Neuroprotection Mediated by Human Blood Plasma in Mouse Hippocampal Slice Cultures and in Oxidatively Stressed Human Neurons
Abstract
:1. Introduction
2. Results
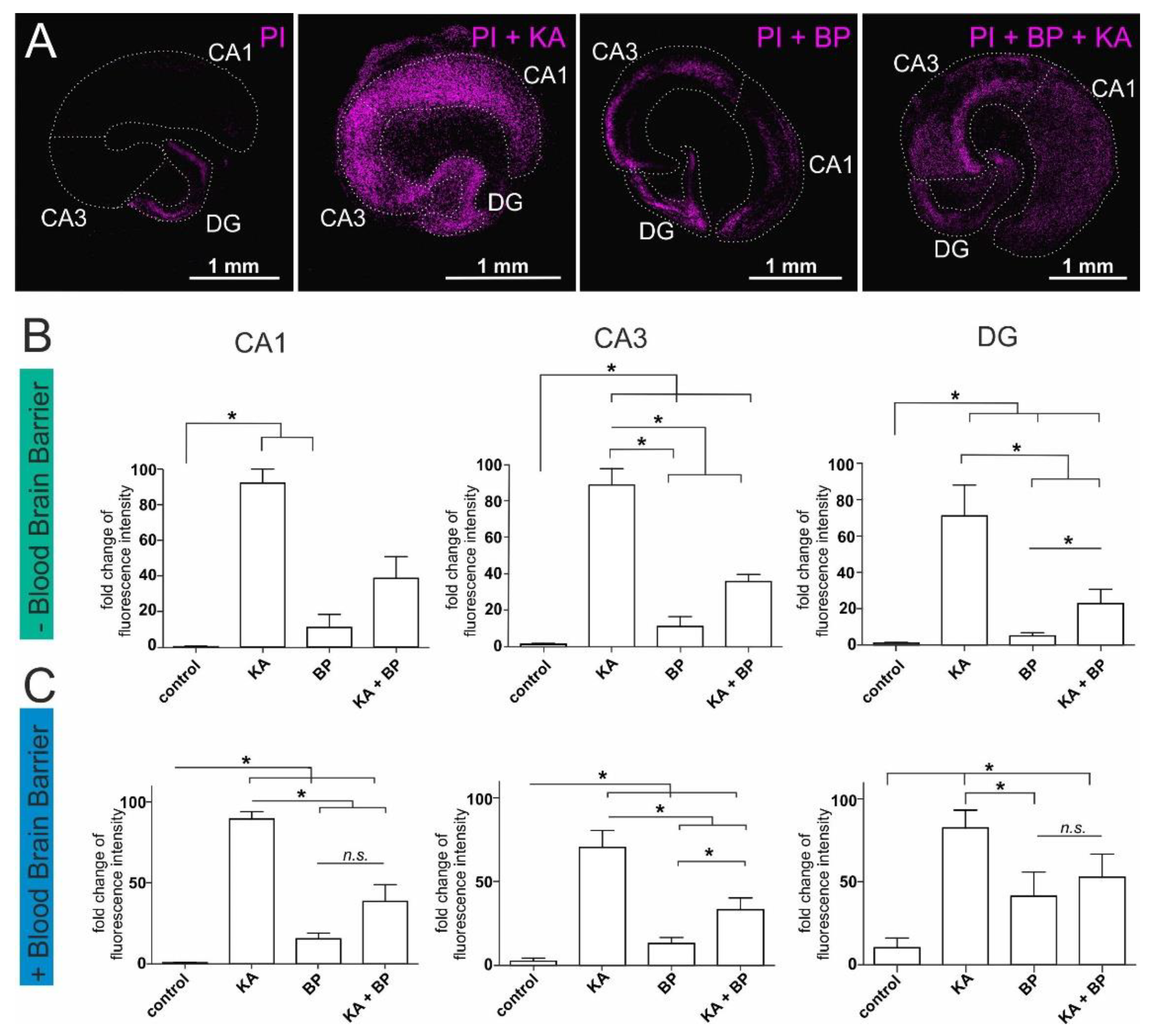
2.1. Human Plasma Protects Ex-Vivo Cultured Mouse Hippocampal Slices from Excitatory Stress
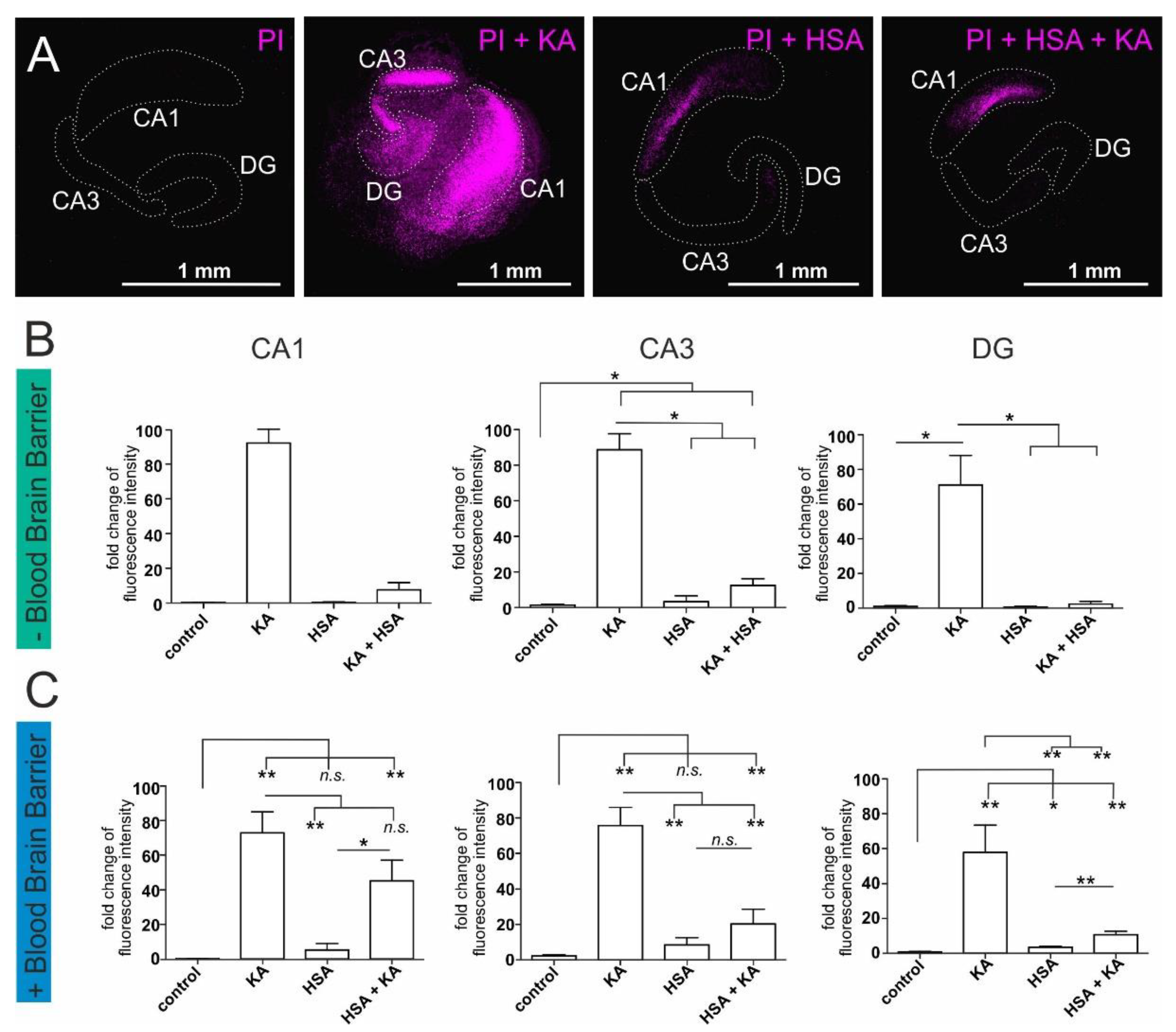
2.2. Human Serum Albumin Has Neuroprotective Effects on Hippocampal Slice Cultures
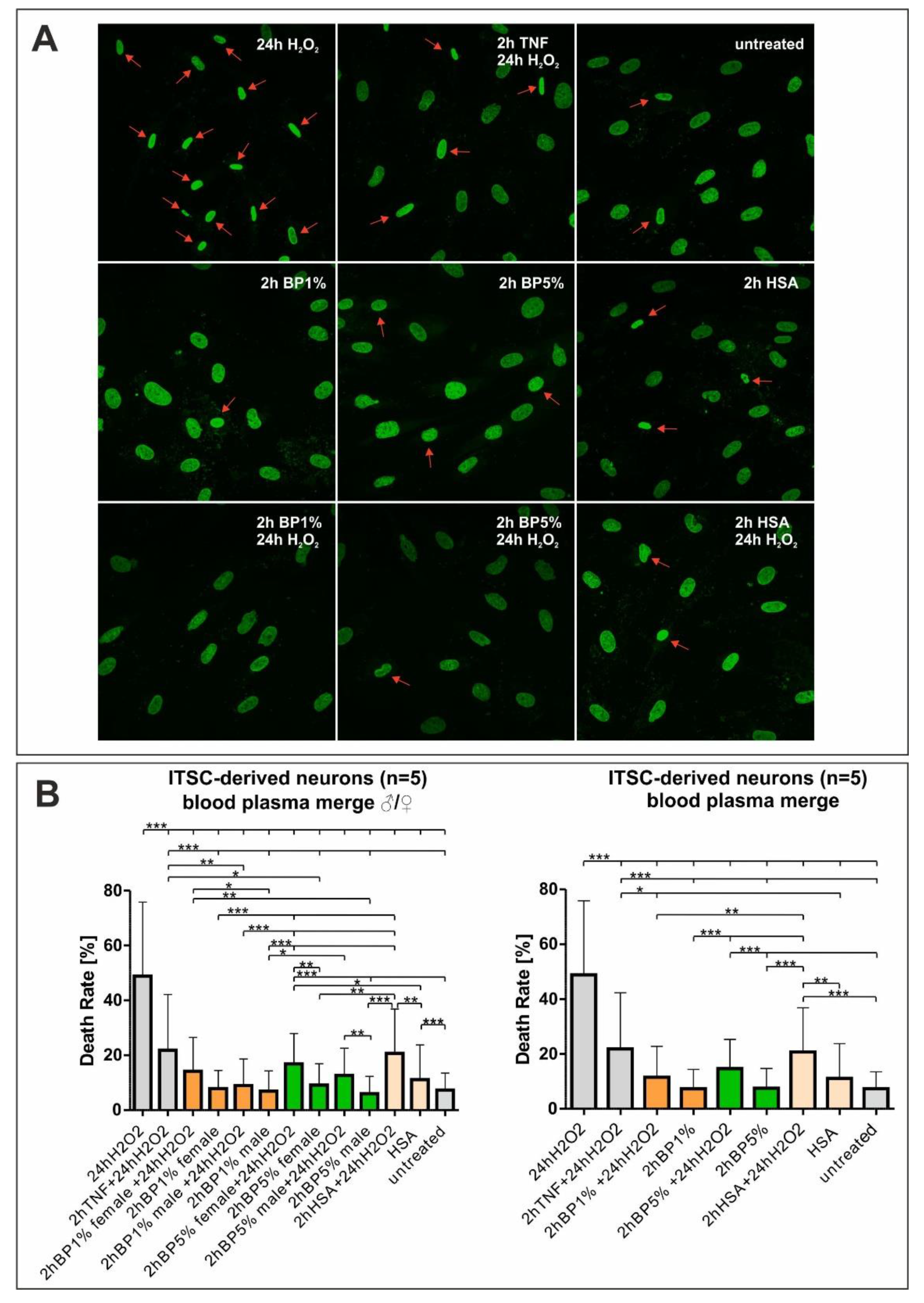
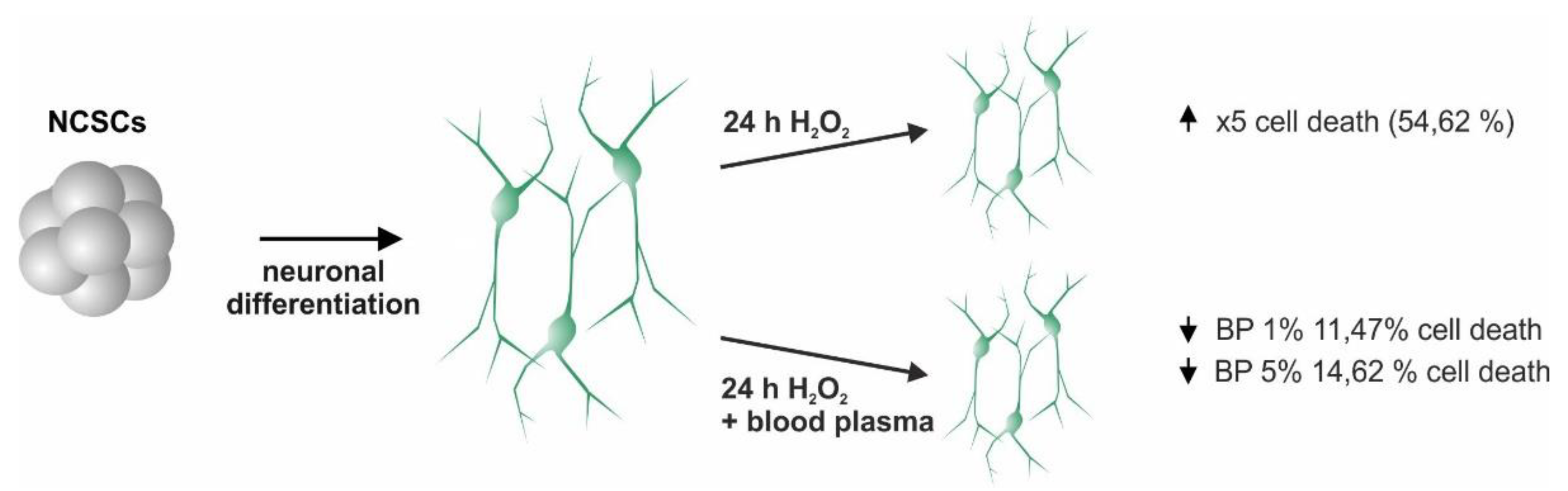
2.3. Plasma Mediates Neuroprotection against Oxidative Stress in ITSC-Derived Human Glutamatergic Neurons
3. Discussion
4. Materials and Methods
4.1. Isolation and Cultivation of Human ITSCs
4.2. Glutamatergic Differentiation of Human NCSCs
4.3. Neuronal Treatments
4.4. Isolation and Culture of Mouse Endothelial Cells
4.5. Ex-Vivo Culture of Mouse Hippocampal Slices
4.6. Staining of Human Nuclei
4.7. Image Analyses and Quantification
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawas, C.H.; Kim, R.C.; Sonnen, J.A.; Bullain, S.S.; Trieu, T.; Corrada, M.M. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology 2015, 85, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Checkoway, H.; Lundin, J.I.; Kelada, S.N. Neurodegenerative Diseases. IARC Sci. Publ. 2011, 163, 407–419. [Google Scholar]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nat. Cell Biol. 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.; Mosher, K.; Abbey, R.J.; McBride, A.A.; James, M.L.; Berdnik, D.; Shen, J.C.; Zou, B.; Xie, X.S.; Tingle, M.; et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nat. Cell Biol. 2017, 544, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Plambeck, K.; Middeldorp, J.; Castellano, J.; Mosher, K.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, S.; Widera, D.; Qunneis, F.; Müller, J.; Zander, C.; Greiner, J.; Strauss, C.; Lüningschrör, P.; Heimann, P.; Schwarze, H.; et al. Isolation of Novel Multipotent Neural Crest-Derived Stem Cells from Adult Human Inferior Turbinate. Stem Cells Dev. 2012, 21, 742–756. [Google Scholar] [CrossRef]
