Novel Approaches Used to Examine and Control Neurogenesis in Parkinson′s Disease
Abstract
1. Introduction: Neurogenesis in the Healthy and Parkinson’s Disease-Affected Brains
1.1. Key Characteristics of Adult Neurogenesis
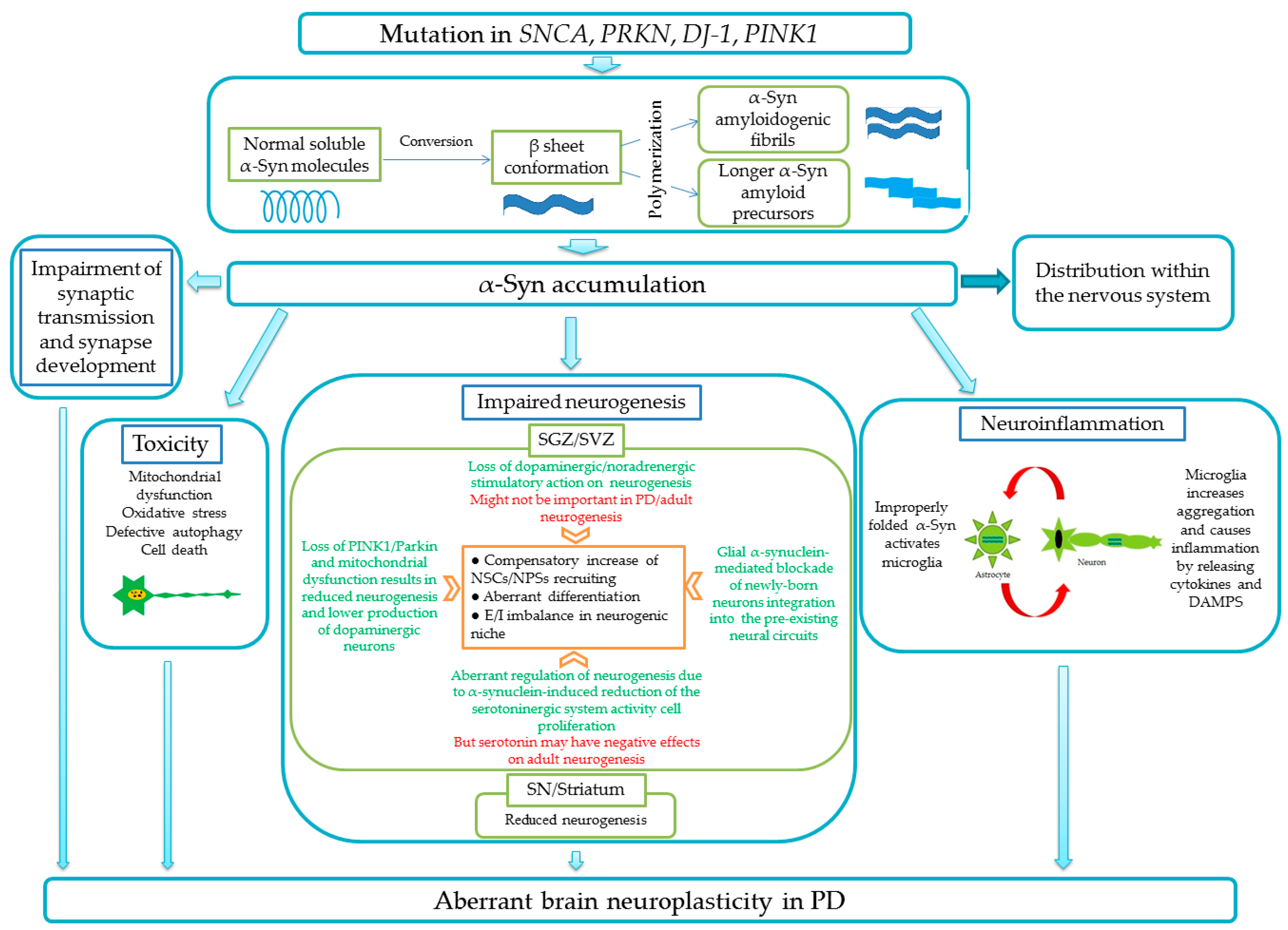
1.2. Aberrant Neurogenesis in Parkinson′s Disease
2. iPSC-Based Platform for Studying PD-Affected Neurogenesis In Vitro
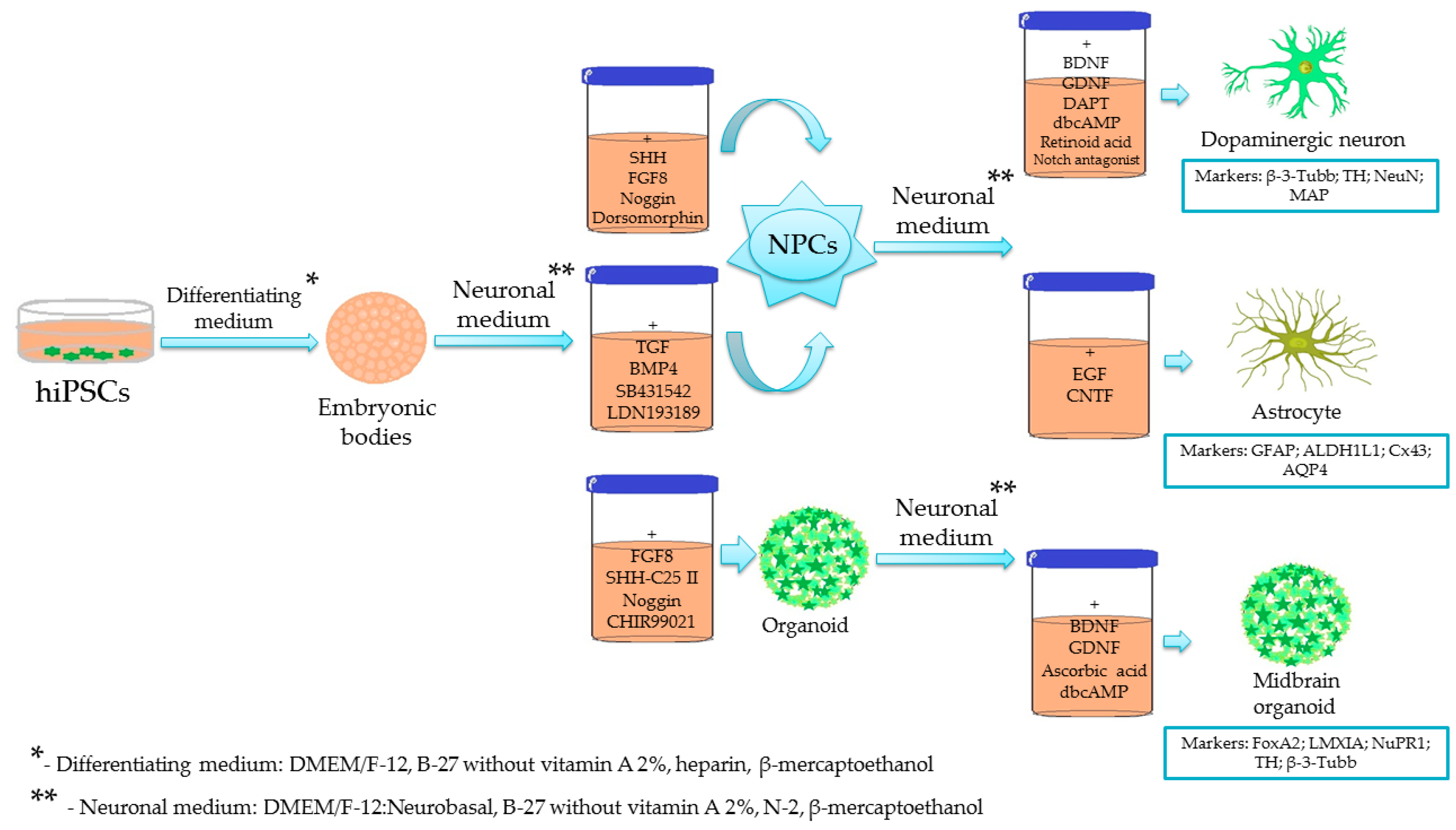
2.1. Generation of iPSC-Derived Dopaminergic Neurons
2.2. Generation of iPSC-Derived Midbrain Astrocytes
2.3. Generation of iPSC-Derived Midbrain Cerebral Organoids
3. Adult Neurogenesis as a Target for Therapy and Optogenetic Control
4. Optogenetic Activation of Niche Astrocytes for the Control of Cell Development in PD
4.1. Astrocytes as Potent Regulators of Neurogenesis and Parkinson’s Type Neurodegeneration
4.2. Optogenetic Targeting of GFAP+ Cells in the Neurogenic Niche: Established and Prospective Approaches to Cells Activation and Signal Propagation
5. Alternative Approaches to Restoring Impaired Neurogenesis in PD
6. Conclusion and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AV | adeno-associated virus |
| ALDH1L1 | aldehyde dehydrogenase 1 family member L1 |
| AMP | adenosine monophosphate |
| ADP | adenosine diphosphate |
| AQ4 | aquaporin 4 |
| ASCL1 | Achaete-Scute homolog 1 |
| ATP | adenosine triphosphate |
| BBB | blood-brain barrier |
| BDNF | brain-derived neurotrophic factor |
| BMECs | brain microvessel endothelial cells |
| BMP4 | bone morphogenetic protein 4 |
| CaMKII | calcium/calmodulin-dependent kinase II |
| CD | cluster of differentiation |
| ChR2 | channelrhodopsin 2 |
| CNTF | ciliary neurotrophic factor |
| CRISPR-Cas9 | clustered regularly interspaced short palindromic repeats-associated protein 9 |
| Cx | connexin |
| 3D | three-dimensional |
| DAMPs | damage-associated molecular patterns |
| DJ-1 | deglycase-1 |
| EB | embryonic body |
| EGF | epidermal growth factor |
| E/I | excitation/inhibition balance |
| FBS | fetal bovine serum |
| FCS | fetal calf serum |
| FGF | fibroblast growth factor |
| Foxa2 | forkhead box protein A2 |
| GABA | gamma-aminobutyric acid |
| GDNF | glia cell line-derived neurotrophic factor |
| GFAP | glial fibrillary acidic protein |
| GPCRs | G-protein-coupled receptors |
| GSK3 | glycogen synthase kinase 3 |
| HDAC | histone deacetylase |
| IGF | insulin-like growth factor |
| IL | interleukin |
| iPSCs | induced pluripotent stem cells |
| LIF | leukemia inhibitory factor |
| LiGluR | light-gated glutamate receptor 6 |
| Lmx1a | LIM homeobox transcription factor 1 alpha |
| LRKK2 | leucine-rich repeat kinase 2 |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| mRNA | messenger ribonucleic acid |
| NAD+ | nicotinamide adenine dinucleotide |
| NeuroD1 | neurogenic differentiation 1 transcription factor |
| Neurog2 | neurogenin 2 |
| NFIA | nuclear factor I A |
| NFIB | nuclear factor I B |
| NMDA | N-methyl-D-aspartate |
| NPCs | neural progenitor cells |
| NSCs | neural stem cells |
| Nurr1 | nuclear receptor related 1 |
| 6-OHDA | 6-hydroxydopamine |
| optoFGFR | light-activatable fibroblast growth factor receptor |
| optoGap | light-activatable gap junction |
| optoGPCR | light-activatable G-protein coupled receptor |
| PARK2 | parkin ubiquitin protein ligase |
| Pax6 | transcription factor paired box protein |
| PCNA | proliferating cell nuclear antigen |
| PD | Parkinson’s disease |
| PDGF-BB | platelet-derived growth factor |
| PINK-1 | PTEN (phosphatase and tensin homolog deleted)-induced kinase 1 |
| PRKN | parkin |
| RGCs | radial glia cells |
| PTBP1 | RNA-binding protein PTB |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| S100β | S100 calcium-binding protein B |
| SGZ | subgranular zone |
| SHH | sonic hedgehog |
| smNPCs | small molecule-treated neural progenitor cells |
| SN | substantia nigra |
| SNCA | synuclein |
| Sox6 | SRY-Box transcription factor 6 |
| Sox9 | SRY-Box transcription factor 9 |
| SVZ | subventricular zone |
| SYN1 | synapsin 1 |
| TGFβ | transforming growth factor-beta |
| TRP | transient receptor potential channel |
| VOCC | voltage-operated calcium channel |
| Wnt | Wingless/Int-1 |
References
- Saxe, M.D.; Battaglia, F.; Wang, J.-W.; Malleret, G.; David, D.J.; Monckton, J.E.; Garcia, A.D.R.; Sofroniew, M.V.; Kandel, E.R.; Santarelli, L.; et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. USA 2006, 103, 17501–17506. [Google Scholar] [CrossRef]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caixeta, A.R.; Guarnieri, L.O.; Medeiros, D.C.; Mendes, E.; Ladeira, L.C.D.; Pereira, M.T.; Moraes, M.F.D.; Pereira, G.S. Inhibiting constitutive neurogenesis compromises long-term social recognition memory. Neurobiol. Learn. Mem. 2018, 155, 92–103. [Google Scholar] [CrossRef]
- Petsophonsakul, P.; Richetin, K.; Andraini, T.; Roybon, L.; Rampon, C. Memory formation orchestrates the wiring of adult-born hippocampal neurons into brain circuits. Brain Struct. Funct. 2017, 222, 2585–2601. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, O.L.; Malinovskaya, N.A.; Komleva, Y.K.; Gorina, Y.V.; Shuvaev, A.N.; Olovyannikova, R.Y.; Belozor, O.S.; Belova, O.A.; Higashida, H.; Salmina, A.B. Excitation/inhibition imbalance and impaired neurogenesis in neurodevelopmental and neurodegenerative disorders. Rev. Neurosci. 2019, 30, 807–820. [Google Scholar] [CrossRef] [PubMed]
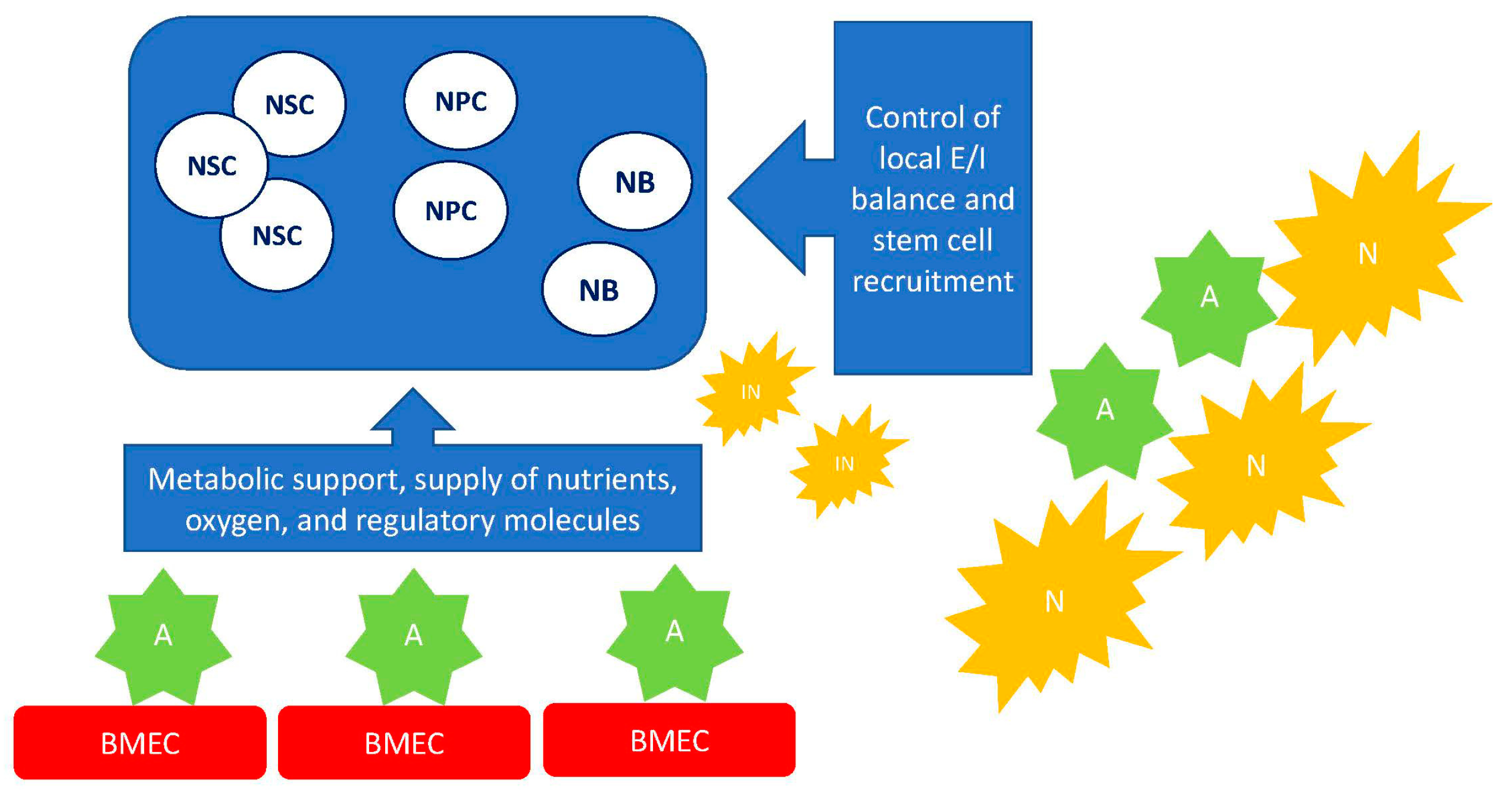
- Pozhilenkova, E.A.; Lopatina, O.L.; Komleva, Y.K.; Salmin, V.V.; Salmina, A.B. Blood-brain barrier-supported neurogenesis in healthy and diseased brain. Rev. Neurosci. 2017, 28, 397–415. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Xi, G.; Zhou, X.; Pan, S.; Ying, Q.-L. Long-term self-renewal of naïve neural stem cells in a defined condition. Biochim. Biophys. Acta (BBA)-Bioenerg. 2019, 1866, 971–977. [Google Scholar] [CrossRef]
- Alexanian, A.R.; Kurpad, S.N. Quiescent neural cells regain multipotent stem cell characteristics influenced by adult neural stem cells in co-culture. Exp. Neurol. 2005, 191, 193–197. [Google Scholar] [CrossRef]
- Semënov, M.V. Adult Hippocampal Neurogenesis Is a Developmental Process Involved in Cognitive Development. Front. Neurosci. 2019, 13, 159. [Google Scholar] [CrossRef]
- Santilli, G.; Lamorte, G.; Carlessi, L.; Ferrari, D.; Rota Nodari, L.; Binda, E.; Delia, D.; Vescovi, A.L.; De Filippis, L. Mild Hypoxia Enhances Proliferation and Multipotency of Human Neural Stem Cells. PLoS ONE 2010, 5, e8575. [Google Scholar] [CrossRef]
- Cipriani, S.; Ferrer, I.; Aronica, E.; Kovacs, G.G.; Verney, C.; Nardelli, J.; Khung, S.; Delezoide, A.-L.; Milenkovic, I.; Rasika, S.; et al. Hippocampal Radial Glial Subtypes and Their Neurogenic Potential in Human Fetuses and Healthy and Alzheimer’s Disease Adults. Cereb. Cortex 2018, 28, 2458–2478. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Saitoh, Y.; Takashima, N.; Murayama, A.; Niibori, Y.; Ageta, H.; Sekiguchi, M.; Sugiyama, H.; Inokuchi, K. Adult Neurogenesis Modulates the Hippocampus-Dependent Period of Associative Fear Memory. Cell 2009, 139, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Xia, F.; Guskjolen, A.J.; Ramsaran, A.I.; Santoro, A.; Josselyn, S.A.; Frankland, P.W. Elevation of Hippocampal Neurogenesis Induces a Temporally Graded Pattern of Forgetting of Contextual Fear Memories. J. Neurosci. 2018, 38, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Nakashiba, T.; Cushman, J.D.; Pelkey, K.A.; Renaudineau, S.; Buhl, D.L.; McHugh, T.J.; Rodriguez Barrera, V.; Chittajallu, R.; Iwamoto, K.S.; McBain, C.J.; et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 2012, 149, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Kim, Y.; Jeong, H.J.; Kang, J.S.; Lee, S.H.; Ho, W.K. Impaired pattern separation in Tg2576 mice is associated with hyperexcitable dentate gyrus caused by Kv4.1 downregulation. Mol. Brain 2021, 14, 62. [Google Scholar] [CrossRef]
- Shulman, J.M.; Jager, P.L.D.; Feany, M.B. Parkinson’s Disease: Genetics and Pathogenesis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 193–222. [Google Scholar] [CrossRef]
- Novosadova, E.V.; Nenasheva, V.V.; Makarova, I.V.; Dolotov, O.V.; Inozemtseva, L.S.; Arsenyeva, E.L.; Chernyshenko, S.V.; Sultanov, R.I.; Illarioshkin, S.N.; Grivennikov, I.A.; et al. Parkinson’s Disease-Associated Changes in the Expression of Neurotrophic Factors and their Receptors upon Neuronal Differentiation of Human Induced Pluripotent Stem Cells. J. Mol. Neurosci. 2020, 70, 514–521. [Google Scholar] [CrossRef]
- Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Urazgildeeva, G.R.; Abaimov, D.A.; Fedotova, E.Y.; Poleschuk, V.V.; Illarioshkin, S.N.; Lokhov, P.G. Parkinson’s Disease: Available Clinical and Promising Omics Tests for Diagnostics, Disease Risk Assessment, and Pharmacotherapy Personalization. Diagnostics 2020, 10, 339. [Google Scholar] [CrossRef]
- Winner, B.; Lie, D.C.; Rockenstein, E.; Aigner, R.; Aigner, L.; Masliah, E.; Kuhn, H.G.; Winkler, J. Human Wild-Type α-Synuclein Impairs Neurogenesis. J. Neuropathol. Exp. Neurol. 2004, 63, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. What does it mean to be natively unfolded? Eur. J. Biochem. 2002, 269, 2–12. [Google Scholar] [CrossRef]
- Burré, J. The Synaptic Function of α-Synuclein. J. Park. Dis. 2015, 5, 699–713. [Google Scholar] [CrossRef]
- Fortin, D.L.; Nemani, V.M.; Voglmaier, S.M.; Anthony, M.D.; Ryan, T.A.; Edwards, R.H. Neural activity controls the synaptic accumulation of alpha-synuclein. J. Neurosci. 2005, 25, 10913–10921. [Google Scholar] [CrossRef]
- Guhathakurta, S.; Bok, E.; Evangelista, B.A.; Kim, Y.-S. Deregulation of α-synuclein in Parkinson’s disease: Insight from epigenetic structure and transcriptional regulation of SNCA. Prog. Neurobiol. 2017, 154, 21–36. [Google Scholar] [CrossRef]
- Hope, A.D.; Myhre, R.; Kachergus, J.; Lincoln, S.; Bisceglio, G.; Hulihan, M.; Farrer, M.J. α-Synuclein missense and multiplication mutations in autosomal dominant Parkinson’s disease. Neurosci. Lett. 2004, 367, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.B.; Kim, S.Y.; Kim, J.Y.; Park, S.S.; Lee, D.S.; Min, H.J.; Kim, Y.K.; Kim, S.E.; Kim, J.M.; Kim, H.J.; et al. alpha-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology 2008, 70, 43–49. [Google Scholar] [CrossRef]
- Baena-Montes, J.M.; Avazzadeh, S.; Quinlan, L.R. α-synuclein pathogenesis in hiPSC models of Parkinson’s disease. Neuronal Signal. 2021, 5, NS20210021. [Google Scholar] [CrossRef]
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. Potential Pathways of Abnormal Tau and α-Synuclein Dissemination in Sporadic Alzheimer’s and Parkinson’s Diseases. Cold Spring Harb. Perspect. Biol. 2016, 8, a023630. [Google Scholar] [CrossRef] [PubMed]
- Okuzumi, A.; Kurosawa, M.; Hatano, T.; Takanashi, M.; Nojiri, S.; Fukuhara, T.; Yamanaka, T.; Miyazaki, H.; Yoshinaga, S.; Furukawa, Y.; et al. Rapid dissemination of alpha-synuclein seeds through neural circuits in an in-vivo prion-like seeding experiment. Acta Neuropathol. Commun. 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.F.; Sackmann, C.; Hoffmann, A.; Svenningsson, P.; Winkler, J.; Ingelsson, M.; Hallbeck, M. Binding of α-synuclein oligomers to Cx32 facilitates protein uptake and transfer in neurons and oligodendrocytes. Acta Neuropathol. 2019, 138, 23–47. [Google Scholar] [CrossRef]
- Salkov, V.N.; Voronkov, D.V.; Khacheva, K.K.; Fedotova, E.Y.; Khudoerkov, R.M.; Illarioshkin, S.N. Clinical and morphological analysis of a caseof Parkinson’s disease. Arch. Patol. 2020, 82, 52–56. [Google Scholar] [CrossRef]
- Schaser, A.J.; Stackhouse, T.L.; Weston, L.J.; Kerstein, P.C.; Osterberg, V.R.; López, C.S.; Dickson, D.W.; Luk, K.C.; Meshul, C.K.; Woltjer, R.L.; et al. Trans-synaptic and retrograde axonal spread of Lewy pathology following pre-formed fibril injection in an in vivo A53T alpha-synuclein mouse model of synucleinopathy. Acta Neuropathol. Commun. 2020, 8, 150. [Google Scholar] [CrossRef]
- Stavrovskaya, A.V.; Voronkov, D.N.; Kutukova, K.A.; Ivanov, M.V.; Gushchina, A.S.; Illarioshkin, S.N. Paraquat-induced model of Parkinson’s disease and detection of phosphorylated A-synuclein in the enteric nervous system of rats. Vestn. RGMU 2019, 6, 63–69. [Google Scholar] [CrossRef]
- Sherstnev, V.V.; Kedrov, A.V.; Solov’eva, O.A.; Gruden’, M.A.; Konovalova, E.V.; Kalinin, I.A.; Proshin, A.T. The effects of α-synuclein oligomers on neurogenesis in the hippocampus and the behavior of aged mice. Neurochem. J. 2017, 11, 282–289. [Google Scholar] [CrossRef]
- Crews, L.; Mizuno, H.; Desplats, P.; Rockenstein, E.; Adame, A.; Patrick, C.; Winner, B.; Winkler, J.; Masliah, E. α-Synuclein Alters Notch-1 Expression and Neurogenesis in Mouse Embryonic Stem Cells and in the Hippocampus of Transgenic Mice. J. Neurosci. 2008, 28, 4250–4260. [Google Scholar] [CrossRef] [PubMed]
- Bender, H.; Fietz, S.A.; Richter, F.; Stanojlovic, M. Alpha-Synuclein Pathology Coincides with Increased Number of Early Stage Neural Progenitors in the Adult Hippocampus. Front. Cell Dev. Biol. 2021, 9, 691560. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Andersen, J.K. Mutant α-synuclein and aging reduce neurogenesis in the acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Aging Cell 2011, 10, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Farzanehfar, P. Towards a Better Treatment Option for Parkinson’s Disease: A Review of Adult Neurogenesis. Neurochem. Res. 2016, 41, 3161–3170. [Google Scholar] [CrossRef]
- Baker, S.A.; Baker, K.A.; Hagg, T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur. J. Neurosci. 2004, 20, 575–579. [Google Scholar] [CrossRef]
- Ermine, C.M.; Wright, J.L.; Frausin, S.; Kauhausen, J.A.; Parish, C.L.; Stanic, D.; Thompson, L.H. Modelling the dopamine and noradrenergic cell loss that occurs in Parkinson’s disease and the impact on hippocampal neurogenesis. Hippocampus 2018, 28, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.D.; Krüger-Gerlach, D.; Hermann, A.; Meyer, A.K.; Kim, K.-S.; Storch, A. Early Postnatal but Not Late Adult Neurogenesis Is Impaired in the Pitx3-Mutant Animal Model of Parkinson’s Disease. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Aponso, P.M.; Faull, R.L.; Connor, B. Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson’s disease. Neuroscience 2008, 151, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.A.; Hagg, T. Serotonin 1A receptor agonist increases species- and region-selective adult CNS proliferation, but not through CNTF. Neuropharmacology 2012, 63, 1238–1247. [Google Scholar] [CrossRef]
- Blum, R.; Lesch, K.-P. Parkinson’s disease, anxious depression and serotonin—Zooming in on hippocampal neurogenesis. J. Neurochem. 2015, 135, 441–444. [Google Scholar] [CrossRef]
- Song, N.-N.; Jia, Y.-F.; Zhang, L.; Zhang, Q.; Huang, Y.; Liu, X.-Z.; Hu, L.; Lan, W.; Chen, L.; Lesch, K.-P.; et al. Reducing central serotonin in adulthood promotes hippocampal neurogenesis. Sci. Rep. 2016, 6, 20338. [Google Scholar] [CrossRef]
- Brown, S.J.; Boussaad, I.; Jarazo, J.; Fitzgerald, J.C.; Antony, P.; Keatinge, M.; Blechman, J.; Schwamborn, J.C.; Krüger, R.; Placzek, M.; et al. PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons. Sci. Rep. 2021, 11, 6617. [Google Scholar] [CrossRef]
- Agnihotri, S.K.; Shen, R.; Li, J.; Gao, X.; Büeler, H. Loss of PINK1 leads to metabolic deficits in adult neural stem cells and impedes differentiation of newborn neurons in the mouse hippocampus. FASEB J. 2017, 31, 2839–2853. [Google Scholar] [CrossRef]
- Marxreiter, F.; Ettle, B.; May, V.E.L.; Esmer, H.; Patrick, C.; Kragh, C.L.; Klucken, J.; Winner, B.; Riess, O.; Winkler, J.; et al. Glial A30P alpha-synuclein pathology segregates neurogenesis from anxiety-related behavior in conditional transgenic mice. Neurobiol. Dis. 2013, 59, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Dziewczapolski, G.; Lie, D.C.; Ray, J.; Gage, F.H.; Shults, C.W. Survival and differentiation of adult rat-derived neural progenitor cells transplanted to the striatum of hemiparkinsonian rats. Exp. Neurol. 2003, 183, 653–664. [Google Scholar] [CrossRef]
- Tattersfield, A.S.; Croon, R.J.; Liu, Y.W.; Kells, A.P.; Faull, R.L.; Connor, B. Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington’s disease. Neuroscience 2004, 127, 319–332. [Google Scholar] [CrossRef]
- Kay, J.N.; Blum, M. Differential Response of Ventral Midbrain and Striatal Progenitor Cells to Lesions of the Nigrostriatal Dopaminergic Projection. Dev. Neurosci. 2000, 22, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Porritt, M.J.; Batchelor, P.E.; Hughes, A.J.; Kalnins, R.; Donnan, G.A.; Howells, D.W. New dopaminergic neurons in Parkinson’s disease striatum. Lancet 2000, 356, 44–45. [Google Scholar] [CrossRef]
