Targeted Disruption of the Inhibitor of DNA Binding 4 (Id4) Gene Alters Photic Entrainment of the Circadian Clock
Abstract
:1. Introduction
2. Results
2.1. Circadian Rhythm Characteristics Are Different in Id4−/− Mice
2.2. Id4−/− Mice Exhibit Reduced Phase Delays in Response to Continuous Light
2.3. Id4−/− Mice Show Smaller Phase Shifts in Response to Nonparametric Entrainment, Using Discrete Saturating Light Pulses and Discrete Low Illuminance/Short Duration Light Pulses
2.4. Id4−/− Phase Angle Is Advanced under Variable Photoperiods
2.5. Histological Analysis of Id4−/− SCN
2.6. Histological Analysis of Id4−/− Retinal Hypothalamic Tract
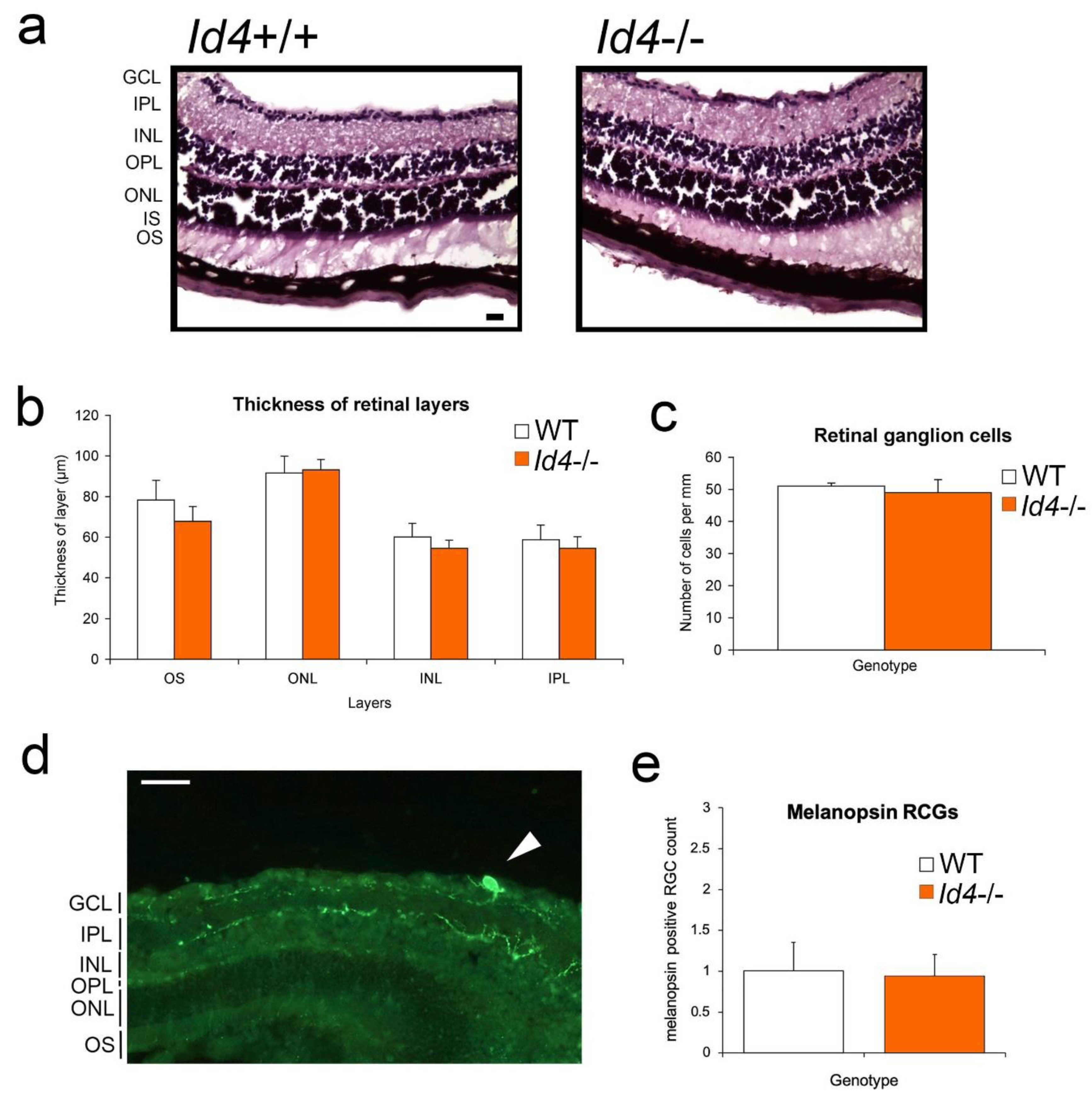
2.7. Retinal Anatomy and Pupil Constriction Responses in Id4−/− Compared to WT Mice
2.8. Gene Expression of Id1 Is Abnormal in Id4−/− SCN
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Locomotor Activity Monitoring, Behavioral Manipulations, and Circadian Phenotype Analysis
4.3. Continuous/Parametric Entrainment Experiments
4.4. Discrete/Nonparametric Entrainment Experiments
4.5. Phase angle Determination under Different Photoperiods
4.6. Whole Brain and SCN Histology
4.7. Retinal Hypothalamic Tract (RHT) Tracing
4.8. Retinal Histology and Anti-Melanopsin Immunohistochemistry
4.9. Pupillometry
4.10. SCN Gene Expression Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.H.; Lazar, M.A. Transcriptional Control of Circadian Rhythms and Metabolism: A Matter of Time and Space. Endocr. Rev. 2020, 41, 707–732. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New Insights Into the Circadian Rhythm and Its Related Diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [Green Version]
- Peirson, S.N.; Butler, J.N.; Duffield, G.E.; Takher, S.; Sharma, P.; Foster, R.G. Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem. Biophys. Res. Commun. 2006, 351, 800–807. [Google Scholar] [CrossRef]
- Dunlap, J.C.; Loros, J.J.; Decoursey, P. Chronobiology: Biological Timekeeping, 1st ed.; Sinauer Assoc.: Sunderland, MA, USA, 2004. [Google Scholar]
- Patel, D.; Morton, D.J.; Carey, J.; Havrda, M.C.; Chaudhary, J. Inhibitor of differentiation 4 (ID4): From development to cancer. Biochim. Biophys. Acta 2015, 1855, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Duffield, G.E.; Watson, N.P.; Mantani, A.; Peirson, S.N.; Robles-Murguia, M.; Loros, J.J.; Israel, M.A.; Dunlap, J.C. A role for Id2 in regulating photic entrainment of the mammalian circadian system. Curr. Biol. 2009, 19, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Duffield, G.E.; Best, J.D.; Meurers, B.H.; Bittner, A.; Loros, J.J.; Dunlap, J.C. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr. Biol. 2002, 12, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.Y.; Ward, S.M.; Murad, J.M.; Watson, N.P.; Israel, M.A.; Duffield, G.E. ID2 (inhibitor of DNA binding 2) is a rhythmically expressed transcriptional repressor required for circadian clock output in mouse liver. J. Biol. Chem. 2009, 284, 31735–31745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.; Antoch, M.P.; Miller, B.H.; Su, A.I.; Schook, A.B.; Straume, M.; Schultz, P.G.; Kay, S.A.; Takahashi, J.S.; Hogenesch, J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002, 109, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.E.; DiTacchio, L.; Hayes, K.R.; Vollmers, C.; Pulivarthy, S.; Baggs, J.E.; Panda, S.; Hogenesch, J.B. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009, 5, e1000442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, A.A.; Fujioka, A.; Nagano, M.; Masumoto, K.H.; Takumi, T.; Yoshimura, T.; Ebihara, S.; Mori, K.; Yokota, Y.; Shigeyoshi, Y. Helix-loop-helix protein Id2 stabilizes mammalian circadian oscillation under constant light conditions. Zoolog. Sci. 2013, 30, 1011–1018. [Google Scholar] [CrossRef]
