Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction
Abstract
1. Introduction
2. Causes of Inflammation in Male Reproductive Tract
2.1. Endogenous Causes of Inflammation
2.1.1. Obesity
2.1.2. Varicocele
2.1.3. Leukocytospermia
2.2. Exogenous Causes of Inflammation
2.2.1. Sexually Transmitted Diseases
2.2.2. Prostatitis
2.2.3. Urethritis
2.2.4. Viral Infections
HIV
Hepatitis
3. Oxidative Stress as Cause and Consequence of Inflammation
4. Oxidative Stress and Male Infertility
4.1. Lipid Peroxidation (LPO)
4.2. Sperm DNA Fragmentation
4.3. Apoptosis
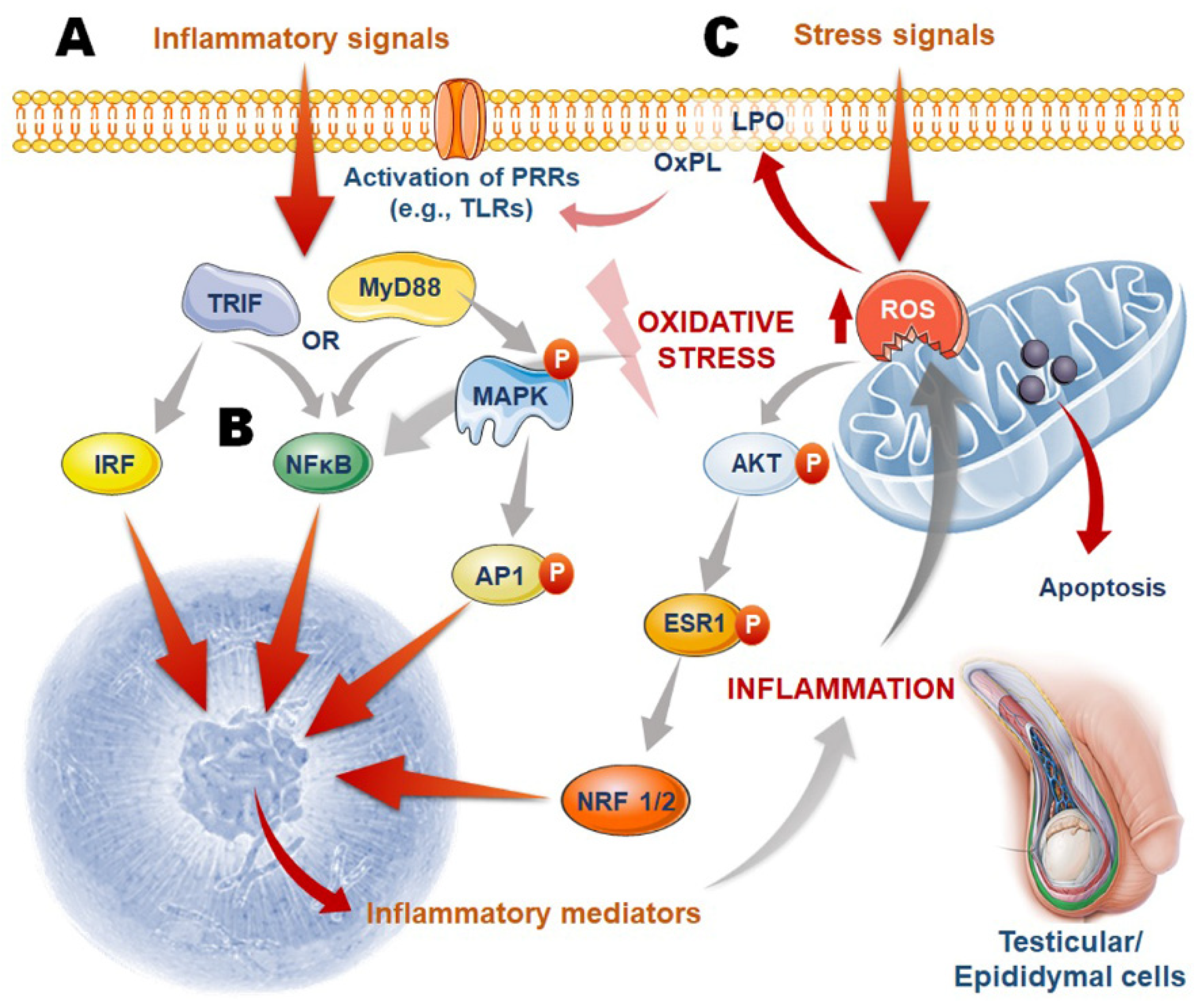
5. Oxidant Sensitive Inflammatory Pathways
5.1. Initiation of Male Reproductive Tract Inflammation
5.2. Inflammation-Mediated Oxidative Stress in Male Reproductive Tissues
5.3. Oxidative Stress-Induced Inflammation in Male Reproductive Tissues
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sengupta, P.; Dutta, S.; Krajewska-Kulak, E. The Disappearing Sperms: Analysis of Reports Published Between 1980 and 2015. Am. J. Mens Health 2017, 11, 1279–1304. [Google Scholar] [CrossRef]
- Sengupta, P.; Nwagha, U.; Dutta, S.; Krajewska-Kulak, E.; Izuka, E. Evidence for decreasing sperm count in African population from 1965 to 2015. Afr. Health Sci. 2017, 17, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Dutta, S.; Tusimin, M.B.; İrez, T.; Krajewska-Kulak, E. Sperm counts in Asian men: Reviewing the trend of past 50 years. Asian Pac. J. Reprod. 2018, 7, 87–92. [Google Scholar] [CrossRef]
- Sengupta, P. Reviewing reports of semen volume and male aging of last 33 years: From 1980 through 2013. Asian Pac. J. Reprod. 2015, 4, 242–246. [Google Scholar] [CrossRef]
- Sengupta, P.; Borges, E., Jr.; Dutta, S.; Krajewska-Kulak, E. Decline in sperm count in European men during the past 50 years. Hum. Exp. Toxicol. 2018, 37, 247–255. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Sengupta, P.; Dutta, S.; Karkada, I.R. Obesity, systemic inflammation and male infertility. Chem. Biol. Lett. 2020, 7, 92–98. [Google Scholar]
- Agarwal, A.; Leisegang, K.; Sengupta, P. Oxidative stress in pathologies of male reproductive disorders. In Pathology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 15–27. [Google Scholar]
- Dutta, S.; Sengupta, P.; Chhikara, B.S. Reproductive inflammatory mediators and male infertility. Chem. Biol. Lett. 2020, 7, 73–74. [Google Scholar]