- Ruiz-Perera, L.M.; Schneider, L.; Windmöller, B.; Müller, J.; Greiner, J.F.W.; Kaltschmidt, C.; Kaltschmidt, B. NF-κB p65 directs sex-specific neuroprotection in human neurons. Sci. Rep. 2018, 8, 16012. [Google Scholar] [CrossRef]
- Baierle, M.; Nascimento, S.N.; Moro, A.M.; Brucker, N.; Freitas, F.; Gauer, B.; Durgante, J.; Bordignon, S.; Zibetti, M.R.; Trentini, C.M.; et al. Relationship between Inflammation and Oxidative Stress and Cognitive Decline in the Institutionalized Elderly. Oxidative Med. Cell. Longev. 2015, 2015, 804198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Gutterridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pr. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Montine, T.J.; Neely, M.; Quinn, J.; Beal, M.; Markesbery, W.R.; Roberts, L.; Morrow, J.D. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic. Biol. Med. 2002, 33, 620–626. [Google Scholar] [CrossRef]
- Doble, A. The Role of Excitotoxicity in Neurodegenerative Disease: Implications for Therapy. Pharmacol. Ther. 1999, 81, 163–221. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Stadelmann, C. Mechanisms of cell death in neurodegenerative disorders. Adv. Dement. Res. 2000, 59, 95–114. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, S.; Simonyi, A.; Sun, G.Y.; Sun, A.Y. Kainic Acid-Mediated Excitotoxicity as a Model for Neurodegeneration. Mol. Neurobiol. 2005, 31, 3–16. [Google Scholar] [CrossRef]
