Inflammation- and Gut-Homing Macrophages, Engineered to De Novo Overexpress Active Vitamin D, Promoted the Regenerative Function of Intestinal Stem Cells
Abstract
:1. Introduction
2. Results
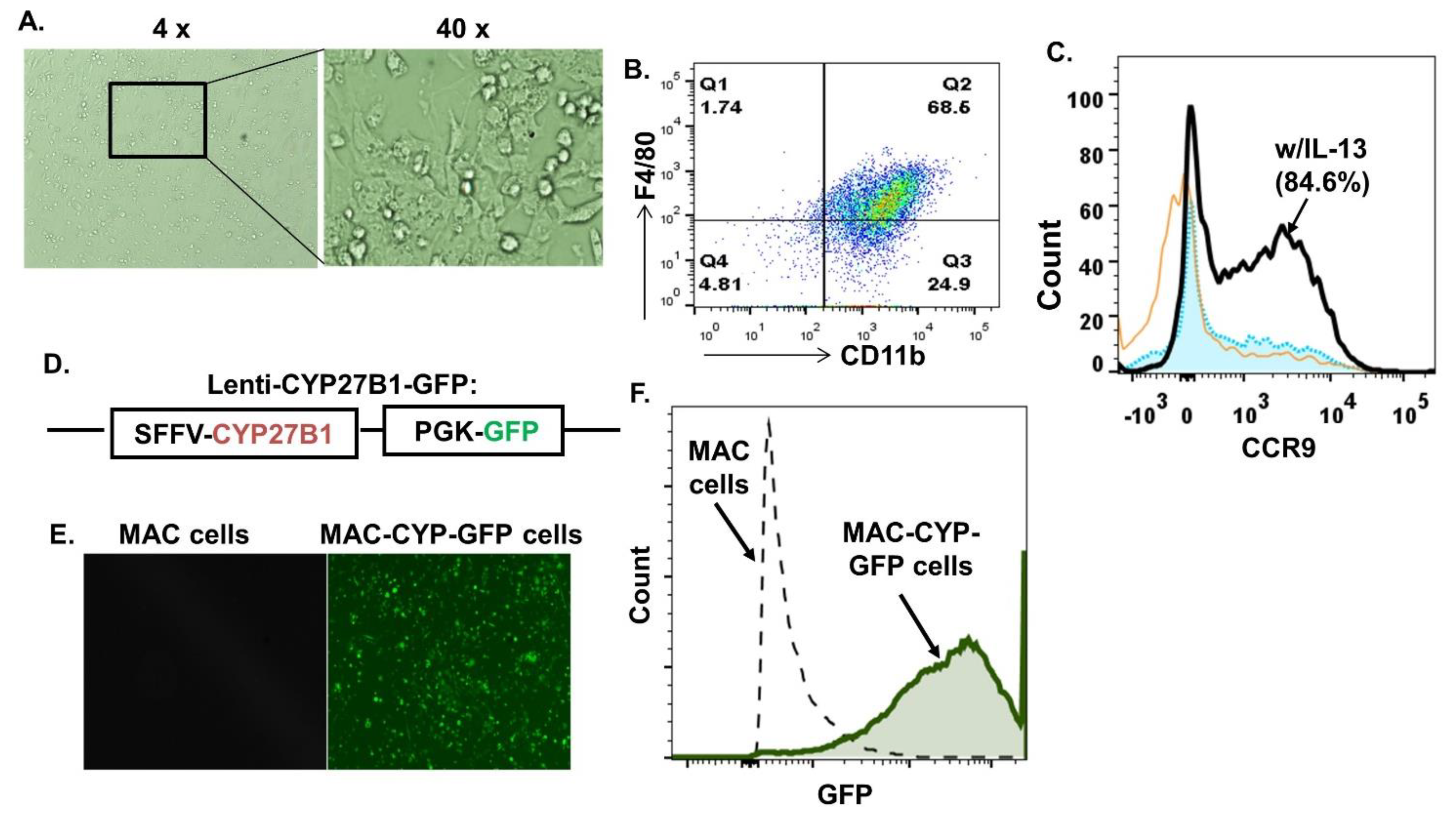
2.1. Generation of Inflammation- and Gut-Homing MAC-CYP Cells In Vitro
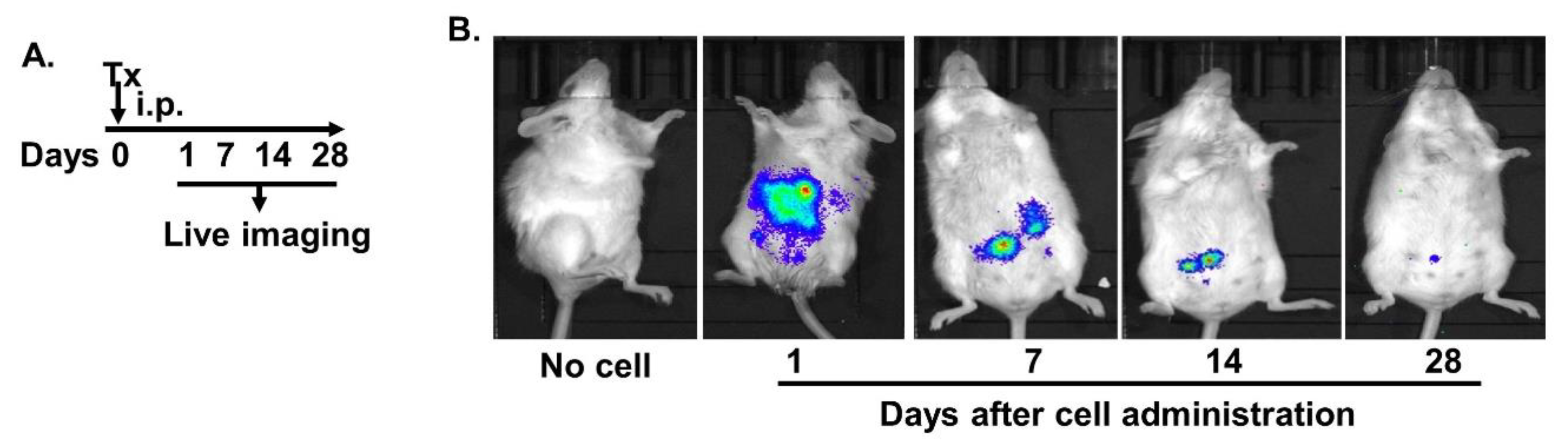
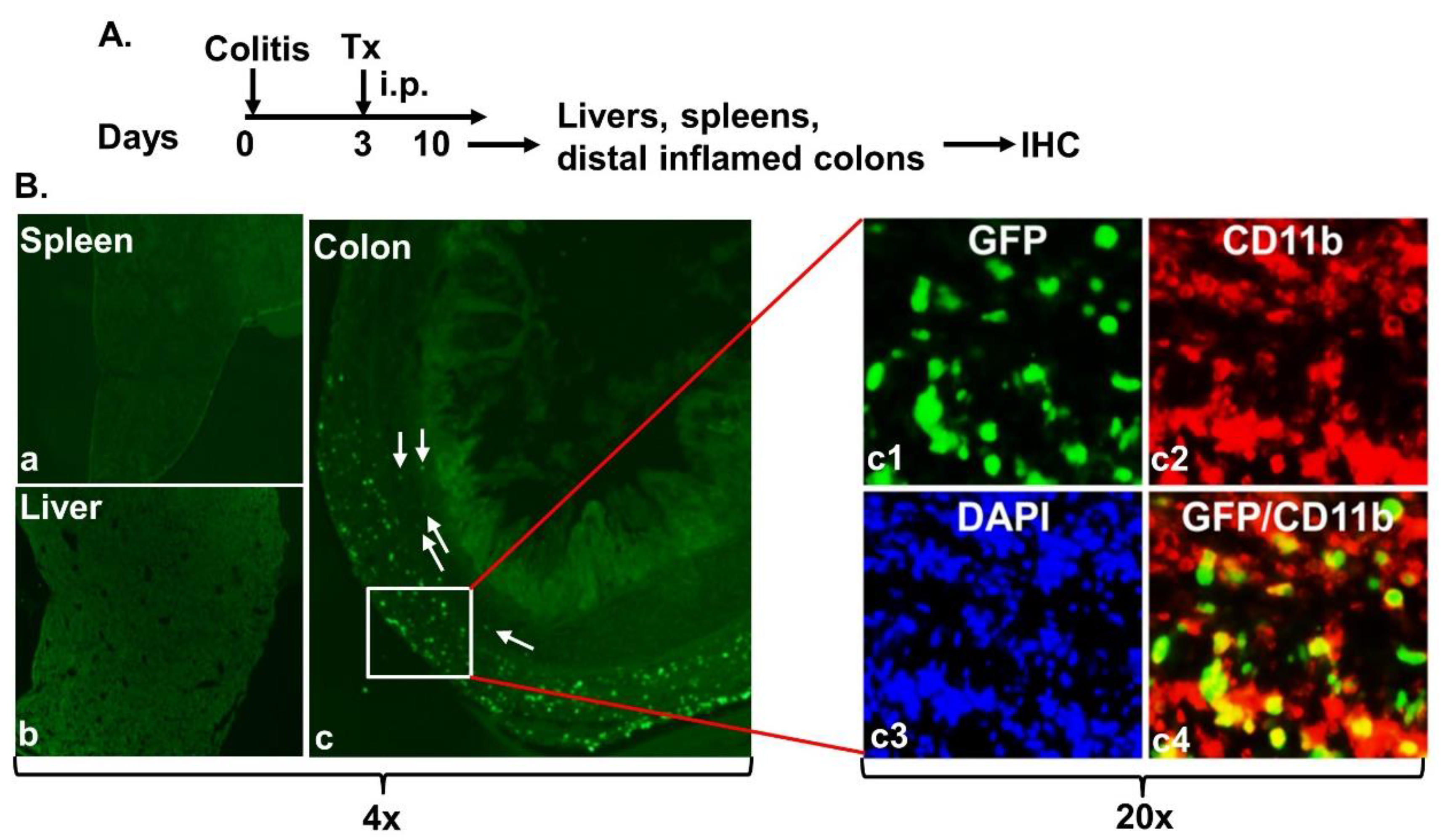
2.2. Intraperitoneally Administered MAC-CYP Cells Migrated Specifically into Inflamed Colons
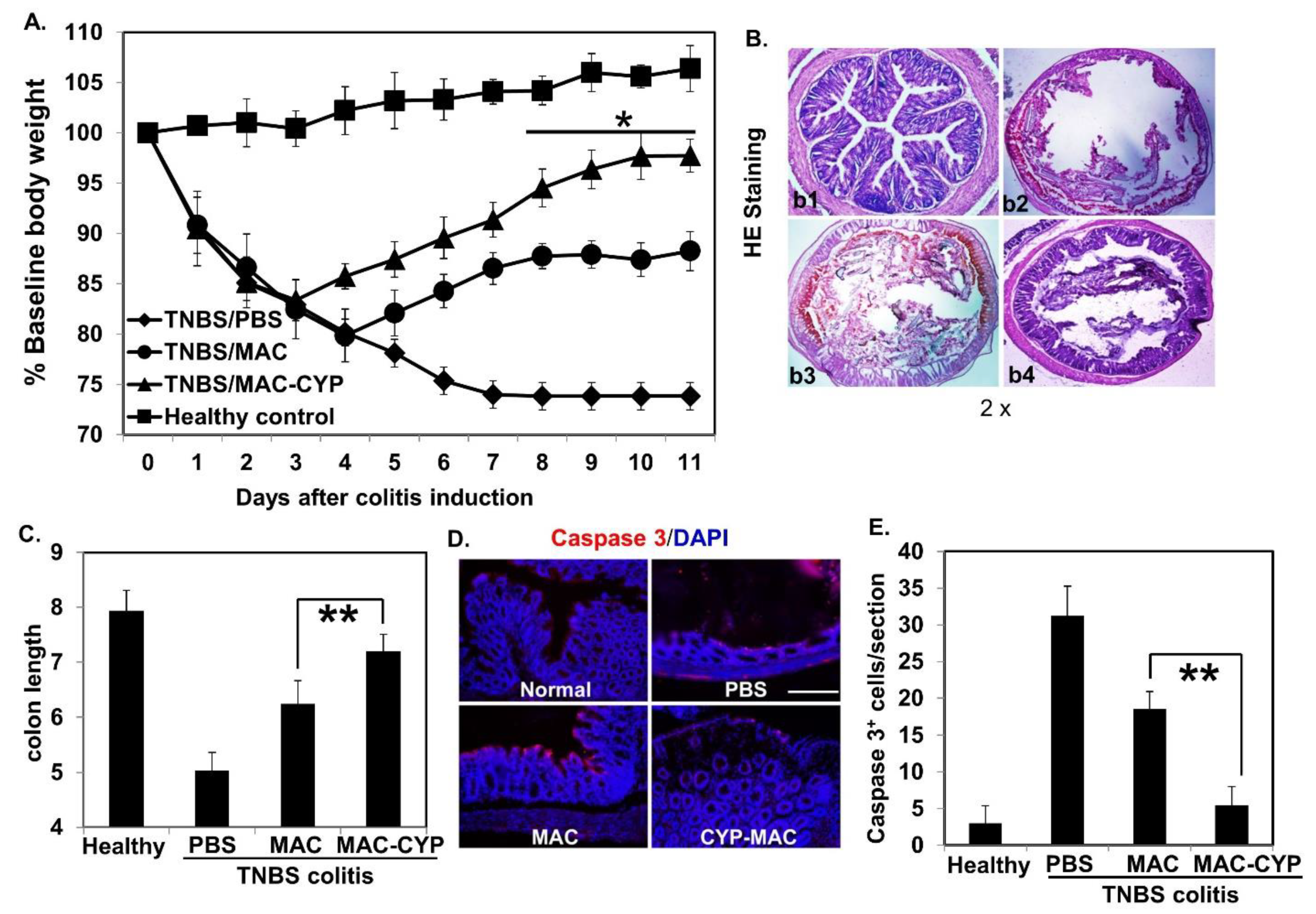
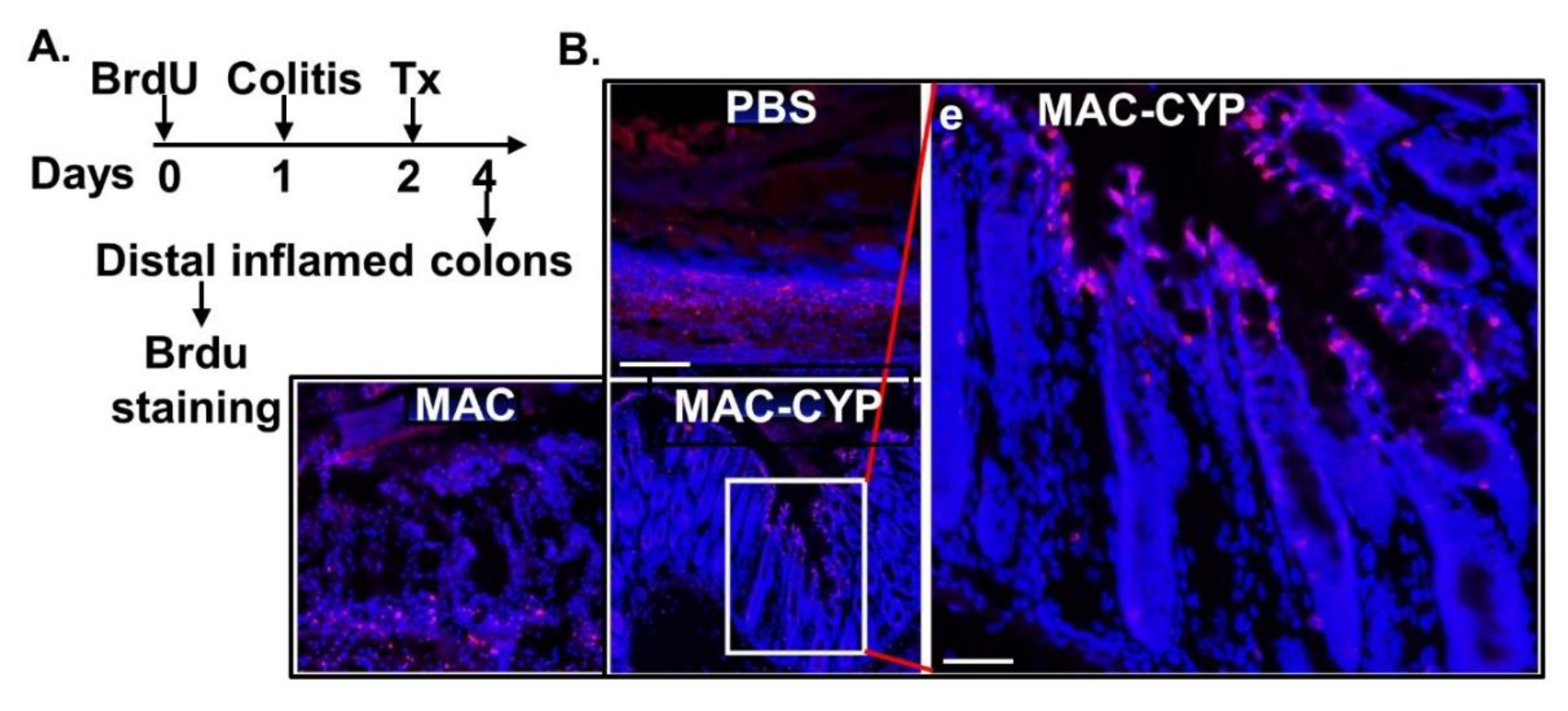
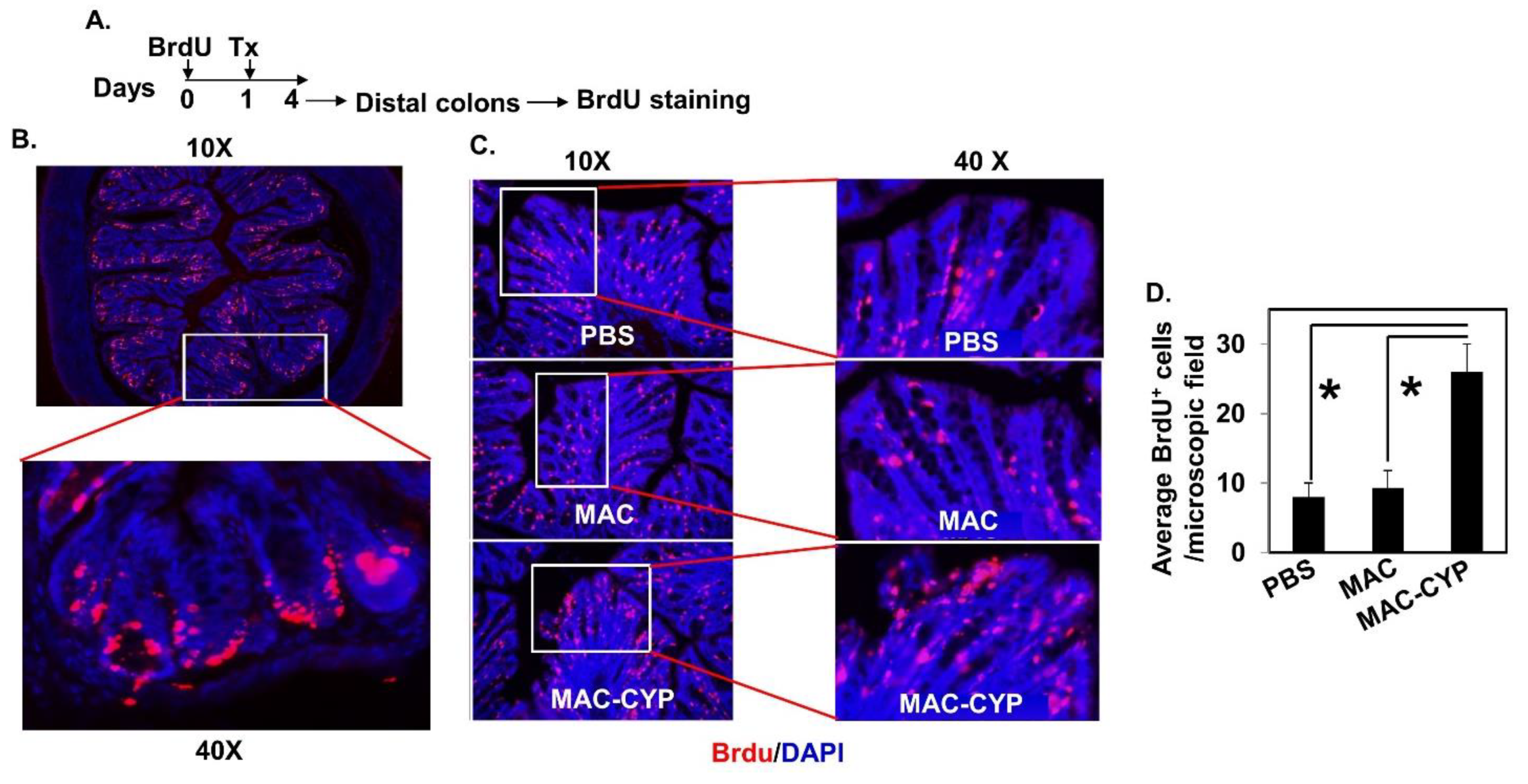
2.3. Intraperitoneally Administered MAC-CYP Cells Accelerated Intestinal Stem Cell Migration
2.4. Migrated Intestinal Stem Cells Differentiated into Mature Epithelial Cells
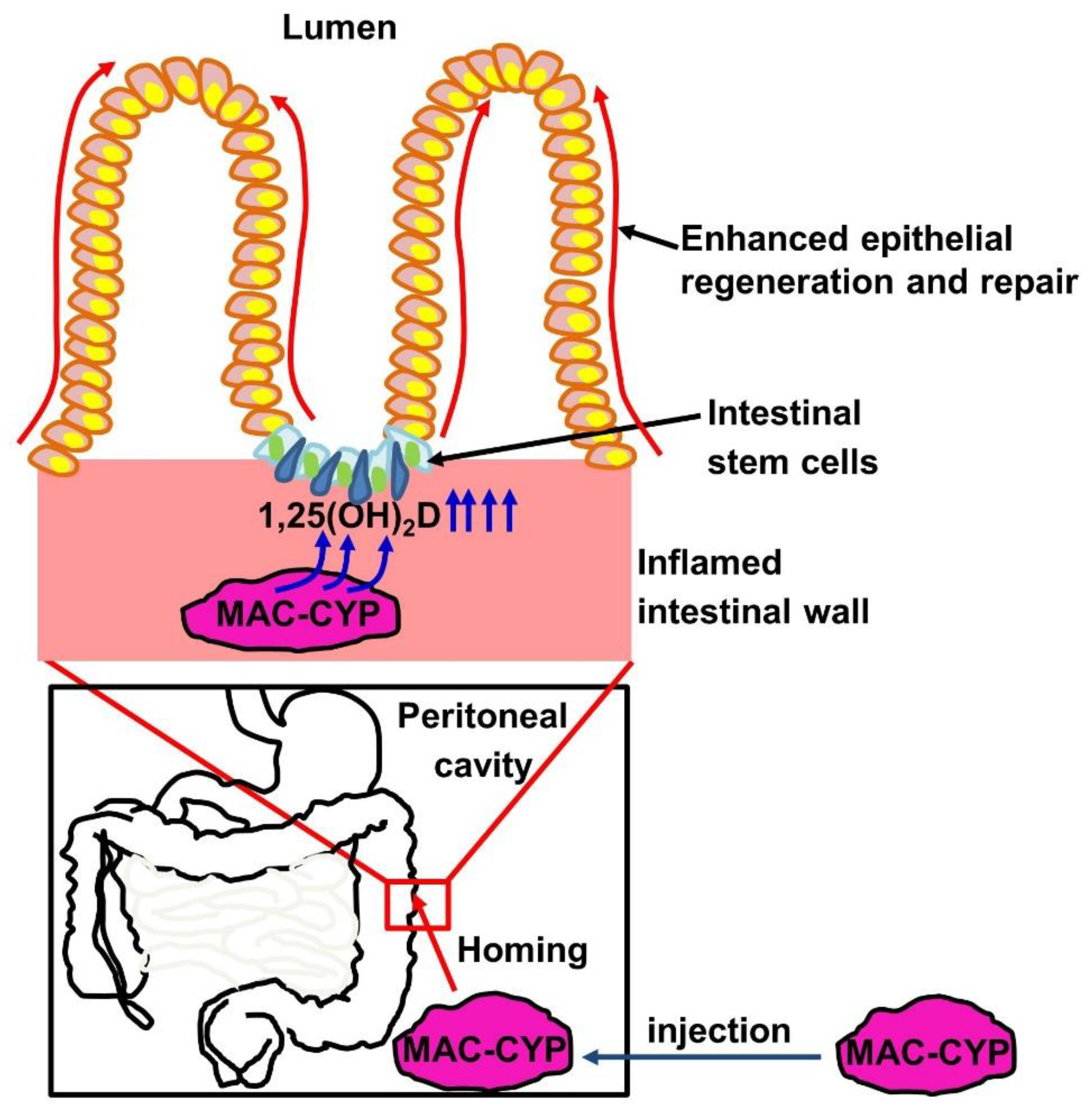
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Induction of Experimental Colitis
4.3. Preparation of Lentiviruses
4.4. Generation of CD11b+Gr1+CCR9+ Macrophages (MAC Cells) from Bone Marrow and Lentivirus Transduction
4.5. Intraperitoneal Administration of the MAC-CYP Cells
4.6. Fluorescence-Activated Cell Sorting (FACS) Analysis
4.7. Live Imaging of MAC-CYP-Luciferase Cells In Vivo
4.8. Histological Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Herrinton, L.J.; Liu, L.; Lafata, J.E.; Allison, J.E.; Andrade, S.E.; Korner, E.J.; Chan, K.A.; Platt, R.; Hiatt, D.; O’Connor, S. Estimation of the period prevalence of inflammatory bowel disease among nine health plans using computerized diagnoses and outpatient pharmacy dispensings. Inflamm. Bowel. Dis. 2007, 13, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.A.; Neurath, M.F. New pathophysiological insights and modern treatment of IBD. J. Gastroenterol. 