- Ward, S.M.; Fernando, S.J.; Hou, T.Y.; Duffield, G.E. The transcriptional repressor ID2 can interact with the canonical clock components CLOCK and BMAL1 and mediate inhibitory effects on mPer1 expression. J. Biol. Chem. 2010, 285, 38987–39000. [Google Scholar] [CrossRef] [Green Version]
- Mathew, D.; Zhou, P.; Pywell, C.M.; van der Veen, D.R.; Shao, J.; Xi, Y.; Bonar, N.A.; Hummel, A.D.; Chapman, S.; Leevy, W.M.; et al. Ablation of the ID2 gene results in altered circadian feeding behavior, and sex-specific enhancement of insulin sensitivity and elevated glucose uptake in skeletal muscle and brown adipose tissue. PLoS ONE 2013, 8, e73064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Hummel, A.D.; Pywell, C.M.; Dong, X.C.; Charlie Dong, X.; Duffield, G.E. High fat diet rescues disturbances to metabolic homeostasis and survival in the Id2 null mouse in a sex-specific manner. Biochem. Biophys. Res. Commun. 2014, 451, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Robles-Murguia, M.; Mathew, D.; Duffield, G.E. Impaired Thermogenesis and a Molecular Signature for Brown Adipose Tissue in Id2 Null Mice. J. Diabetes Res. 2016, 2016, 6785948. [Google Scholar] [CrossRef] [Green Version]
- Duffield, G.E.; Han, S.; Hou, T.Y.; de la Iglesia, H.O.; McDonald, K.A.; Mecklenburg, K.L.; Robles-Murguia, M. Regulates Photic Entrainment Responses in Mice: Differential Responses of the Id2−/− Mouse Circadian System Are Dependent on Circadian Phase and on Duration and Intensity of Light. J. Biol. Rhythms 2020, 35, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Baker, N.E. E Proteins and ID Proteins: Helix-Loop-Helix Partners in Development and Disease. Dev. Cell 2015, 35, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.A.; Williams, J.; Taylor, L.; Thomson, R.J.; Nolan, P.M.; Foster, R.G.; Peirson, S.N. Meta-analysis of transcriptomic datasets identifies genes enriched in the mammalian circadian pacemaker. Nucleic Acids Res. 2017, 45, 9860–9873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.H.; Elliott, J.A.; Foster, R. Entrainment of circadian programs. Chronobiol. Int. 2003, 20, 741–774. [Google Scholar] [CrossRef]
- Foster, R.G.; Hughes, S.; Peirson, S.N. Circadian Photoentrainment in Mice and Humans. Biology 2020, 9, 180. [Google Scholar] [CrossRef]
- Pittendrigh, C.S.; Daan, S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as Clock. J. Comp. Physiol. 1976, 106, 291–331. [Google Scholar] [CrossRef]
- Winfree, A. The Geometry of Biological Time, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
- Aschoff, J. Circadian rhythms: Influences of internal and external factors on the period measured in constant conditions. Z. Tierpsychol. 1979, 49, 225–249. [Google Scholar] [CrossRef] [PubMed]
- von Gall, C.; Duffield, G.E.; Hastings, M.H.; Kopp, M.D.; Dehghani, F.; Korf, H.W.; Stehle, J.H. CREB in the mouse SCN: A molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access. J. Neurosci. 1998, 18, 10389–10397. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.; Mantani, A.; Garel, S.; Rubenstein, J.; Israel, M.A. Id4 regulates neural progenitor proliferation and differentiation in vivo. Development 2004, 131, 5441–5448. [Google Scholar] [CrossRef] [Green Version]