- Bleakman, D.; Lodge, D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology 1998, 37, 1187–1204. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Al-Dalain, S.M.; Castillo, R.; Martínez, G.; Fernández, O.S.L. Selective vulnerability to kainate-induced oxidative damage in different rat brain regions. J. Appl. Toxicol. 2001, 21, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Sun, A.Y. Oxidative mechanisms involved in kainate-induced cytotoxicity in cortical neurons. Neurochem. Res. 1994, 19, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Y.; Cheng, Y.; Sun, G.Y. Chapter 23: Kainic acid-induced excitotoxicity in neurons and glial cells. Prog. Brain Res. 1992, 94, 271–280. [Google Scholar] [CrossRef]
- Grooms, S.Y.; Opitz, T.; Bennett, M.V.L.; Zukin, R.S. Status epilepticus decreases glutamate receptor 2 mRNA and protein expression in hippocampal pyramidal cells before neuronal death. Proc. Natl. Acad. Sci. USA 2000, 97, 3631–3636. [Google Scholar] [CrossRef]
- Albers, D.S.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. Adv. Dement. Res. 2000, 59, 133–154. [Google Scholar] [CrossRef]
- Michaelis, E.K. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog. Neurobiol. 1998, 54, 369–415. [Google Scholar] [CrossRef]
- Milatovic, D.; Gupta, R.C.; Dettbarn, W.-D. Involvement of nitric oxide in kainic acid-induced excitotoxicity in rat brain. Brain Res. 2002, 957, 330–337. [Google Scholar] [CrossRef]
- Weiss, J.H.; Sensi, S.L. Ca2+–Zn2+ permeable AMPA or kainate receptors: Possible key factors in selective neurodegeneration. Trends Neurosci. 2000, 23, 365–371. [Google Scholar] [CrossRef]
- Ke, C.; Sun, L.; Qiao, D.; Wang, D.; Zeng, X. Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem. Toxicol. 2011, 49, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.W.; Gilchrist, C.; Fehlings, M.G. High molecular weight hyaluronan reduces lipopolysaccharide mediated microglial activation. J. Neurochem. 2012, 122, 344–355. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Leinonen, J.S.; Ahonen, J.-P.; Lonnrot, K.; Jehkonen, M.; Dastidar, P.; Molnár, G.; Alho, H. Low Plasma Antioxidant Activity Is Associated with High Lesion Volume and Neurological Impairment in Stroke. Stroke 2000, 31, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Sapojnikova, N.; Asatiani, N.; Kartvelishvili, T.; Kalandadze, I.; Tsiskaridze, I.K.A.A. Plasma Antioxidant Activity as a Marker for a Favourable Outcome in Acute Ischemic Stroke. Antioxid. Enzym. 2012. [Google Scholar] [CrossRef] [Green Version]
- Imam, A.; Jin, G.; Sillesen, M.; Dekker, S.E.; Bambakidis, T.; Hwabejire, J.O.; Jepsen, C.H.; Halaweish, I.; Alam, H.B. Fresh Frozen Plasma Resuscitation Provides Neuroprotection Compared to Normal Saline in a Large Animal Model of Traumatic Brain Injury and Polytrauma. J. Neurotrauma 2015, 32, 307–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, G.; Demoya, M.A.; Duggan, M.; Knightly, T.; Mejaddam, A.Y.; Hwabejire, J.; Lu, J.; Smith, W.M.; Kasotakis, G.; Velmahos, G.C.; et al. Traumatic Brain Injury and Hemorrhagic Shock: Evaluation of Different Resuscitation Strategies in a Large Animal Model of Combined Insults. Shock 2012, 38, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Ossig, C.; Greiner, J.; Hauser, S.; Fauser, M.; Widera, D.; Kaltschmidt, C.; Storch, A.; Kaltschmidt, B. Intrastriatal Transplantation of Adult Human Neural Crest-Derived Stem Cells Improves Functional Outcome in Parkinsonian Rats. STEM CELLS Transl. Med. 2015, 4, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Honoki, K. Preventing aging with stem cell rejuvenation: Feasible or infeasible? World J. Stem Cells 2017, 9, 1–8. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Höving, A.L.; Schmidt, K.E.; Merten, M.; Hamidi, J.; Rott, A.-K.; Faust, I.; Greiner, J.F.W.; Gummert, J.; Kaltschmidt, B.; Kaltschmidt, C.; et al. Blood Serum Stimulates p38-mediated Proliferation and Changes in Global Gene Expression of Adult Human Cardiac Stem Cells. Cells 2020, 9, 1472. [Google Scholar] [CrossRef]