2010, 45, 571–583. [Google Scholar] [CrossRef]
- Lanzoni, G.; Roda, G.; Belluzzi, A.; Roda, E.; Bagnara, G.P. Inflammatory bowel disease: Moving toward a stem cell-based therapy. World J. Gastroenterol. 2008, 14, 4616–4626. [Google Scholar] [CrossRef] [PubMed]
- Leitner, G.C.; Vogelsang, H. Pharmacological and non-pharmacological therapeutic approaches in inflammatory bowel disease in adults. World J. Gastrointest Pharmacol. Ther. 2016, 7, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Vermeire, S.; Van Assche, G. Mucosal healing in inflammatory bowel disease: Impossible ideal or therapeutic target? Gut 2007, 56, 453–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Davis, H.; Irshad, S.; Sandberg, T.; Worthley, D.; Leedham, S. Microenvironmental control of stem cell fate in intestinal homeostasis and disease. J. Pathol. 2015, 237, 135–145. [Google Scholar] [CrossRef]
- Schepers, A.G.; Snippert, H.J.; Stange, D.E.; van den Born, M.; van Es, J.H.; van de Wetering, M.; Clevers, H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 2012, 337, 730–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umar, S. Intestinal stem cells. Curr. Gastroenterol. Rep. 2010, 12, 340–348. [Google Scholar] [CrossRef]