- Shan, X.; Chi, L.; Bishop, M.; Luo, C.; Lien, L.; Zhang, Z.; Liu, R. Enhanced de novo neurogenesis and dopaminergic neurogenesis in the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease-like mice. Stem Cells 2006, 24, 1280–1287. [Google Scholar] [CrossRef]
- Fauser, M.; Pan-Montojo, F.; Richter, C.; Kahle, P.J.; Schwarz, S.C.; Schwarz, J.; Storch, A.; Hermann, A. Chronic-Progressive Dopaminergic Deficiency Does Not Induce Midbrain Neurogenesis. Cells 2021, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Kohl, Z.; Ben Abdallah, N.; Vogelgsang, J.; Tischer, L.; Deusser, J.; Amato, D.; Anderson, S.; Müller, C.P.; Riess, O.; Masliah, E.; et al. Severely impaired hippocampal neurogenesis associates with an early serotonergic deficit in a BAC α-synuclein transgenic rat model of Parkinson’s disease. Neurobiol. Dis. 2016, 85, 206–217. [Google Scholar] [CrossRef]
- Mori, K.; Kaneko, Y.S.; Nakashima, A.; Nagasaki, H.; Nagatsu, T.; Nagatsu, I.; Ota, A. Subventricular zone under the neuroinflammatory stress and Parkinson’s disease. Cell. Mol. Neurobiol. 2012, 32, 777–785. [Google Scholar] [CrossRef]
- Regensburger, M.; Prots, I.; Winner, B. Adult Hippocampal Neurogenesis in Parkinson’s Disease: Impact on Neuronal Survival and Plasticity. Neural Plast. 2014, 2014, 454696. [Google Scholar] [CrossRef] [PubMed]
- Jurkowski, M.P.; Bettio, L.; Woo, E.K.; Patten, A.; Yau, S.-Y.; Gil-Mohapel, J. Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain. Front. Cell. Neurosci. 2020, 14, 576444. [Google Scholar] [CrossRef]
- Mourtzi, T.; Dimitrakopoulos, D.; Kakogiannis, D.; Salodimitris, C.; Botsakis, K.; Meri, D.K.; Anesti, M.; Dimopoulou, A.; Charalampopoulos, I.; Gravanis, A.; et al. Characterization of substantia nigra neurogenesis in homeostasis and dopaminergic degeneration: Beneficial effects of the microneurotrophin BNN-20. Stem Cell Res. Ther. 2021, 12, 335. [Google Scholar] [CrossRef]
- Zhao, M.; Momma, S.; Delfani, K.; Carlen, M.; Cassidy, R.M.; Johansson, C.B.; Brismar, H.; Shupliakov, O.; Frisen, J.; Janson, A.M. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc. Natl. Acad. Sci. USA 2003, 100, 7925–7930. [Google Scholar] [CrossRef]
- Frielingsdorf, H.; Schwarz, K.; Brundin, P.; Mohapel, P. No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc. Natl. Acad. Sci. USA 2004, 101, 10177–10182. [Google Scholar] [CrossRef]
- Arzate, D.M.; Guerra-Crespo, M.; Covarrubias, L. Induction of typical and atypical neurogenesis in the adult substantia nigra after mouse embryonic stem cells transplantation. Neuroscience 2019, 408, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Salvi, R.; Steigleder, T.; Schlachetzki, J.C.M.; Waldmann, E.; Schwab, S.; Winner, B.; Winkler, J.; Kohl, Z. Distinct Effects of Chronic Dopaminergic Stimulation on Hippocampal Neurogenesis and Striatal Doublecortin Expression in Adult Mice. Front. Neurosci. 2016, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, J.M.; Robertson, H.A. A possible role for dopamine D3 receptor stimulation in the induction of neurogenesis in the adult rat substantia nigra. Neuroscience 2005, 136, 381–386. [Google Scholar] [CrossRef]
- Lie, D.C.; Dziewczapolski, G.; Willhoite, A.R.; Kaspar, B.K.; Shults, C.W.; Gage, F.H. The adult substantia nigra contains progenitor cells with neurogenic potential. J. Neurosci. 2002, 22, 6639–6649. [Google Scholar] [CrossRef] [PubMed]
- Seah, Y.F.; El Farran, C.A.; Warrier, T.; Xu, J.; Loh, Y.H. Induced Pluripotency and Gene Editing in Disease Modelling: Perspectives and Challenges. Int. J. Mol. Sci. 2015, 16, 28614–28634. [Google Scholar] [CrossRef]
- Shuvalova, L.D.; Eremeev, A.V.; Bogomazova, A.N.; Novosadova, E.V.; Zerkalenkova, E.A.; Olshanskaya, Y.V.; Fedotova, E.Y.; Glagoleva, E.S.; Illarioshkin, S.N.; Lebedeva, O.S.; et al. Generation of induced pluripotent stem cell line RCPCMi004-A derived from patient with Parkinson’s disease with deletion of the exon 2 in PARK2 gene. Stem Cell Res. 2020, 44, 101733. [Google Scholar] [CrossRef]
- Novosadova, E.; Antonov, S.; Arsenyeva, E.; Kobylanskiy, A.; Vanyushina, Y.; Malova, T.; Khaspekov, L.; Bobrov, M.; Bezuglov, V.; Tarantul, V.; et al. Neuroprotective and neurotoxic effects of endocannabinoid-like compounds, N-arachidonoyl dopamine and N-docosahexaenoyl dopamine in differentiated cultures of induced pluripotent stem cells derived from patients with Parkinson’s disease. Neurotoxicology 2021, 82, 108–118. [Google Scholar] [CrossRef]
- Novosadova, E.V.; Arsenyeva, E.L.; Manuilova, E.S.; Khaspekov, L.G.; Bobrov, M.Y.; Bezuglov, V.V.; Illarioshkin, S.N.; Grivennikov, I.A. Neuroprotective Properties of Endocannabinoids N-Arachidonoyl Dopamine and N-Docosahexaenoyl Dopamine Examined in Neuronal Precursors Derived from Human Pluripotent Stem Cells. Biochemistry 2017, 82, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Antonov, S.A.; Novosadova, E.V. Current State-of-the-Art and Unresolved Problems in Using Human Induced Pluripotent Stem Cell-Derived Dopamine Neurons for Parkinson’s Disease Drug Development. Int. J. Mol. Sci. 2021, 22, 3381. [Google Scholar] [CrossRef] [PubMed]
- de Rus Jacquet, A. Preparation and Co-Culture of iPSC-Derived Dopaminergic Neurons and Astrocytes. Curr. Protoc. Cell Biol. 2019, 85, e98. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Ganat, Y.M.; Kishinevsky, S.; Bowman, R.L.; Liu, B.; Tu, E.Y.; Mandal, P.; Vera, E.; Shim, J.-W.; Kriks, S.; et al. Human iPSC-Based Modeling of Late-Onset Disease via Progerin-Induced Aging. Cell Stem Cell 2013, 13, 691–705. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, L.; Wagner, A.M.; Marchetto, M.C.N.; Muotri, A.R.; Gage, F.H.; Chen, G. Astroglial cells regulate the developmental timeline of human neurons differentiated from induced pluripotent stem cells. Stem Cell Res. 2013, 11, 743–757. [Google Scholar] [CrossRef]
- Nadadhur, A.G.; Alsaqati, M.; Gasparotto, L.; Cornelissen-Steijger, P.; van Hugte, E.; Dooves, S.; Harwood, A.J.; Heine, V.M. Neuron-Glia Interactions Increase Neuronal Phenotypes in Tuberous Sclerosis Complex Patient iPSC-Derived Models. Stem Cell Rep. 2019, 12, 42–56. [Google Scholar] [CrossRef]
- Taga, A.; Dastgheyb, R.; Habela, C.; Joseph, J.; Richard, J.-P.; Gross, S.K.; Lauria, G.; Lee, G.; Haughey, N.; Maragakis, N.J. Role of Human-Induced Pluripotent Stem Cell-Derived Spinal Cord Astrocytes in the Functional Maturation of Motor Neurons in a Multielectrode Array System. STEM CELLS Transl. Med. 2019, 8, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Klapper, S.D.; Garg, P.; Dagar, S.; Lenk, K.; Gottmann, K.; Nieweg, K. Astrocyte lineage cells are essential for functional neuronal differentiation and synapse maturation in human iPSC-derived neural networks. Glia 2019, 67, 1893–1909. [Google Scholar] [CrossRef]
- Coccia, E.; Ahfeldt, T. Towards physiologically relevant human pluripotent stem cell (hPSC) models of Parkinson’s disease. Stem Cell Res. Ther. 2021, 12, 253. [Google Scholar] [CrossRef]
- Tagliafierro, L.; Chiba-Falek, O. Up-regulation of SNCA gene expression: Implications to synucleinopathies. Neurogenetics 2016, 17, 145–157. [Google Scholar] [CrossRef]
- Fukusumi, H.; Togo, K.; Sumida, M.; Nakamori, M.; Obika, S.; Baba, K.; Shofuda, T.; Ito, D.; Okano, H.; Mochizuki, H.; et al. Alpha-synuclein dynamics in induced pluripotent stem cell-derived dopaminergic neurons from a Parkinson’s disease patient (PARK4) with SNCA triplication. FEBS Open Bio 2021, 11, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.; Falomir-Lockhart, L.J.; Botelho, M.G.; Lin, K.H.; Wales, P.; Koch, J.C.; Gerhardt, E.; Taschenberger, H.; Outeiro, T.F.; Lingor, P.; et al. Elevated α-synuclein caused by SNCA gene triplication impairs neuronal differentiation and maturation in Parkinson’s patient-derived induced pluripotent stem cells. Cell Death Dis. 2015, 6, e1994. [Google Scholar] [CrossRef]
- Gribaudo, S.; Tixador, P.; Bousset, L.; Fenyi, A.; Lino, P.; Melki, R.; Peyrin, J.-M.; Perrier, A.L. Propagation of α-Synuclein Strains within Human Reconstructed Neuronal Network. Stem Cell Rep. 2019, 12, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Soubannier, V.; Maussion, G.; Chaineau, M.; Sigutova, V.; Rouleau, G.; Durcan, T.M.; Stifani, S. Characterization of human iPSC-derived astrocytes with potential for disease modeling and drug discovery. Neurosci. Lett. 2020, 731, 135028. [Google Scholar] [CrossRef]