- Teselkin, I.O.; Babenkova, I.V.; Liubitskiĭ, O.B.; Klebanov, G.I.; Vladimirov, I.A. The measuring of blood plasma antioxidant activity by the hemoglobin-hydrogen peroxide-luminol system. Vopr. Meditsinskoi Khimii 1998, 44, 70–76. [Google Scholar]
- Rusina, E.; Bernard, C.; Williamson, A. The Kainic Acid Models of Temporal Lobe Epilepsy. eNeuro 2021, 8. [Google Scholar] [CrossRef]
- Henning, L.; Steinhäuser, C.; Bedner, P. Initiation of Experimental Temporal Lobe Epilepsy by Early Astrocyte Uncoupling Is Independent of TGFβR1/ALK5 Signaling. Front. Neurol. 2021, 12, 660591. [Google Scholar] [CrossRef]
- Muzio, L.; Sirtori, R.; Gornati, D.; Eleuteri, S.; Fossaghi, A.; Brancaccio, D.; Manzoni, L.; Ottoboni, L.; De Feo, L.; Quattrini, A.; et al. Retromer stabilization results in neuroprotection in a model of Amyotrophic Lateral Sclerosis. Nat. Commun. 2020, 11, 3848. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, Z.; Wang, F.; Han, Z.; Huang, S.; Hu, T.; Guo, M.; Lei, P. Hydrogen exerts neuroprotection by activation of the miR-21/PI3K/AKT/GSK-3β pathway in an in vitro model of traumatic brain injury. J. Cell. Mol. Med. 2020, 24, 4061–4071. [Google Scholar] [CrossRef] [Green Version]
- Cha, M.-K.; Kim, I.-H. Glutathione-Linked Thiol Peroxidase Activity of Human Serum Albumin: A Possible Antioxidant Role of Serum Albumin in Blood Plasma. Biochem. Biophys. Res. Commun. 1996, 222, 619–625. [Google Scholar] [CrossRef]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Sarnatskaya, V.V.; Yushko, L.A.; Sakhno, L.A.; Nikolaev, V.; Nikolaev, A.V.; Grinenko, D.V.; Mikhalovsky, S. New Approaches to the Removal of Protein-Bound Toxins from Blood Plasma of Uremic Patients. Artif. Cells Blood Substit. Biotechnol. 2007, 35, 287–308. [Google Scholar] [CrossRef]