- Wang, F.; Scoville, D.; He, X.C.; Mahe, M.M.; Box, A.; Perry, J.M.; Smith, N.R.; Lei, N.Y.; Davies, P.S.; Fuller, M.K.; et al. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 2013, 145, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Barry, E.R.; Morikawa, T.; Butler, B.L.; Shrestha, K.; de la Rosa, R.; Yan, K.S.; Fuchs, C.S.; Magness, S.T.; Smits, R.; Ogino, S.; et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 2013, 493, 106–110. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Peregrina, K.; Houston, M.; Daroqui, C.; Dhima, E.; Sellers, R.S.; Augenlicht, L.H. Vitamin D is a determinant of mouse intestinal Lgr5 stem cell functions. Carcinogenesis 2015, 36, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Barral, A.; Costales-Carrera, A.; Buira, S.P.; Jung, P.; Ferrer-Mayorga, G.; Larriba, M.J.; Bustamante-Madrid, P.; Dominguez, O.; Real, F.X.; Guerra-Pastrian, L.; et al. Vitamin D differentially regulates colon stem cells in patient-derived normal and tumor organoids. FEBS J. 2020, 287, 53–72. [Google Scholar] [CrossRef] [Green Version]
- Cantorna, M.T.; Munsick, C.; Bemiss, C.; Mahon, B.D. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J. Nutr. 2000, 130, 2648–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Zhang, H.; Wu, H.; Li, H.; Liu, L.; Guo, J.; Li, C.; Shih, D.Q.; Zhang, X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Mahon, B.D.; Froicu, M.; Cantorna, M.T. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur. J. Immunol. 2005, 35, 217–224. [Google Scholar] [CrossRef]
- Wasnik, S.; Sharma, I.; Baylink, D.J.; Tang, X. Vitamin D as a Potential Therapy for Multiple Sclerosis: Where Are We? Int. J. Mol. Sci. 2020, 21, 3102. [Google Scholar] [CrossRef] [PubMed]