- Tcw, J.; Wang, M.; Pimenova, A.A.; Bowles, K.R.; Hartley, B.J.; Lacin, E.; Machlovi, S.I.; Abdelaal, R.; Karch, C.M.; Phatnani, H.; et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 600–614. [Google Scholar] [CrossRef]
- Serio, A.; Bilican, B.; Barmada, S.J.; Ando, D.M.; Zhao, C.; Siller, R.; Burr, K.; Haghi, G.; Story, D.; Nishimura, A.L.; et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc. Natl. Acad. Sci. USA 2013, 110, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.S.; Lee, C.O.; Oh, M.; Cha, D.; Kim, W.-K.; Oh, K.-J.; Bae, K.-H.; Lee, S.-C.; Han, B.-S. Rapid differentiation of astrocytes from human embryonic stem cells. Neurosci. Lett. 2020, 716, 134681. [Google Scholar] [CrossRef]
- Caiazzo, M.; Giannelli, S.; Valente, P.; Lignani, G.; Carissimo, A.; Sessa, A.; Colasante, G.; Bartolomeo, R.; Massimino, L.; Ferroni, S.; et al. Direct Conversion of Fibroblasts into Functional Astrocytes by Defined Transcription Factors. Stem Cell Rep. 2015, 4, 25–36. [Google Scholar] [CrossRef]
- Reinhardt, P.; Glatza, M.; Hemmer, K.; Tsytsyura, Y.; Thiel, C.S.; Höing, S.; Moritz, S.; Parga, J.A.; Wagner, L.; Bruder, J.M.; et al. Derivation and Expansion Using Only Small Molecules of Human Neural Progenitors for Neurodegenerative Disease Modeling. PLoS ONE 2013, 8, e59252. [Google Scholar] [CrossRef]
- Barbuti, P.A.; Antony, P.; Novak, G.; Larsen, S.B.; Berenguer-Escuder, C.; Santos, B.F.; Massart, F.; Grossmann, D.; Shiga, T.; Ishikawa, K.-I.; et al. IPSC-derived midbrain astrocytes from Parkinson’s disease patients carrying pathogenic SNCA mutations exhibit alpha-synuclein aggregation, mitochondrial fragmentation and excess calcium release. bioRxiv 2020. [Google Scholar] [CrossRef]
- di Domenico, A.; Carola, G.; Calatayud, C.; Pons-Espinal, M.; Muñoz, J.P.; Richaud-Patin, Y.; Fernandez-Carasa, I.; Gut, M.; Faella, A.; Parameswaran, J.; et al. Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson’s Disease. Stem Cell Rep. 2019, 12, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Griffiths, R.; Price, D.J.; Mason, J.O. Cerebral organoids as tools to identify the developmental roots of autism. Mol. Autism 2020, 11, 58. [Google Scholar] [CrossRef]
- Khakipoor, S.; Crouch, E.E.; Mayer, S. Human organoids to model the developing human neocortex in health and disease. Brain Res. 2020, 1742, 146803. [Google Scholar] [CrossRef]
- Di Lullo, E.; Kriegstein, A.R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 2017, 18, 573–584. [Google Scholar] [CrossRef]
- Karzbrun, E.; Reiner, O. Brain Organoids-A Bottom-Up Approach for Studying Human Neurodevelopment. Bioengineering 2019, 6, 9. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Saia-Cereda, V.M.; Sartore, R.C.; da Costa, R.M.; Schitine, C.S.; Freitas, H.R.; Murgu, M.; de Melo Reis, R.A.; Rehen, S.K.; Martins-de-Souza, D. Human Cerebral Organoids and Fetal Brain Tissue Share Proteomic Similarities. Front. Cell Dev. Biol. 2019, 7, 303. [Google Scholar] [CrossRef]
- Vargas-Valderrama, A.; Messina, A.; Mitjavila-Garcia, M.T.; Guenou, H. The endothelium, a key actor in organ development and hPSC-derived organoid vascularization. J. Biomed. Sci. 2020, 27, 67. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.T.; Pollock, K.M.; Rose, M.D.; Cary, W.A.; Stewart, H.R.; Zhou, P.; Nolta, J.A.; Waldau, B. Generation of human vascularized brain organoids. Neuroreport 2018, 29, 588–593. [Google Scholar] [CrossRef]
- Fagerlund, I.; Dougalis, A.; Shakirzyanova, A.; Gómez-Budia, M.; Konttinen, H.; Ohtonen, S.; Feroze, F.; Koskuvi, M.; Kuusisto, J.; Hernández, D.; et al. Microglia orchestrate neuronal activity in brain organoids. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ormel, P.R.; Vieira de Sá, R.; van Bodegraven, E.J.; Karst, H.; Harschnitz, O.; Sneeboer, M.A.M.; Johansen, L.E.; van Dijk, R.E.; Scheefhals, N.; Berdenis van Berlekom, A.; et al. Microglia innately develop within cerebral organoids. Nat. Commun. 2018, 9, 4167. [Google Scholar] [CrossRef] [PubMed]
- Raja, W.K.; Mungenast, A.E.; Lin, Y.-T.; Ko, T.; Abdurrob, F.; Seo, J.; Tsai, L.-H. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer’s Disease Phenotypes. PLoS ONE 2016, 11, e0161969. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Kritskiy, O.; Watson, L.A.; Barker, S.J.; Dey, D.; Raja, W.K.; Lin, Y.-T.; Ko, T.; Cho, S.; Penney, J.; et al. Inhibition of p25/Cdk5 Attenuates Tauopathy in Mouse and iPSC Models of Frontotemporal Dementia. J. Neurosci. 2017, 37, 9917–9924. [Google Scholar] [CrossRef] [PubMed]
- Grenier, K.; Kao, J.; Diamandis, P. Three-dimensional modeling of human neurodegeneration: Brain organoids coming of age. Mol. Psychiatry 2020, 25, 254–274. [Google Scholar] [CrossRef]
- Galet, B.; Cheval, H.; Ravassard, P. Patient-Derived Midbrain Organoids to Explore the Molecular Basis of Parkinson’s Disease. Front. Neurol. 2020, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Nickels, S.L.; Modamio, J.; Mendes-Pinheiro, B.; Monzel, A.S.; Betsou, F.; Schwamborn, J.C. Reproducible generation of human midbrain organoids for in vitro modeling of Parkinson’s disease. Stem Cell Res. 2020, 46, 101870. [Google Scholar] [CrossRef]
- Sánchez-Danés, A.; Consiglio, A.; Richaud, Y.; Rodríguez-Pizà, I.; Dehay, B.; Edel, M.; Bové, J.; Memo, M.; Vila, M.; Raya, A.; et al. Efficient generation of A9 midbrain dopaminergic neurons by lentiviral delivery of LMX1A in human embryonic stem cells and induced pluripotent stem cells. Hum. Gene Ther. 2012, 23, 56–69. [Google Scholar] [CrossRef]
- Kwak, T.H.; Kang, J.H.; Hali, S.; Kim, J.; Kim, K.P.; Park, C.; Lee, J.H.; Ryu, H.K.; Na, J.E.; Jo, J.; et al. Generation of homogeneous midbrain organoids with in vivo-like cellular composition facilitates neurotoxin-based Parkinson’s disease modeling. Stem Cells 2020, 38, 727–740. [Google Scholar] [CrossRef]
- Zanetti, C.; Spitz, S.; Berger, E.; Bolognin, S.; Smits, L.M.; Crepaz, P.; Rothbauer, M.; Rosser, J.M.; Marchetti-Deschmann, M.; Schwarmborn, J.C.; et al. Monitoring the neurotransmitter release of human midbrain organoids using a redox cycling microsensor as a novel tool for personalized Parkinson’s disease modelling and drug screening. Analyst. 2021, 146, 2358–2367. [Google Scholar] [CrossRef]
- Schultz, E.M.; Jones, T.J.; Xu, S.; Dean, D.D.; Zechmann, B.; Barr, K.L. Cerebral Organoids Derived from a Parkinson’s Patient Exhibit Unique Pathogenesis from Chikungunya Virus Infection When Compared to a Non-Parkinson’s Patient. Pathogens 2021, 10, 913. [Google Scholar] [CrossRef]
- Jarazo, J.; Barmpa, K.; Rosety, I.; Smits, L.M.; Arias-Fuenzalida, J.; Walter, J.; Gomez-Giro, G.; Monzel, A.S.; Qing, X.; Cruciani, G.; et al. Parkinson’s disease phenotypes in patient specific brain organoids are improved by HP-β-CD treatment. bioRxiv 2019, 813089. [Google Scholar] [CrossRef]
- Calatayud, C.; Carola, G.; Fernández-Carasa, I.; Valtorta, M.; Jiménez-Delgado, S.; Díaz, M.; Soriano-Fradera, J.; Cappelletti, G.; García-Sancho, J.; Raya, Á.; et al. CRISPR/Cas9-mediated generation of a tyrosine hydroxylase reporter iPSC line for live imaging and isolation of dopaminergic neurons. Sci. Rep. 2019, 9, 6811. [Google Scholar] [CrossRef]
- Chlebanowska, P.; Tejchman, A.; Sułkowski, M.; Skrzypek, K.; Majka, M. Use of 3D Organoids as a Model to Study Idiopathic Form of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 694. [Google Scholar] [CrossRef]
- Katt, M.E.; Mayo, L.N.; Ellis, S.E.; Mahairaki, V.; Rothstein, J.D.; Cheng, L.; Searson, P.C. The role of mutations associated with familial neurodegenerative disorders on blood-brain barrier function in an iPSC model. Fluids Barriers CNS 2019, 16, 20. [Google Scholar] [CrossRef]
- Kane, K.I.W.; Jarazo, J.; Moreno, E.L.; Fleming, R.M.T.; Schwamborn, J.C. Passive controlled flow for Parkinson’s disease neuronal cell culture in 3D microfluidic devices. Organs-on-a-Chip 2020, 2, 100005. [Google Scholar] [CrossRef]
- Doi, D.; Magotani, H.; Kikuchi, T.; Ikeda, M.; Hiramatsu, S.; Yoshida, K.; Amano, N.; Nomura, M.; Umekage, M.; Morizane, A.; et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 2020, 11, 3369. [Google Scholar] [CrossRef] [PubMed]
