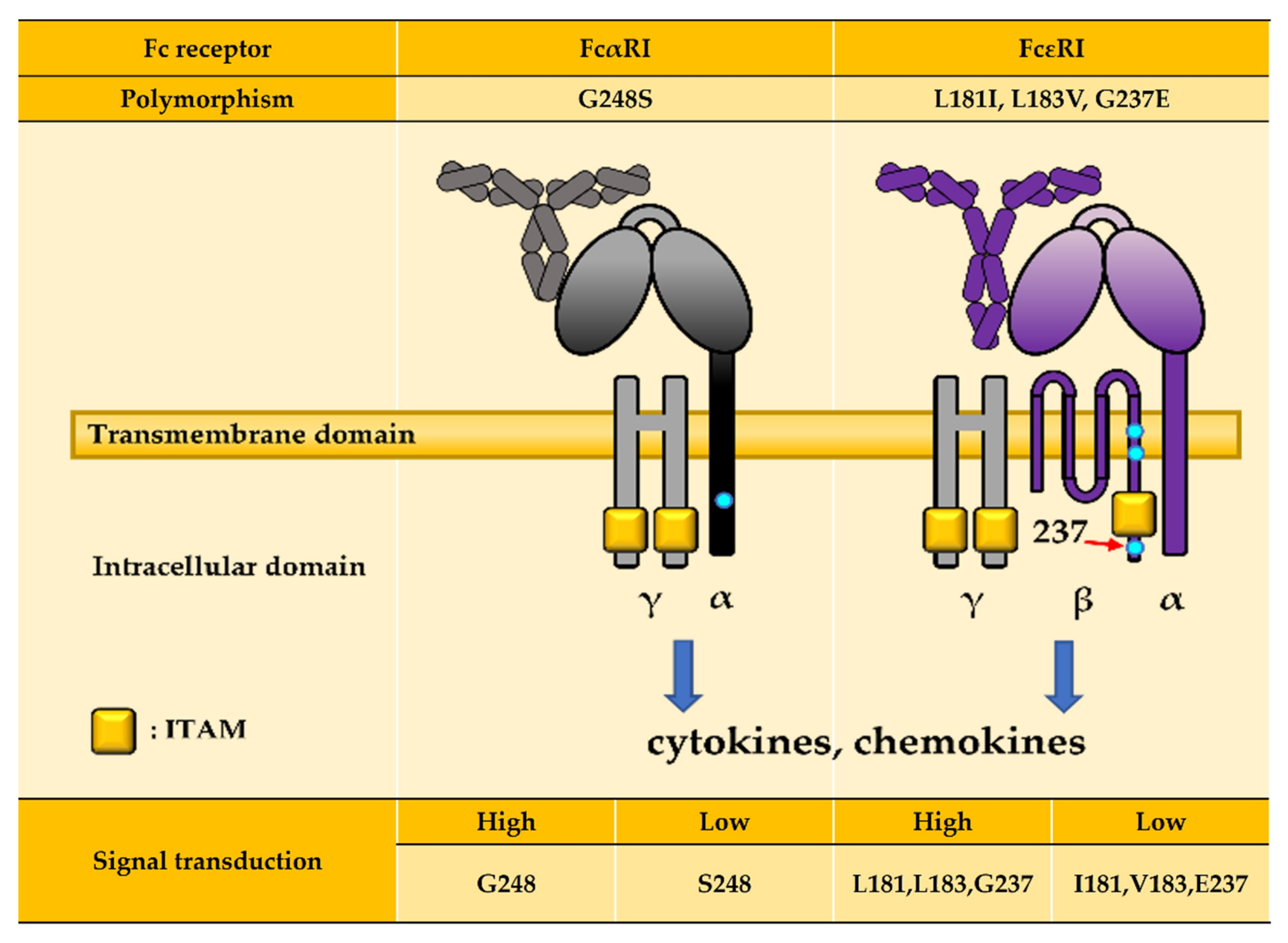
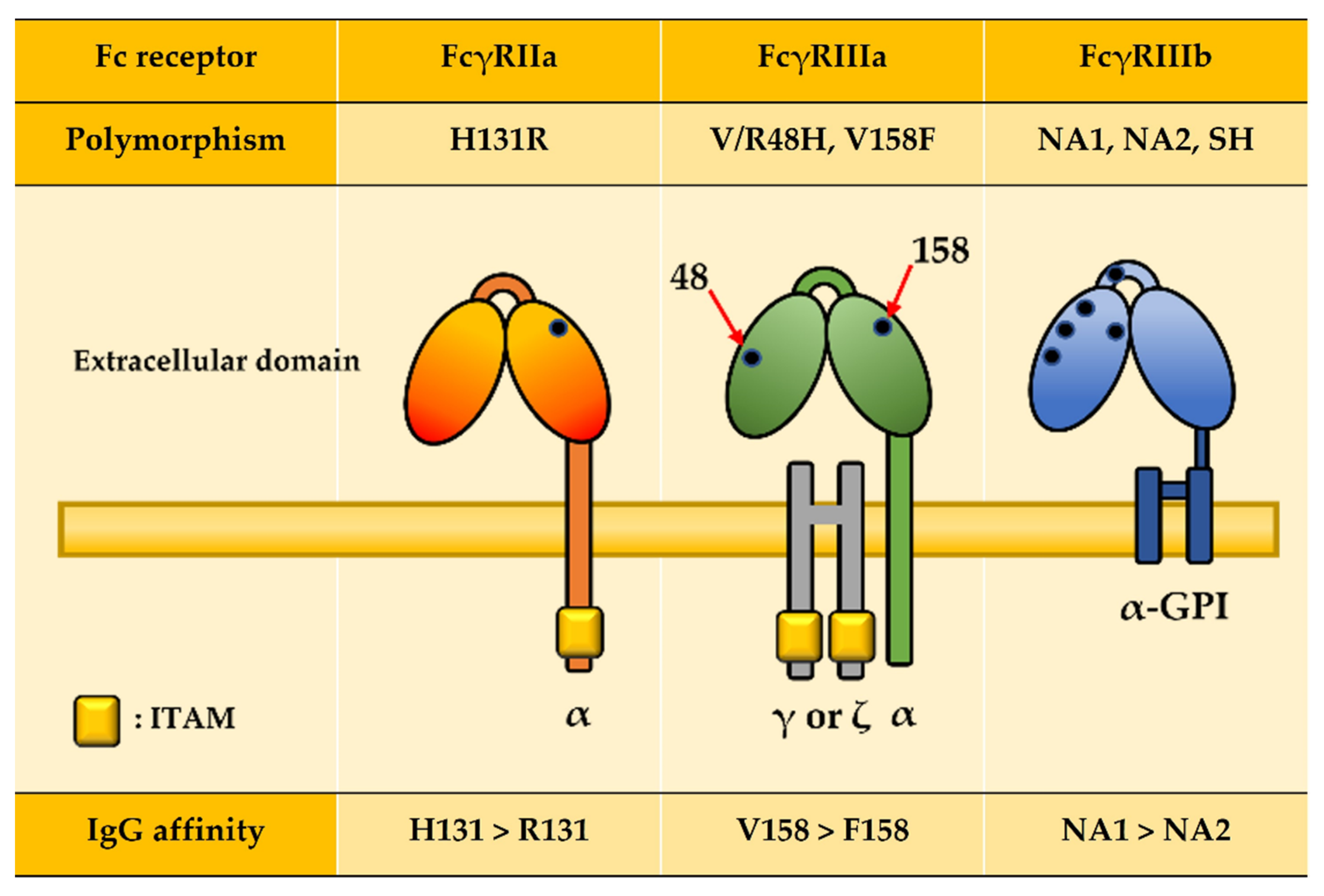
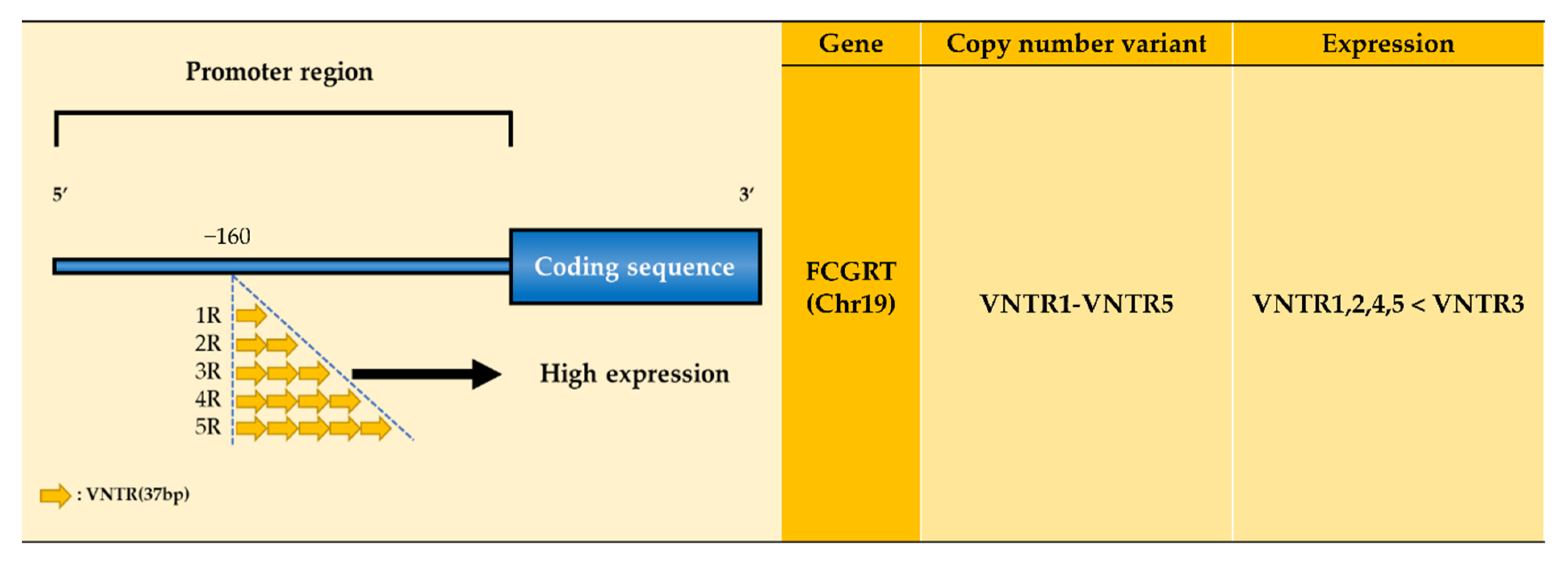
| Receptor | Ig Isotype and Subclass Binding Affinity | Function | Expression | |||
|---|---|---|---|---|---|---|
| Myeloid Cell | Lymphoid Cell | Non-Immune Cell | ||||
| FcγRI | IgG1,2,3,4 | High affinity (IgG1 = IgG3 > IgG4 >>> IgG2) | Activation | Monocyte, Macrophage, Neutrophil, DC, Mast cell, Eosinophil | - | - |
| FcγRIIa | Low affinity (H131: IgG3 > IgG1 > IgG2 > IgG4 R131: IgG3 > IgG1 > IgG4 > IgG2) | Activation/Inhibition | Monocyte, Macrophage, Neutrophil, DC, Mast cell, Basophil, Eosinophil | T cell | Platelet, Endothelium | |
| FcγRIIb | Low affinity (IgG3 > IgG1 > IgG4 >> IgG2) | Inhibition | Monocyte, Macrophage, Neutrophil, DC, Mast cell, Basophil, Eosinophil | B cell, Plasma cell | - | |
| FcγRIIc | Low affinity (IgG3 > IgG1 > IgG4 >> IgG2) | Activation | Monocyte, Macrophage, Neutrophil | NK cell | - | |
| FcγRIIIa | Low affinity (IgG3 >> IgG1 > IgG4 > IgG2) | Activation/Inhibition | Monocyte, Macrophage | NK cell, T cell | - | |