- Probert, C. Commentary: Vitamin D supplementation in Crohn’s disease. Aliment. Pharmacol. Ther. 2020, 51, 201–202. [Google Scholar] [CrossRef] [Green Version]
- Guzman-Prado, Y.; Samson, O.; Segal, J.P.; Limdi, J.K.; Hayee, B. Vitamin D Therapy in Adults With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, 1819–1830. [Google Scholar] [CrossRef]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Baylink, D.J.; Walter, M.H.; Lau, K.H.; Meng, X.; Wang, J.; Cherkas, A.; Tang, X.; Qin, X. Targeted 25-hydroxyvitamin D3 1alpha-hydroxylase adoptive gene therapy ameliorates dss-induced colitis without causing hypercalcemia in mice. Mol. Ther. 2015, 23, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Highfill, S.L.; Rodriguez, P.C.; Zhou, Q.; Goetz, C.A.; Koehn, B.H.; Veenstra, R.; Taylor, P.A.; Panoskaltsis-Mortari, A.; Serody, J.S.; Munn, D.H.; et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 2010, 116, 5738–5747. [Google Scholar] [CrossRef]
- Lopez-Pacheco, C.; Soldevila, G.; Du Pont, G.; Hernandez-Pando, R.; Garcia-Zepeda, E.A. CCR9 Is a Key Regulator of Early Phases of Allergic Airway Inflammation. Mediat. Inflamm. 2016, 2016, 3635809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoyama, W.; Kohsaka, H.; Kaneko, K.; Walters, M.; Takayasu, A.; Fukuda, S.; Miyabe, C.; Miyabe, Y.; Love, P.E.; Nakamoto, N.; et al. Abrogation of CC chemokine receptor 9 ameliorates collagen-induced arthritis of mice. Arthritis Res. Ther. 2014, 16, 445. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wang, D.; Wang, X.; Zhang, Y.; Liu, T.; Chen, Y.; Tang, Y.; Wang, T.; Hu, D.; Huang, C. Abrogation of CC chemokine receptor 9 ameliorates ventricular remodeling in mice after myocardial infarction. Sci. Rep. 2016, 6, 32660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamoto, N.; Ebinuma, H.; Kanai, T.; Chu, P.S.; Ono, Y.; Mikami, Y.; Ojiro, K.; Lipp, M.; Love, P.E.; Saito, H.; et al. CCR9+ macrophages are required for acute liver inflammation in mouse models of hepatitis. Gastroenterology 2012, 142, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, J.; Wu, M.; Xu, L.; Wang, Y.; Yuan, W.; Hua, F.; Fan, H.; Dong, F.; Qu, X.; et al. Toll-Like Receptor 4 Promotes Th17 Lymphocyte Infiltration Via CCL25/CCR9 in Pathogenesis of Experimental Autoimmune Encephalomyelitis. J. Neuroimmune Pharmacol. 2019, 14, 493–502. [Google Scholar] [CrossRef]
- Takeuchi, H.; Yokota, A.; Ohoka, Y.; Kagechika, H.; Kato, C.; Song, S.Y.; Iwata, M. Efficient induction of CCR9 on T cells requires coactivation of retinoic acid receptors and retinoid X receptors (RXRs): Exaggerated T Cell homing to the intestine by RXR activation with organotins. J. Immunol. 2010, 185, 5289–5299. [Google Scholar] [CrossRef] [Green Version]
- Pathak, M.; Lal, G. The Regulatory Function of CCR9(+) Dendritic Cells in Inflammation and Autoimmunity. Front. Immunol. 2020, 11, 536326. [Google Scholar] [CrossRef]
- Neurath, M.F.; Fuss, I.; Kelsall, B.L.; Stuber, E.; Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995, 182, 1281–1290. [Google Scholar] [CrossRef] [Green Version]