- DeCarolis, N.A.; Eisch, A.J. Hippocampal neurogenesis as a target for the treatment of mental illness: A critical evaluation. Neuropharmacology 2010, 58, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Bortolasci, C.C.; Spolding, B.; Kidnapillai, S.; Connor, T.; Truong, T.T.T.; Liu, Z.S.J.; Panizzutti, B.; Richardson, M.F.; Gray, L.; Berk, M.; et al. Transcriptional Effects of Psychoactive Drugs on Genes Involved in Neurogenesis. Int. J. Mol. Sci. 2020, 21, 8333. [Google Scholar] [CrossRef]
- Latchney, S.E.; Eisch, A.J. Therapeutic application of neural stem cells and adult neurogenesis for neurodegenerative disorders: Regeneration and beyond. Eur. J. Neurodegener. Dis. 2012, 1, 335–351. [Google Scholar] [PubMed]
- Kreutzmann, J.C.; Havekes, R.; Abel, T.; Meerlo, P. Sleep deprivation and hippocampal vulnerability: Changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience 2015, 309, 173–190. [Google Scholar] [CrossRef]
- Venna, V.R.; Xu, Y.; Doran, S.J.; Patrizz, A.; McCullough, L.D. Social interaction plays a critical role in neurogenesis and recovery after stroke. Transl. Psychiatry 2014, 4, e351. [Google Scholar] [CrossRef]
- Salmin, V.V.; Komleva, Y.K.; Kuvacheva, N.V.; Morgun, A.V.; Khilazheva, E.D.; Lopatina, O.L.; Pozhilenkova, E.A.; Shapovalov, K.A.; Uspenskaya, Y.A.; Salmina, A.B. Differential Roles of Environmental Enrichment in Alzheimer’s Type of Neurodegeneration and Physiological Aging. Front. Aging Neurosci. 2017, 9, 245. [Google Scholar] [CrossRef]
- Salmina, A.B.; Komleva, Y.K.; Salmin, V.V.; Lopatina, O.L.; Belova, O.A. Environmental enrichment and physiological aging. In The Neuroscience of Aging, 1st ed.; Martin, C., Preedy, V., Rajendram, R., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-Lactate Promotes Adult Hippocampal Neurogenesis. Front. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef]
- Mainardi, M.; Fusco, S.; Grassi, C. Modulation of Hippocampal Neural Plasticity by Glucose-Related Signaling. Neural Plast. 2015, 2015, 657928. [Google Scholar] [CrossRef]
- Storer, M.A.; Gallagher, D.; Fatt, M.P.; Simonetta, J.V.; Kaplan, D.R.; Miller, F.D. Interleukin-6 Regulates Adult Neural Stem Cell Numbers during Normal and Abnormal Post-natal Development. Stem Cell Rep. 2018, 10, 1464–1480. [Google Scholar] [CrossRef]
- Wang, S.N.; Xu, T.Y.; Li, W.L.; Miao, C.Y. Targeting Nicotinamide Phosphoribosyltransferase as a Potential Therapeutic Strategy to Restore Adult Neurogenesis. CNS Neurosci. Ther. 2016, 22, 431–439. [Google Scholar] [CrossRef]
- Asano, T.; Teh, D.B.L.; Yawo, H. Application of Optogenetics for Muscle Cells and Stem Cells. Adv. Exp. Med. Biol. 2021, 1293, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Boltze, J.; Stroh, A. Optogenetics in Stem Cell Research: Focus on the Central Nervous System. In Optogenetics: A Roadmap; Stroh, A., Ed.; Springer: New York, NY, USA, 2018; pp. 75–87. [Google Scholar]
- Teh, D.B.L.; Prasad, A.; Jiang, W.; Zhang, N.; Wu, Y.; Yang, H.; Han, S.; Yi, Z.; Yeo, Y.; Ishizuka, T.; et al. Driving Neurogenesis in Neural Stem Cells with High Sensitivity Optogenetics. NeuroMolecular Med. 2020, 22, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, E.; Palmero-Canton, D.; Martinez-Rojas, B.; Sanchez-Martin, M.D.M.; Moreno-Manzano, V. Optogenetic Modulation of Neural Progenitor Cells Improves Neuroregenerative Potential. Int. J. Mol. Sci. 2020, 22, 365. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; George, J.H.; Nagel, D.A.; Ye, H.; Kueberuwa, G.; Seymour, L.W. Optogenetic control of iPS cell-derived neurons in 2D and 3D culture systems using channelrhodopsin-2 expression driven by the synapsin-1 and calcium-calmodulin kinase II promoters. J. Tissue Eng. Regen. Med. 2019, 13, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Daadi, M.M.; Klausner, J.Q.; Bajar, B.; Goshen, I.; Lee-Messer, C.; Lee, S.Y.; Winge, M.C.; Ramakrishnan, C.; Lo, M.; Sun, G.; et al. Optogenetic Stimulation of Neural Grafts Enhances Neurotransmission and Downregulates the Inflammatory Response in Experimental Stroke Model. Cell Transpl. 2016, 25, 1371–1380. [Google Scholar] [CrossRef]
- Klapper, S.D.; Sauter, E.J.; Swiersy, A.; Hyman, M.A.E.; Bamann, C.; Bamberg, E.; Busskamp, V. On-demand optogenetic activation of human stem-cell-derived neurons. Sci. Rep. 2017, 7, 14450. [Google Scholar] [CrossRef]
- Ryu, J.; Vincent, P.F.Y.; Ziogas, N.K.; Xu, L.; Sadeghpour, S.; Curtin, J.; Alexandris, A.S.; Stewart, N.; Sima, R.; du Lac, S.; et al. Optogenetically transduced human ES cell-derived neural progenitors and their neuronal progenies: Phenotypic characterization and responses to optical stimulation. PLoS ONE 2019, 14, e0224846. [Google Scholar] [CrossRef]
- Avaliani, N.; Sørensen, A.T.; Ledri, M.; Bengzon, J.; Koch, P.; Brüstle, O.; Deisseroth, K.; Andersson, M.; Kokaia, M. Optogenetics reveal delayed afferent synaptogenesis on grafted human-induced pluripotent stem cell-derived neural progenitors. Stem Cells 2014, 32, 3088–3098. [Google Scholar] [CrossRef]
- Shohayeb, B.; Diab, M.; Ahmed, M.; Ng, D.C.H. Factors that influence adult neurogenesis as potential therapy. Transl. Neurodegener. 2018, 7, 4. [Google Scholar] [CrossRef]
- Isaev, N.K.; Stelmashook, E.V.; Genrikhs, E.E. Neurogenesis and brain aging. Rev. Neurosci. 2019, 30, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. Activity Dependency and Aging in the Regulation of Adult Neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, a018929. [Google Scholar] [CrossRef]
- Berdugo-Vega, G.; Arias-Gil, G.; López-Fernández, A.; Artegiani, B.; Wasielewska, J.M.; Lee, C.-C.; Lippert, M.T.; Kempermann, G.; Takagaki, K.; Calegari, F. Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat. Commun. 2020, 11, 135. [Google Scholar] [CrossRef]
- Qi, C.; Varga, S.; Oh, S.-J.; Lee, C.J.; Lee, D. Optogenetic Rescue of Locomotor Dysfunction and Dopaminergic Degeneration Caused by Alpha-Synuclein and EKO Genes. Exp. Neurobiol. 2017, 26, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Inoshita, T.; Meng, H.; Shiba-Fukushima, K.; Hara, K.Y.; Sawamura, N.; Hattori, N. Light-driven activation of mitochondrial proton-motive force improves motor behaviors in a Drosophila model of Parkinson’s disease. Commun. Biol. 2019, 2, 424. [Google Scholar] [CrossRef] [PubMed]
- Bérard, M.; Sheta, R.; Malvaut, S.; Turmel, R.; Alpaugh, M.; Dubois, M.; Dahmene, M.; Salesse, C.; Profes, M.; Lamontagne-Proulx, J.; et al. Optogenetic-Mediated Spatiotemporal Control of α-Synuclein Aggregation Disrupts Nigrostriatal Transmission and Precipitates Neurodegeneration. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Qian, H.; Kang, X.; Hu, J.; Zhang, D.; Liang, Z.; Meng, F.; Zhang, X.; Xue, Y.; Maimon, R.; Dowdy, S.F.; et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 2020, 582, 550–556. [Google Scholar] [CrossRef]
- Cassé, F.; Richetin, K.; Toni, N. Astrocytes’ Contribution to Adult Neurogenesis in Physiology and Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 432. [Google Scholar] [CrossRef]
- Araki, T.; Ikegaya, Y.; Koyama, R. The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur. J. Neurosci. 2020, 54, 5880–5901. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, U.; Faiz, M.; de Pablo, Y.; Sjöqvist, M.; Andersson, D.; Widestrand, Å.; Potokar, M.; Stenovec, M.; Smith, P.L.P.; Shinjyo, N.; et al. Astrocytes Negatively Regulate Neurogenesis Through the Jagged1-Mediated Notch Pathway. Stem Cells 2012, 30, 2320–2329. [Google Scholar] [CrossRef]
- Rizor, A.; Pajarillo, E.; Johnson, J.; Aschner, M.; Lee, E. Astrocytic Oxidative/Nitrosative Stress Contributes to Parkinson’s Disease Pathogenesis: The Dual Role of Reactive Astrocytes. Antioxidants 2019, 8, 265. [Google Scholar] [CrossRef]
- Hindeya Gebreyesus, H.; Gebrehiwot Gebremichael, T. The Potential Role of Astrocytes in Parkinson’s Disease (PD). Med. Sci. 2020, 8, 7. [Google Scholar] [CrossRef]
- Mosher, K.I.; Schaffer, D.V. Influence of hippocampal niche signals on neural stem cell functions during aging. Cell Tissue Res. 2018, 371, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, B.; L’Episcopo, F.; Morale, M.C.; Tirolo, C.; Testa, N.; Caniglia, S.; Serapide, M.F.; Pluchino, S. Uncovering novel actors in astrocyte-neuron crosstalk in Parkinson’s disease: The Wnt/β-catenin signaling cascade as the common final pathway for neuroprotection and self-repair. Eur. J. Neurosci. 2013, 37, 1550–1563. [Google Scholar] [CrossRef]