| FcγRIIIb | Low affinity (IgG1 = IgG3 >>> IgG2 = IgG4) | Activation | Neutrophil, Basophil, Eosinophil | - | - | |
| FcRn | High affinity (pH ≤ 6.5)/Low affinity (pH 7.4) (IgG4 > IgG1 > IgG3 > IgG2) | Transcytosis/Recycling | Monocyte, Macrophage, Neutrophil, DC | - | Endothelium, Epithelium | |
| FcεRI | IgE | High affinity | Activation | Langerhans cell, Eosinophil, Mast cell, Basophil, DC, Monocyte | - | Platelet |
| FcεRII | Low affinity | Activation | Langerhans cell, Monocyte, DC, Macrophage, Eosinophil, | B cell, T cell, NK cell | Platelet | |
| FcαRI | IgA1,2 | Low affinity (IgA1 = IgA2) | Activation/Inhibition | Monocyte, Macrophage, Neutrophil, DC, Kupffer cell | - | - |
| FcμRI | IgM | Low affinity (monomeric IgM)/High avidity (pentameric IgM) | NA | - | B cell, T cell, NK cell | - |
| Fcα/μR | IgA/IgM | High affinity (IgM > IgA) | NA | Macrophage, DC | B cell | - |
| FcδR | IgD | NA | ||||
| plgR | Dimeric IgA/pentameric IgM | High affinity | Transcytosis | - | - | Endothelium, Epithelium |
| Fc Receptor | Genetic Variant | Related Diseases | |||||
|---|---|---|---|---|---|---|---|