- Harbour, S.N.; Maynard, C.L.; Zindl, C.L.; Schoeb, T.R.; Weaver, C.T. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc. Natl. Acad. Sci. USA 2015, 112, 7061–7066. [Google Scholar] [CrossRef] [Green Version]
- Basak, O.; van de Born, M.; Korving, J.; Beumer, J.; van der Elst, S.; van Es, J.H.; Clevers, H. Mapping early fate determination in Lgr5+ crypt stem cells using a novel Ki67-RFP allele. EMBO J. 2014, 33, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Hancock, A.; Priester, C.; Kidder, E.; Keith, J.R. Does 5-bromo-2’-deoxyuridine (BrdU) disrupt cell proliferation and neuronal maturation in the adult rat hippocampus in vivo? Behav. Brain Res. 2009, 199, 218–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Cheng, Y.; Baylink, D.J.; Wasnik, S.; Goel, G.; Huang, M.; Cao, H.; Qin, X.; Lau, K.W.; Chan, C.; et al. In Vivo Generation of Gut-Homing Regulatory T Cells for the Suppression of Colitis. J. Immunol. 2019, 202, 3447–3457. [Google Scholar] [CrossRef] [PubMed]
- Schewe, M.; Franken, P.F.; Sacchetti, A.; Schmitt, M.; Joosten, R.; Bottcher, R.; van Royen, M.E.; Jeammet, L.; Payre, C.; Scott, P.M.; et al. Secreted Phospholipases A2 Are Intestinal Stem Cell Niche Factors with Distinct Roles in Homeostasis, Inflammation, and Cancer. Cell. Stem. Cell 2016, 19, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef] [Green Version]