- Chen, X.; Rivard, L.; Naqvi, S.; Nakada, S.; Padbury, J.; Sanchez-Esteban, J.; Stopa, E.; Lim, Y.-P.; Stonestreet, B. Expression and localization of Inter-alpha Inhibitors in rodent brain. Neuroscience 2016, 324, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Nakada, S.; Donahue, J.E.; Chen, R.H.; Tucker, R.; Qiu, J.; Lim, Y.-P.; Stopa, E.G.; Stonestreet, B.S. Neuroprotective effects of inter-alpha inhibitor proteins after hypoxic-ischemic brain injury in neonatal rats. Exp. Neurol. 2019, 317, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Perlman, R.L. Mouse Models of Human Disease: An Evolutionary Perspective. Evol. Med. Public Health 2016, 2016, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Greiner, J.F.; Merten, M.; Kaltschmidt, C.; Kaltschmidt, B. Sexual dimorphisms in adult human neural, mesoderm-derived, and neural crest-derived stem cells. FEBS Lett. 2019, 593, 3338–3352. [Google Scholar] [CrossRef] [Green Version]
- Höving, A.L.; Sielemann, K.; Greiner, J.F.W.; Kaltschmidt, B.; Knabbe, C.; Kaltschmidt, C. Transcriptome Analysis Reveals High Similarities between Adult Human Cardiac Stem Cells and Neural Crest-Derived Stem Cells. Biology 2020, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Höving, A.L.; Windmöller, B.A.; Knabbe, C.; Kaltschmidt, B.; Kaltschmidt, C.; Greiner, J.F.W. Between Fate Choice and Self-Renewal—Heterogeneity of Adult Neural Crest-Derived Stem Cells. Front. Cell Dev. Biol. 2021, 9, 662754. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Liu, Y.; Zhao, W.; Busto, R.; Ginsberg, M.D. Human Albumin Therapy of Acute Ischemic Stroke: Marked Neuroprotective Efficacy at Moderate Doses and with a Broad Therapeutic Window. Stroke 2001, 32, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, R.H.; Yeatts, S.D.; Hill, M.; Moy, C.S.; Ginsberg, M.D.; Palesch, Y.Y. ALIAS Parts 1 and 2 and NETT Investigators. ALIAS (Albumin in Acute Ischemic Stroke) Trials: Analysis of the Combined Data from Parts 1 and 2. Stroke 2016, 47, 2355–2359. [Google Scholar] [CrossRef] [Green Version]
- Suarez, J.I.; Martin, R.H.; Calvillo, E.; Bershad, E.M.; Rao, C.P.V. Effect of Human Albumin on TCD Vasospasm, DCI, and Cerebral Infarction in Subarachnoid Hemorrhage: The ALISAH Study. Neurosurg. Med. Ethics 2015, 120, 287–290. [Google Scholar] [CrossRef]
- Macdonald, R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014, 10, 44–58. [Google Scholar] [CrossRef]
- SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health; Myburgh, J.; Cooper, D.J.; Finfer, S.; Bellomo, R.; Norton, R.; Bishop, N.; et al. Saline or Albumin for Fluid Resuscitation in Patients with Traumatic Brain Injury. N. Engl. J. Med. 2007, 357, 874–884. [Google Scholar] [CrossRef] [Green Version]
- Greiner, J.; Widera, D.; Müller, J.; Qunneis, F.; Zander, C.; Martin, I.; Mallah, J.; Schuetzmann, D.; Prante, C.; Schwarze, H.; et al. Efficient animal-serum free 3D cultivation method for adult human neural crest-derived stem cell therapeutics. Eur. Cells Mater. 2011, 22, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Duport, S.; Robert, F.; Muller, D.; Grau, G.; Parisi, L.; Stoppini, L. An in vitro blood-brain barrier model: Cocultures between endothelial cells and organotypic brain slice cultures. Proc. Natl. Acad. Sci. USA 1998, 95, 1840–1845. [Google Scholar] [CrossRef] [Green Version]
- De Simoni, A.; Yu, L.M.Y. Preparation of organotypic hippocampal slice cultures: Interface method. Nat. Protoc. 2006, 1, 1439–1445. [Google Scholar] [CrossRef]