| Polymorphism | Copy Number Variant | ||||||
| Promoter Region | Extracellular Domain | Transmembrane Domain | Intracellular Domain | ||||
| FcγRIIa | H131R | R131 allele: Bacterial infection (Low affinity to IgG2) | |||||
| FcγRIIb | −120T/A −343G/C −386G/C | −343G, −386C−120A allele: SLE (High FcγRIIb expression) | |||||
| FcγRIIIa | 48L/R/H V158F | F131 allele: Cancer (Low affinity to IgG) | |||||
| FcγRIIIb | NA1 NA2 SH | NA2 allele: CGD (Low affinity to IgG1 and IgG3) | |||||
| FcRn | VNTR1- VNTR5 | NA | |||||
| FcεRI | α-subunit | −66T/C −344C/T | −66T, −344T allele: Allergic diseases (High FcεRI expression) | ||||
| β-subunit | −109C/T | I181L V183L | E237G | −109T allele: Asthma (High FcεRI expression) L181, L183 allele: Atopy, Asthma (Altered signaling pathway leading to cell activation) G237 allele: Allergy diseases (Activating signal induction) | |||
| γ-subunit | −237A/G | −237A allele: AIA (High FcεRI expression) | |||||
| FcαRI | −114C/T +56C/T | S248G | −114C, +56C allele: IgAN (High FcαRI expression) G248 allele: SLE (Altered signaling pathway leading to cell activation) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, J.Y.; Kim, H.G.; Kwak, M.W.; Kang, T.H. Fc Receptor Variants and Disease: A Crucial Factor to Consider in the Antibody Therapeutics in Clinic. Int. J. Mol. Sci. 2021, 22, 9489. https://doi.org/10.3390/ijms22179489
Kim J, Lee JY, Kim HG, Kwak MW, Kang TH. Fc Receptor Variants and Disease: A Crucial Factor to Consider in the Antibody Therapeutics in Clinic. International Journal of Molecular Sciences. 2021; 22(17):9489. https://doi.org/10.3390/ijms22179489
Chicago/Turabian StyleKim, Jin, Ji Young Lee, Han Gil Kim, Min Woo Kwak, and Tae Hyun Kang. 2021. "Fc Receptor Variants and Disease: A Crucial Factor to Consider in the Antibody Therapeutics in Clinic" International Journal of Molecular Sciences 22, no. 17: 9489. https://doi.org/10.3390/ijms22179489
APA StyleKim, J., Lee, J. Y., Kim, H. G., Kwak, M. W., & Kang, T. H. (2021). Fc Receptor Variants and Disease: A Crucial Factor to Consider in the Antibody Therapeutics in Clinic. International Journal of Molecular Sciences, 22(17), 9489. https://doi.org/10.3390/ijms22179489