- Cianferotti, L.; Cox, M.; Skorija, K.; Demay, M.B. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc. Natl. Acad. Sci. USA 2007, 104, 9428–9433. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, A.G.; Errea, O.; van Wijngaarden, P.; Gonzalez, G.A.; Kerninon, C.; Jarjour, A.A.; Lewis, H.J.; Jones, C.A.; Nait-Oumesmar, B.; Zhao, C.; et al. Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J. Cell. Biol. 2015, 211, 975–985. [Google Scholar] [CrossRef] [Green Version]
- Cortes, M.; Chen, M.J.; Stachura, D.L.; Liu, S.Y.; Kwan, W.; Wright, F.; Vo, L.T.; Theodore, L.N.; Esain, V.; Frost, I.M.; et al. Developmental Vitamin D Availability Impacts Hematopoietic Stem Cell Production. Cell. Rep. 2016, 17, 458–468. [Google Scholar] [CrossRef] [Green Version]
- Klotz, B.; Mentrup, B.; Regensburger, M.; Zeck, S.; Schneidereit, J.; Schupp, N.; Linden, C.; Merz, C.; Ebert, R.; Jakob, F. 1,25-dihydroxyvitamin D3 treatment delays cellular aging in human mesenchymal stem cells while maintaining their multipotent capacity. PLoS ONE 2012, 7, e29959. [Google Scholar] [CrossRef]
- Abdelbaset-Ismail, A.; Pedziwiatr, D.; Suszynska, E.; Sluczanowska-Glabowska, S.; Schneider, G.; Kakar, S.S.; Ratajczak, M.Z. Vitamin D3 stimulates embryonic stem cells but inhibits migration and growth of ovarian cancer and teratocarcinoma cell lines. J. Ovarian Res. 2016, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matiasova, A.; Sevc, J.; Mikes, J.; Jendzelovsky, R.; Daxnerova, Z.; Fedorocko, P. Flow cytometric determination of 5-bromo-2’-deoxyuridine pharmacokinetics in blood serum after intraperitoneal administration to rats and mice. Histochem. Cell. Biol. 2014, 142, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tamamaki, N.; Noda, T.; Kimura, K.; Itokazu, Y.; Matsumoto, N.; Dezawa, M.; Ide, C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp. Neurol. 