| Donor | Sex | Age |
|---|---|---|
| K243 | ♂ | 53 |
| K248 | ♂ | 45 |
| K246 | ♂ | 26 |
| K325 | ♀ | 68 |
| K395 | ♀ | 61 |
| Donor | Sex |
|---|---|
| Ym12 | ♂ |
| Ym15 | ♂ |
| Ym17 | ♂ |
| Yf8 | ♀ |
| Yf7 | ♀ |
| Yf2 | ♀ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Perera, L.M.; Höving, A.L.; Schmidt, K.E.; Cenan, S.; Wohllebe, M.; Greiner, J.F.W.; Kaltschmidt, C.; Simon, M.; Knabbe, C.; Kaltschmidt, B. Neuroprotection Mediated by Human Blood Plasma in Mouse Hippocampal Slice Cultures and in Oxidatively Stressed Human Neurons. Int. J. Mol. Sci. 2021, 22, 9567. https://doi.org/10.3390/ijms22179567
Ruiz-Perera LM, Höving AL, Schmidt KE, Cenan S, Wohllebe M, Greiner JFW, Kaltschmidt C, Simon M, Knabbe C, Kaltschmidt B. Neuroprotection Mediated by Human Blood Plasma in Mouse Hippocampal Slice Cultures and in Oxidatively Stressed Human Neurons. International Journal of Molecular Sciences. 2021; 22(17):9567. https://doi.org/10.3390/ijms22179567
Chicago/Turabian StyleRuiz-Perera, Lucia M., Anna L. Höving, Kazuko E. Schmidt, Sule Cenan, Max Wohllebe, Johannes F. W. Greiner, Christian Kaltschmidt, Matthias Simon, Cornelius Knabbe, and Barbara Kaltschmidt. 2021. "Neuroprotection Mediated by Human Blood Plasma in Mouse Hippocampal Slice Cultures and in Oxidatively Stressed Human Neurons" International Journal of Molecular Sciences 22, no. 17: 9567. https://doi.org/10.3390/ijms22179567
APA StyleRuiz-Perera, L. M., Höving, A. L., Schmidt, K. E., Cenan, S., Wohllebe, M., Greiner, J. F. W., Kaltschmidt, C., Simon, M., Knabbe, C., & Kaltschmidt, B. (2021). Neuroprotection Mediated by Human Blood Plasma in Mouse Hippocampal Slice Cultures and in Oxidatively Stressed Human Neurons. International Journal of Molecular Sciences, 22(17), 9567. https://doi.org/10.3390/ijms22179567