- L’Episcopo, F.; Tirolo, C.; Testa, N.; Caniglia, S.; Morale, M.C.; Cossetti, C.; D’Adamo, P.; Zardini, E.; Andreoni, L.; Ihekwaba, A.E.; et al. Reactive astrocytes and Wnt/β-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiol. Dis. 2011, 41, 508–527. [Google Scholar] [CrossRef]
- Marchetti, B. Wnt/β-Catenin Signaling Pathway Governs a Full Program for Dopaminergic Neuron Survival, Neurorescue and Regeneration in the MPTP Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 3743. [Google Scholar] [CrossRef]
- Faigle, R.; Song, H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 2013, 1830, 2435–2448. [Google Scholar] [CrossRef] [PubMed]
- Hitoshi, S.; Alexson, T.; Tropepe, V.; Donoviel, D.; Elia, A.J.; Nye, J.S.; Conlon, R.A.; Mak, T.W.; Bernstein, A.; van der Kooy, D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002, 16, 846–858. [Google Scholar] [CrossRef]
- Bejoy, J.; Bijonowski, B.; Marzano, M.; Jeske, R.; Ma, T.; Li, Y. Wnt-Notch Signaling Interactions During Neural and Astroglial Patterning of Human Stem Cells. Tissue Eng. Part A 2020, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Berwick, D.C.; Harvey, K. The regulation and deregulation of Wnt signaling by PARK genes in health and disease. J. Mol. Cell Biol. 2014, 6, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Rawal, N.; Corti, O.; Sacchetti, P.; Ardilla-Osorio, H.; Sehat, B.; Brice, A.; Arenas, E. Parkin protects dopaminergic neurons from excessive Wnt/beta-catenin signaling. Biochem. Biophys. Res. Commun. 2009, 388, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Nouri, N.; Patel, M.J.; Joksimovic, M.; Poulin, J.F.; Anderegg, A.; Taketo, M.M.; Ma, Y.C.; Awatramani, R. Excessive Wnt/beta-catenin signaling promotes midbrain floor plate neurogenesis, but results in vacillating dopamine progenitors. Mol. Cell. Neurosci. 2015, 68, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Salašová, A.; Yokota, C.; Potěšil, D.; Zdráhal, Z.; Bryja, V.; Arenas, E. A proteomic analysis of LRRK2 binding partners reveals interactions with multiple signaling components of the WNT/PCP pathway. Mol. Neurodegener. 2017, 12, 54. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Tu, J.; Wan, J.; Zhang, J.; Wu, B.; Chen, S.; Zhou, J.; Mu, Y.; Wang, L. Activated astrocytes enhance the dopaminergic differentiation of stem cells and promote brain repair through bFGF. Nat. Commun. 2014, 5, 5627. [Google Scholar] [CrossRef]
- Saarimäki-Vire, J.; Peltopuro, P.; Lahti, L.; Naserke, T.; Blak, A.A.; Vogt Weisenhorn, D.M.; Yu, K.; Ornitz, D.M.; Wurst, W.; Partanen, J. Fibroblast growth factor receptors cooperate to regulate neural progenitor properties in the developing midbrain and hindbrain. J. Neurosci. 2007, 27, 8581–8592. [Google Scholar] [CrossRef] [PubMed]
- Puelles, E.; Annino, A.; Tuorto, F.; Usiello, A.; Acampora, D.; Czerny, T.; Brodski, C.; Ang, S.L.; Wurst, W.; Simeone, A. Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development 2004, 131, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, J.M.; Heo, W.D. Optogenetic Control of Fibroblast Growth Factor Receptor Signaling. Methods Mol. Biol. 2016, 1408, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, J.M.; Lee, M.; Kim, C.Y.; Chang, K.-Y.; Heo, W.D. Spatiotemporal Control of Fibroblast Growth Factor Receptor Signals by Blue Light. Chem. Biol. 2014, 21, 903–912. [Google Scholar] [CrossRef]
- Cheli, V.T.; Santiago González, D.A.; Smith, J.; Spreuer, V.; Murphy, G.G.; Paez, P.M. L-type voltage-operated calcium channels contribute to astrocyte activation in vitro. Glia 2016, 64, 1396–1415. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Wang, N.; Bol, M.; Decrock, E.; Ponsaerts, R.; Bultynck, G.; Dupont, G.; Leybaert, L. Connexin 43 hemichannels contribute to cytoplasmic Ca2+ oscillations by providing a bimodal Ca2+-dependent Ca2+ entry pathway. J. Biol. Chem. 2012, 287, 12250–12266. [Google Scholar] [CrossRef]
- Shigetomi, E.; Tong, X.; Kwan, K.Y.; Corey, D.P.; Khakh, B.S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 2012, 15, 70–80. [Google Scholar] [CrossRef]
- Banerjee, S.; Walseth, T.F.; Borgmann, K.; Wu, L.; Bidasee, K.R.; Kannan, M.S.; Ghorpade, A. CD38/cyclic ADP-ribose regulates astrocyte calcium signaling: Implications for neuroinflammation and HIV-1-associated dementia. J. Neuroimmune Pharm. 2008, 3, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Perea, G.; de Sevilla, D.F.; Gómez-Gonzalo, M.; Núñez, A.; Martín, E.D.; Araque, A. Astrocytes Mediate In Vivo Cholinergic-Induced Synaptic Plasticity. PLOS Biol. 2012, 10, e1001259. [Google Scholar] [CrossRef]
- Guerra-Gomes, S.; Sousa, N.; Pinto, L.; Oliveira, J.F. Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front. Cell. Neurosci. 2018, 11, 427. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Wu, J.; Zhu, Z.; Feng, X.; Qin, L.; Zhu, Y.; Sun, L.; Liu, Y.; Qiu, Z.; et al. Activation of astrocytes in hippocampus decreases fear memory through adenosine A(1) receptors. Elife 2020, 9, 9. [Google Scholar] [CrossRef]
- Ono, K.; Suzuki, H.; Higa, M.; Tabata, K.; Sawada, M. Glutamate release from astrocyte cell-line GL261 via alterations in the intracellular ion environment. J. Neural Transm. 2014, 121, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Iino, M. Visualization of astrocytic intracellular Ca(2+) mobilization. J. Physiol. 2020, 598, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Lane, S.; Tang, F.; Liu, B.H.; Hewinson, J.; Marina, N.; Kasymov, V.; Souslova, E.A.; Chudakov, D.M.; Gourine, A.V.; et al. Optogenetic experimentation on astrocytes. Exp. Physiol. 2011, 96, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Salmina, A.B.; Gorina, Y.V.; Erofeev, A.I.; Balaban, P.M.; Bezprozvanny, I.B.; Vlasova, O.L. Optogenetic and chemogenetic modulation of astroglial secretory phenotype. Rev. Neurosci. 2021, 32, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Borodinova, A.A.; Balaban, P.M.; Bezprozvanny, I.B.; Salmina, A.B.; Vlasova, O.L. Genetic Constructs for the Control of Astrocytes’ Activity. Cells 2021, 10, 1600. [Google Scholar] [CrossRef]
- Bang, J.; Kim, H.Y.; Lee, H. Optogenetic and Chemogenetic Approaches for Studying Astrocytes and Gliotransmitters. Exp. Neurobiol. 2016, 25, 205–221. [Google Scholar] [CrossRef]
- Chai, H.; Diaz-Castro, B.; Shigetomi, E.; Monte, E.; Octeau, J.C.; Yu, X.; Cohn, W.; Rajendran, P.S.; Vondriska, T.M.; Whitelegge, J.P.; et al. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 2017, 95, 531–549.e539. [Google Scholar] [CrossRef]
- Morgun, A.V.; Malinovskaya, N.A.; Komleva, Y.K.; Lopatina, O.L.; Kuvacheva, N.V.; Panina, Y.A.; Taranushenko, T.Y.; Solonchuk, Y.R.; Salmina, A.B. Structural and functional heterogeneity of astrocytes in the brain: Role in neurodegeneration and neuroinflammation. Bull. Sib. Med. 2014, 13, 138–148. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef]
- Figueiredo, M.; Lane, S.; Stout, R.F.J.; Liu, B.; Parpura, V.; Teschemacher, A.G.; Kasparov, S. Comparative analysis of optogenetic actuators in cultured astrocytes. Cell Calcium 2014, 56, 208–214. [Google Scholar] [CrossRef]
- Shuvaev, A.N.; Belozor, O.S.; Mozhei, O.; Yakovleva, D.A.; Potapenko, I.V.; Shuvaev, A.N.; Smolnikova, M.V.; Salmin, V.V.; Salmina, A.B.; Hirai, H.; et al. Chronic optogenetic stimulation of Bergman glia leads to dysfunction of EAAT1 and Purkinje cell death, mimicking the events caused by expression of pathogenic ataxin-1. Neurobiol. Dis. 2021, 154, 105340. [Google Scholar] [CrossRef]
- Morgun, A.V.; Osipova, E.D.; Boytsova, E.B.; Shuvaev, A.N.; Komleva, Y.K.; Trufanova, L.V.; Vais, E.F.; Salmina, A.B. Astroglia-mediated regulation of cell development in the model of neurogenic niche in vitro treated with Aβ1-42. Biomed. Khim. 2019, 65, 366–373. [Google Scholar] [CrossRef]
- Morgun, A.V.; Osipova, E.D.; Boitsova, E.B.; Shuvaev, A.N.; Malinovskaya, N.A.; Mosiagina, A.I.; Salmina, A.B. Neurogenic Potential of Implanted Neurospheres Is Regulated by Optogenetic Stimulation of Hippocampal Astrocytes Ex Vivo. Bull. Exp. Biol. Med. 2021, 170, 693–698. [Google Scholar] [CrossRef]
- Hedegaard, A.; Monzón-Sandoval, J.; Newey, S.E.; Whiteley, E.S.; Webber, C.; Akerman, C.J. Pro-maturational Effects of Human iPSC-Derived Cortical Astrocytes upon iPSC-Derived Cortical Neurons. Stem Cell Rep. 2020, 15, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiao, J. Molecular Biomarkers for Embryonic and Adult Neural Stem Cell and Neurogenesis. Biomed. Res. Int. 2015, 2015, 727542. [Google Scholar] [CrossRef] [PubMed]