- Bedford, L.; Walker, R.; Kondo, T.; van Crüchten, I.; King, E.R.; Sablitzky, F. Id4 is required for the correct timing of neural differentiation. Dev. Biol. 2005, 280, 386–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.E.; Takahashi, J.S. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). J. Physiol. 1991, 439, 115–145. [Google Scholar] [CrossRef] [PubMed]
- Comas, M.; Beersma, D.G.; Spoelstra, K.; Daan, S. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J. Biol. Rhythms 2006, 21, 362–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronauer, R.E.; St Hilaire, M.A.; Rahman, S.A.; Czeisler, C.A.; Klerman, E.B. An Exploration of the Temporal Dynamics of Circadian Resetting Responses to Short- and Long-Duration Light Exposures: Cross-Species Consistencies and Differences. J. Biol. Rhythms 2019, 34, 497–514. [Google Scholar] [CrossRef]
- Gronfier, C.; Wright, K.P.; Kronauer, R.E.; Czeisler, C.A. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc. Natl. Acad. Sci. USA 2007, 104, 9081–9086. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.R.; Campbell, S.S.; Zone, S.E.; Cooper, F.; DeSano, A.; Murphy, P.J.; Jones, B.; Czajkowski, L.; Ptácek, L.J. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat. Med. 1999, 5, 1062–1065. [Google Scholar] [CrossRef]
- Lee, K.; Shiva Kumar, P.; McQuade, S.; Lee, J.Y.; Park, S.; An, Z.; Piccoli, B. Experimental and Mathematical Analyses Relating Circadian Period and Phase of Entrainment in Neurospora crassa. J. Biol. Rhythms 2017, 32, 550–559. [Google Scholar] [CrossRef]
- Murad, J.M.; Place, C.S.; Ran, C.; Hekmatyar, S.K.; Watson, N.P.; Kauppinen, R.A.; Israel, M.A. Inhibitor of DNA binding 4 (ID4) regulation of adipocyte differentiation and adipose tissue formation in mice. J. Biol. Chem. 2010, 285, 24164–24173. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Larkin, D.W.; Albrecht, U.; Sun, Z.S.; Sage, M.; Eichele, G.; Lee, C.C.; Bradley, A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 1999, 400, 169–173. [Google Scholar] [CrossRef]
- Masubuchi, S.; Kataoka, N.; Sassone-Corsi, P.; Okamura, H. Mouse Period1 (mPER1) acts as a circadian adaptor to entrain the oscillator to environmental light/dark cycles by regulating mPER2 protein. J. Neurosci. 2005, 25, 4719–4724. [Google Scholar] [CrossRef] [Green Version]
- van der Horst, G.T.; Muijtjens, M.; Kobayashi, K.; Takano, R.; Kanno, S.; Takao, M.; de Wit, J.; Verkerk, A.; Eker, A.P.; van Leenen, D.; et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 1999, 398, 627–630. [Google Scholar] [CrossRef]
- Cermakian, N.; Monaco, L.; Pando, M.P.; Dierich, A.; Sassone-Corsi, P. Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO J. 2001, 20, 3967–3974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.Y.; Dunn, F.A.; Berson, D.M. Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron 2005, 48, 1001–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, M.P.; Silver, R. Divergent photic thresholds in the non-image-forming visual system: Entrainment, masking and pupillary light reflex. Proc. Biol. Sci. 2011, 278, 745–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lall, G.S.; Revell, V.L.; Momiji, H.; Al Enezi, J.; Altimus, C.M.; Güler, A.D.; Aguilar, C.; Cameron, M.A.; Allender, S.; Hankins, M.W.; et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron 2010, 66, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Haugen, M.J.; Woods, D.C. Role for inhibitor of differentiation/deoxyribonucleic acid-binding (Id) proteins in granulosa cell differentiation. Endocrinology 2008, 149, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Knowell, A.E.; Chinaranagari, S.; Komaragiri, S.; Nagappan, P.; Patel, D.; Havrda, M.C.; Chaudhary, J. Id4 deficiency attenuates prostate development and promotes PIN-like lesions by regulating androgen receptor activity and expression of NKX3.1 and PTEN. Mol. Cancer 2013, 12, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Chinaranagari, S.; Chaudhary, J. Inhibitor of differentiation 4 (ID4) acts as an inhibitor of ID-1, -2 and -3 and promotes basic helix loop helix (bHLH) E47 DNA binding and transcriptional activity. Biochimie 2015, 112, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Lee, J.; Kwon, I.; Nakajima, Y.; Ohmiya, Y.; Son, G.H.; Lee, K.H.; Kim, K. Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J. Cell Sci. 2010, 123, 3547–3557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shearman, L.P.; Weaver, D.R. Photic induction of Period gene expression is reduced in Clock mutant mice. Neuroreport 1999, 10, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Duffield, G.E.; Hastings, M.H.; Ebling, F.J. Investigation into the regulation of the circadian system by dopamine and melatonin in the adult Siberian hamster (Phodopus sungorus). J. Neuroendocrinol. 1998, 10, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Duffield, G.E.; Dickerson, J.M.; Alexander, I.H.; Ebling, F.J. Ontogeny of a photic response in the suprachiasmatic nucleus in the Siberian hamster (Phodopus sungorus). Eur. J. Neurosci. 1995, 7, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Provencio, I.; Rollag, M.D.; Castrucci, A.M. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature 2002, 415, 493. [Google Scholar] [CrossRef]
- Van Gelder, R.N. Nonvisual ocular photoreception in the mammal. Methods Enzymol. 2005, 393, 746–755. [Google Scholar] [CrossRef]
- Hattar, S.; Lucas, R.J.; Mrosovsky, N.; Thompson, S.; Douglas, R.H.; Hankins, M.W.; Lem, J.; Biel, M.; Hofmann, F.; Foster, R.G.; et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 2003, 424, 76–81. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duffield, G.E.; Robles-Murguia, M.; Hou, T.Y.; McDonald, K.A. Targeted Disruption of the Inhibitor of DNA Binding 4 (Id4) Gene Alters Photic Entrainment of the Circadian Clock. Int. J. Mol. Sci. 2021, 22, 9632. https://doi.org/10.3390/ijms22179632
Duffield GE, Robles-Murguia M, Hou TY, McDonald KA. Targeted Disruption of the Inhibitor of DNA Binding 4 (Id4) Gene Alters Photic Entrainment of the Circadian Clock. International Journal of Molecular Sciences. 2021; 22(17):9632. https://doi.org/10.3390/ijms22179632
Chicago/Turabian StyleDuffield, Giles E., Maricela Robles-Murguia, Tim Y. Hou, and Kathleen A. McDonald. 2021. "Targeted Disruption of the Inhibitor of DNA Binding 4 (Id4) Gene Alters Photic Entrainment of the Circadian Clock" International Journal of Molecular Sciences 22, no. 17: 9632. https://doi.org/10.3390/ijms22179632
APA StyleDuffield, G. E., Robles-Murguia, M., Hou, T. Y., & McDonald, K. A. (2021). Targeted Disruption of the Inhibitor of DNA Binding 4 (Id4) Gene Alters Photic Entrainment of the Circadian Clock. International Journal of Molecular Sciences, 22(17), 9632. https://doi.org/10.3390/ijms22179632