2005, 192, 251–264. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Baylink, D.J.; Cao, H.; Xiao, J.; Abdalla, M.I.; Wasnik, S.; Tang, X. Inflammation- and Gut-Homing Macrophages, Engineered to De Novo Overexpress Active Vitamin D, Promoted the Regenerative Function of Intestinal Stem Cells. Int. J. Mol. Sci. 2021, 22, 9516. https://doi.org/10.3390/ijms22179516
Xu Y, Baylink DJ, Cao H, Xiao J, Abdalla MI, Wasnik S, Tang X. Inflammation- and Gut-Homing Macrophages, Engineered to De Novo Overexpress Active Vitamin D, Promoted the Regenerative Function of Intestinal Stem Cells. International Journal of Molecular Sciences. 2021; 22(17):9516. https://doi.org/10.3390/ijms22179516
Chicago/Turabian StyleXu, Yi, David J. Baylink, Huynh Cao, Jeffrey Xiao, Maisa I. Abdalla, Samiksha Wasnik, and Xiaolei Tang. 2021. "Inflammation- and Gut-Homing Macrophages, Engineered to De Novo Overexpress Active Vitamin D, Promoted the Regenerative Function of Intestinal Stem Cells" International Journal of Molecular Sciences 22, no. 17: 9516. https://doi.org/10.3390/ijms22179516
APA StyleXu, Y., Baylink, D. J., Cao, H., Xiao, J., Abdalla, M. I., Wasnik, S., & Tang, X. (2021). Inflammation- and Gut-Homing Macrophages, Engineered to De Novo Overexpress Active Vitamin D, Promoted the Regenerative Function of Intestinal Stem Cells. International Journal of Molecular Sciences, 22(17), 9516. https://doi.org/10.3390/ijms22179516