- Kotterman, M.A.; Vazin, T.; Schaffer, D.V. Enhanced selective gene delivery to neural stem cells in vivo by an adeno-associated viral variant. Development 2015, 142, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Parr-Brownlie, L.C.; Bosch-Bouju, C.; Schoderboeck, L.; Sizemore, R.J.; Abraham, W.C.; Hughes, S.M. Lentiviral vectors as tools to understand central nervous system biology in mammalian model organisms. Front. Mol. Neurosci. 2015, 8, 14. [Google Scholar] [CrossRef]
- Abdolahi, S.; Khodakaram-Tafti, A.; Aligholi, H.; Ziaei, S.; Khaleghi Ghadiri, M.; Stummer, W.; Gorji, A. Lentiviral vector-mediated transduction of adult neural stem/progenitor cells isolated from the temporal tissues of epileptic patients. Iran. J. Basic Med. Sci. 2020, 23, 354–361. [Google Scholar] [CrossRef]
- Jandial, R.; Singec, I.; Ames, C.P.; Snyder, E.Y. Genetic Modification of Neural Stem Cells. Mol. Ther. 2008, 16, 450–457. [Google Scholar] [CrossRef]
- Mayorquin, L.C.; Rodriguez, A.V.; Sutachan, J.-J.; Albarracín, S.L. Connexin-Mediated Functional and Metabolic Coupling between Astrocytes and Neurons. Front. Mol. Neurosci. 2018, 11, 118. [Google Scholar] [CrossRef]
- Fujii, Y.; Maekawa, S.; Morita, M. Astrocyte calcium waves propagate proximally by gap junction and distally by extracellular diffusion of ATP released from volume-regulated anion channels. Sci. Rep. 2017, 7, 13115. [Google Scholar] [CrossRef]
- De Bock, M.; Decrock, E.; Wang, N.; Bol, M.; Vinken, M.; Bultynck, G.; Leybaert, L. The dual face of connexin-based astroglial Ca2+ communication: A key player in brain physiology and a prime target in pathology. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2211–2232. [Google Scholar] [CrossRef]
- Li, D.; Hérault, K.; Isacoff, E.Y.; Oheim, M.; Ropert, N. Optogenetic activation of LiGluR-expressing astrocytes evokes anion channel-mediated glutamate release. J. Physiol. 2012, 590, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Agulhon, C.; Sun, M.-Y.; Murphy, T.; Myers, T.; Lauderdale, K.; Fiacco, T.A. Calcium Signaling and Gliotransmission in Normal vs. Reactive Astrocytes. Front. Pharmacol. 2012, 3, 139. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Hayashi, T.; Nakachi, K.; Trosko, J.E.; Sugihara, K.; Kotake, Y.; Ohta, S. Modulation of connexin 43 in rotenone-induced model of Parkinson’s disease. Neuroscience 2009, 160, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Fushiki, S.; Naus, C.C. Astrocytic gap junctions composed of connexin 43 reduce apoptotic neuronal damage in cerebral ischemia. Stroke 2003, 34, 1987–1993. [Google Scholar] [CrossRef]
- Salmina, A.B.; Komleva, Y.K.; Lopatina, O.L.; Gorina, Y.V.; Malinovskaya, N.A.; Pozhilenkova, E.A.; Panina, Y.A.; Zhukov, E.L.; Medvedeva, N.N. CD38 and CD157 expression: Glial control of neurodegeneration and neuroinflammation. Messenger 2014, 3, 78–85. [Google Scholar] [CrossRef]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol. Rev. 2008, 88, 841–886. [Google Scholar] [CrossRef] [PubMed]
- Schöndorf, D.C.; Ivanyuk, D.; Baden, P.; Sanchez-Martinez, A.; De Cicco, S.; Yu, C.; Giunta, I.; Schwarz, L.K.; Di Napoli, G.; Panagiotakopoulou, V.; et al. The NAD+ Precursor Nicotinamide Riboside Rescues Mitochondrial Defects and Neuronal Loss in iPSC and Fly Models of Parkinson’s Disease. Cell Rep. 2018, 23, 2976–2988. [Google Scholar] [CrossRef]
- Song, E.K.; Rah, S.Y.; Lee, Y.R.; Yoo, C.H.; Kim, Y.R.; Yeom, J.H.; Park, K.H.; Kim, J.S.; Kim, U.H.; Han, M.K. Connexin-43 hemichannels mediate cyclic ADP-ribose generation and its Ca2+-mobilizing activity by NAD+/cyclic ADP-ribose transport. J. Biol. Chem. 2011, 286, 44480–44490. [Google Scholar] [CrossRef]
- Greer, K.; Chen, J.; Brickler, T.; Gourdie, R.; Theus, M.H. Modulation of gap junction-associated Cx43 in neural stem/progenitor cells following traumatic brain injury. Brain Res. Bull. 2017, 134, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Jäderstad, J.; Jäderstad, L.M.; Herlenius, E. Dynamic changes in connexin expression following engraftment of neural stem cells to striatal tissue. Exp. Cell Res. 2011, 317, 70–81. [Google Scholar] [CrossRef]
- Jäderstad, J.; Jäderstad, L.M.; Li, J.; Chintawar, S.; Salto, C.; Pandolfo, M.; Ourednik, V.; Teng, Y.D.; Sidman, R.L.; Arenas, E.; et al. Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proc. Natl. Acad. Sci. USA 2010, 107, 5184–5189. [Google Scholar] [CrossRef]
- Habibey, R.; Sharma, K.; Swiersy, A.; Busskamp, V. Optogenetics for neural transplant manipulation and functional analysis. Biochem. Biophys. Res. Commun. 2020, 527, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Guida, L.; Zocchi, E.; Franco, L.; De Flora, A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001, 15, 10–12. [Google Scholar] [CrossRef]
- Díaz, E.F.; Labra, V.C.; Alvear, T.F.; Mellado, L.A.; Inostroza, C.A.; Oyarzún, J.E.; Salgado, N.; Quintanilla, R.A.; Orellana, J.A. Connexin 43 hemichannels and pannexin-1 channels contribute to the α-synuclein-induced dysfunction and death of astrocytes. Glia 2019, 67, 1598–1619. [Google Scholar] [CrossRef] [PubMed]
- Jäderstad, J.; Brismar, H.; Herlenius, E. Hypoxic preconditioning increases gap-junctional graft and host communication. Neuroreport 2010, 21, 1126–1132. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, Q.; Song, D.; Quan, Z.; Qing, H. Optogenetic manipulation of astrocytes from synapses to neuronal networks: A potential therapeutic strategy for neurodegenerative diseases. Glia 2020, 68, 215–226. [Google Scholar] [CrossRef]
- Salmina, A.B.; Morgun, A.V.; Kuvacheva, N.V.; Lopatina, O.L.; Komleva, Y.K.; Malinovskaya, N.A.; Pozhilenkova, E.A. Establishment of neurogenic microenvironment in the neurovascular unit: The connexin 43 story. Rev. Neurosci. 2014, 25, 97–111. [Google Scholar] [CrossRef]
- Kunze, A.; Congreso, M.R.; Hartmann, C.; Wallraff-Beck, A.; Hüttmann, K.; Bedner, P.; Requardt, R.; Seifert, G.; Redecker, C.; Willecke, K.; et al. Connexin expression by radial glia-like cells is required for neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2009, 106, 11336–11341. [Google Scholar] [CrossRef] [PubMed]
- Parekkadan, B.; Berdichevsky, Y.; Irimia, D.; Leeder, A.; Yarmush, G.; Toner, M.; Levine, J.B.; Yarmush, M.L. Cell-cell interaction modulates neuroectodermal specification of embryonic stem cells. Neurosci. Lett. 2008, 438, 190–195. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hartfield, E.M.; Crompton, L.A.; Badger, J.L.; Glover, C.P.; Kelly, C.M.; Rosser, A.E.; Uney, J.B.; Caldwell, M.A. Cross-regulation of Connexin43 and β-catenin influences differentiation of human neural progenitor cells. Cell Death Dis. 2014, 5, e1017. [Google Scholar] [CrossRef]
- Talaverón, R.; Matarredona, E.R.; Herrera, A.; Medina, J.M.; Tabernero, A. Connexin43 Region 266–283, via Src Inhibition, Reduces Neural Progenitor Cell Proliferation Promoted by EGF and FGF-2 and Increases Astrocytic Differentiation. Int. J. Mol. Sci. 2020, 21, 8852. [Google Scholar] [CrossRef]
- Lagos-Cabré, R.; Brenet, M.; Díaz, J.; Pérez, R.D.; Pérez, L.A.; Herrera-Molina, R.; Quest, A.F.G.; Leyton, L. Intracellular Ca(2+) Increases and Connexin 43 Hemichannel Opening Are Necessary but Not Sufficient for Thy-1-Induced Astrocyte Migration. Int. J. Mol. Sci. 2018, 19, 2179. [Google Scholar] [CrossRef]
- Hou, X.; Khan, M.R.A.; Turmaine, M.; Thrasivoulou, C.; Becker, D.L.; Ahmed, A. Wnt signaling regulates cytosolic translocation of connexin 43. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2019, 317, R248–R261. [Google Scholar] [CrossRef]
- Koulakoff, A.; Ezan, P.; Giaume, C. Neurons control the expression of connexin 30 and connexin 43 in mouse cortical astrocytes. Glia 2008, 56, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K.; Singla, S.; Toda, H.; Monje, M.; Palmer, T.D.; Malenka, R.C. Excitation-Neurogenesis Coupling in Adult Neural Stem/Progenitor Cells. Neuron 2004, 42, 535–552. [Google Scholar] [CrossRef]
- Wu, L.; Dong, A.; Dong, L.; Wang, S.-Q.; Li, Y. PARIS, an optogenetic method for functionally mapping gap junctions. bioRxiv 2018, 465781. [Google Scholar] [CrossRef]
- Boyle, P.M.; Yu, J.; Klimas, A.; Williams, J.C.; Trayanova, N.A.; Entcheva, E. OptoGap is an optogenetics-enabled assay for quantification of cell–cell coupling in multicellular cardiac tissue. Sci. Rep. 2021, 11, 9310. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.B.; Siderovski, D.P.; Hooks, S.B. The G betagamma dimer as a novel source of selectivity in G-protein signaling: GGL-ing at convention. Mol. Interv. 2004, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- McKinley, J.W.; Shi, Z.; Kawikova, I.; Hur, M.; Bamford, I.J.; Sudarsana Devi, S.P.; Vahedipour, A.; Darvas, M.; Bamford, N.S. Dopamine Deficiency Reduces Striatal Cholinergic Interneuron Function in Models of Parkinson’s Disease. Neuron 2019, 103, 1056–1072.e6. [Google Scholar] [CrossRef]
- Bernácer, J.; Prensa, L.; Giménez-Amaya, J.M. Distribution of GABAergic interneurons and dopaminergic cells in the functional territories of the human striatum. PLoS ONE 2012, 7, e30504. [Google Scholar] [CrossRef]
- Lane, E.M.; Handley, O.J.; Rosser, A.E.; Dunnett, S.B. Potential cellular and regenerative approaches for the treatment of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 835–845. [Google Scholar] [CrossRef][Green Version]
- Xenias, H.S.; Ibáñez-Sandoval, O.; Koós, T.; Tepper, J.M. Are striatal tyrosine hydroxylase interneurons dopaminergic? J. Neurosci. 2015, 35, 6584–6599. [Google Scholar] [CrossRef] [PubMed]
- Ünal, B.; Shah, F.; Kothari, J.; Tepper, J.M. Anatomical and electrophysiological changes in striatal TH interneurons after loss of the nigrostriatal dopaminergic pathway. Brain Struct. Funct. 2015, 220, 331–349. [Google Scholar] [CrossRef]
- Busceti, C.L.; Bucci, D.; Molinaro, G.; Di Pietro, P.; Zangrandi, L.; Gradini, R.; Moratalla, R.; Battaglia, G.; Bruno, V.; Nicoletti, F.; et al. Lack or inhibition of dopaminergic stimulation induces a development increase of striatal tyrosine hydroxylase-positive interneurons. PLoS ONE 2012, 7, e44025. [Google Scholar] [CrossRef]
- Lenz, J.D.; Lobo, M.K. Optogenetic insights into striatal function and behavior. Behav. Brain Res. 2013, 255, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Caravaggio, F.; Hahn, M.; Nakajima, S.; Gerretsen, P.; Remington, G.; Graff-Guerrero, A. Reduced insulin-receptor mediated modulation of striatal dopamine release by basal insulin as a possible contributing factor to hyperdopaminergia in schizophrenia. Med. Hypotheses 2015, 85, 391–396. [Google Scholar] [CrossRef]
- Stouffer, M.A.; Woods, C.A.; Patel, J.C.; Lee, C.R.; Witkovsky, P.; Bao, L.; Machold, R.P.; Jones, K.T.; de Vaca, S.C.; Reith, M.E.A.; et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat. Commun. 2015, 6, 8543. [Google Scholar] [CrossRef]
- Harrison, N.J.; Connolly, E.; Gascón Gubieda, A.; Yang, Z.; Altenhein, B.; Losada Perez, M.; Moreira, M.; Sun, J.; Hidalgo, A. Regenerative neurogenic response from glia requires insulin-driven neuron-glia communication. Elife 2021, 10, e58756. [Google Scholar] [CrossRef]
- Ziegler, A.N.; Levison, S.W.; Wood, T.L. Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat. Rev. Endocrinol. 2015, 11, 161–170. [Google Scholar] [CrossRef]
- Spinelli, M.; Fusco, S.; Grassi, C. Brain Insulin Resistance and Hippocampal Plasticity: Mechanisms and Biomarkers of Cognitive Decline. Front. Neurosci. 2019, 13, 788. [Google Scholar] [CrossRef]
- Fiory, F.; Perruolo, G.; Cimmino, I.; Cabaro, S.; Pignalosa, F.C.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. The Relevance of Insulin Action in the Dopaminergic System. Front. Neurosci. 2019, 13, 868. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, W.; Vodovozova, E.; Tretiakova, D.; Boldyrevd, I.; Li, Y.; Kürths, J.; Yu, T.; Semyachkina-Glushkovskaya, O.; Zhu, D. Photodynamic opening of the blood-brain barrier to high weight molecules and liposomes through an optical clearing skull window. Biomed. Opt. Express 2018, 9, 4850–4862. [Google Scholar] [CrossRef]
- Nishijima, T.; Piriz, J.; Duflot, S.; Fernandez, A.M.; Gaitan, G.; Gomez-Pinedo, U.; Verdugo, J.M.G.; Leroy, F.; Soya, H.; Nuñez, A.; et al. Neuronal Activity Drives Localized Blood-Brain-Barrier Transport of Serum Insulin-like Growth Factor-I into the CNS. Neuron 2010, 67, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Alkass, K.; Bernard, S.; Salehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisén, J. Neurogenesis in the Striatum of the Adult Human Brain. Cell 2014, 156, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Farzanehfar, P. Comparative review of adult midbrain and striatum neurogenesis with classical neurogenesis. Neurosci. Res. 2018, 134, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Inta, D.; Cameron, H.A.; Gass, P. New neurons in the adult striatum: From rodents to humans. Trends Neurosci. 2015, 38, 517–523. [Google Scholar] [CrossRef]
- Magnusson, J.P.; Göritz, C.; Tatarishvili, J.; Dias, D.O.; Smith, E.M.; Lindvall, O.; Kokaia, Z.; Frisén, J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science 2014, 346, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-Y.D.; Shetty, A.K. Treating Parkinson’s disease by astrocyte reprogramming: Progress and challenges. Sci. Adv. 2021, 7, eabg3198. [Google Scholar] [CrossRef]
- Niu, W.; Zang, T.; Zou, Y.; Fang, S.; Smith, D.K.; Bachoo, R.; Zhang, C.-L. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 2013, 15, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Masserdotti, G.; Gillotin, S.; Sutor, B.; Drechsel, D.; Irmler, M.; Jørgensen, H.; Sass, S.; Theis, F.; Beckers, J.; Berninger, B.; et al. Transcriptional Mechanisms of Proneural Factors and REST in Regulating Neuronal Reprogramming of Astrocytes. Cell Stem Cell 2015, 17, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Rivetti di Val Cervo, P.; Romanov, R.A.; Spigolon, G.; Masini, D.; Martín-Montañez, E.; Toledo, E.M.; La Manno, G.; Feyder, M.; Pifl, C.; Ng, Y.-H.; et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat. Biotechnol. 2017, 35, 444–452. [Google Scholar] [CrossRef]
- Ghasemi-Kasman, M.; Hajikaram, M.; Baharvand, H.; Javan, M. MicroRNA-Mediated In Vitro and In Vivo Direct Conversion of Astrocytes to Neuroblasts. PLoS ONE 2015, 10, e0127878. [Google Scholar] [CrossRef]
- Mohapel, P.; Frielingsdorf, H.; Häggblad, J.; Zachrisson, O.; Brundin, P. Platelet-Derived Growth Factor (PDGF-BB) and Brain-Derived Neurotrophic Factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience 2005, 132, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Fallon, J.; Reid, S.; Kinyamu, R.; Opole, I.; Opole, R.; Baratta, J.; Korc, M.; Endo, T.L.; Duong, A.; Nguyen, G.; et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc. Natl. Acad. Sci. USA 2000, 97, 14686–14691. [Google Scholar] [CrossRef] [PubMed]
- Huot, P.; Lévesque, M.; Parent, A. The fate of striatal dopaminergic neurons in Parkinson’s disease and Huntington’s chorea. Brain 2006, 130, 222–232. [Google Scholar] [CrossRef][Green Version]
- Hermann, A.; Storch, A. Endogenous regeneration in Parkinson’s disease: Do we need orthotopic dopaminergic neurogenesis? Stem Cells 2008, 26, 2749–2752. [Google Scholar] [CrossRef]
- Papanikolaou, T.; Lennington, J.B.; Betz, A.; Figueiredo, C.; Salamone, J.D.; Conover, J.C. In vitro generation of dopaminergic neurons from adult subventricular zone neural progenitor cells. Stem Cells Dev. 2008, 17, 157–172. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmina, A.B.; Kapkaeva, M.R.; Vetchinova, A.S.; Illarioshkin, S.N. Novel Approaches Used to Examine and Control Neurogenesis in Parkinson′s Disease. Int. J. Mol. Sci. 2021, 22, 9608. https://doi.org/10.3390/ijms22179608
Salmina AB, Kapkaeva MR, Vetchinova AS, Illarioshkin SN. Novel Approaches Used to Examine and Control Neurogenesis in Parkinson′s Disease. International Journal of Molecular Sciences. 2021; 22(17):9608. https://doi.org/10.3390/ijms22179608
Chicago/Turabian StyleSalmina, Alla B., Marina R. Kapkaeva, Anna S. Vetchinova, and Sergey N. Illarioshkin. 2021. "Novel Approaches Used to Examine and Control Neurogenesis in Parkinson′s Disease" International Journal of Molecular Sciences 22, no. 17: 9608. https://doi.org/10.3390/ijms22179608
APA StyleSalmina, A. B., Kapkaeva, M. R., Vetchinova, A. S., & Illarioshkin, S. N. (2021). Novel Approaches Used to Examine and Control Neurogenesis in Parkinson′s Disease. International Journal of Molecular Sciences, 22(17), 9608. https://doi.org/10.3390/ijms22179608