- Leisegang, K.; Dutta, S. Do lifestyle practices impede male fertility? Andrologia 2021, 53, e13595. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. 2016, 14, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sengupta, P. Oxidative stress and its association with male infertility. In Male Infertility; Springer: Berlin/Heidelberg, Germany, 2020; pp. 57–68. [Google Scholar]
- Azenabor, A.; Ekun, A.O.; Akinloye, O. Impact of inflammation on male reproductive tract. J. Reprod. Infertil. 2015, 16, 123. [Google Scholar]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191. [Google Scholar] [CrossRef]
- Selvam, M.K.P.; Sengupta, P.; Agarwal, A. Sperm DNA fragmentation and male infertility. In Genetics of Male Infertility; Springer: Berlin/Heidelberg, Germany, 2020; pp. 155–172. [Google Scholar]
- Dutta, S.; Henkel, R.; Sengupta, P.; Agarwal, A. Physiological role of ROS in sperm function. In Male Infertility; Springer: Berlin/Heidelberg, Germany, 2020; pp. 337–345. [Google Scholar]
- Thompson, A.; Agarwal, A.; du Plessis, S.S. Physiological Role of Reactive Oxygen Species in Sperm Function: A Review. Antioxidants in Male Infertility: A Guide for Clinicians and Researchers; Springer Science and Business Media: New York, NY, USA, 2014; pp. 69–89. [Google Scholar]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Griveau, J.F.; Le Lannou, D. Reactive oxygen species and human spermatozoa: Physiology and pathology. Int. J. Androl. 1997, 20, 61–69. [Google Scholar] [CrossRef]
- Balhorn, R. A model for the structure of chromatin in mammalian sperm. J. Cell Biol. 1982, 93, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Saowaros, W.; Panyim, S. The formation of disulfide bonds in human protamines during sperm maturation. Experientia 1979, 35, 191–192. [Google Scholar] [CrossRef]
- Fujii, J.; Tsunoda, S. Redox regulation of fertilisation and the spermatogenic process. Asian J. Androl. 2011, 13, 420–423. [Google Scholar] [CrossRef]
- Hamada, A.; Sharma, R.; Du Plessis, S.S.; Willard, B.; Yadav, S.P.; Sabanegh, E.; Agarwal, A. Two-dimensional differential in-gel electrophoresis–based proteomics of male gametes in relation to oxidative stress. Fertil. Steril. 2013, 99, 1216–1226.e2. [Google Scholar] [CrossRef] [PubMed]
- Khosrowbeygi, A.; Zarghami, N. Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins Leukot. Essent. 2007, 77, 117–121. [Google Scholar] [CrossRef]
- Izuka, E.; Menuba, I.; Sengupta, P.; Dutta, S.; Nwagha, U. Antioxidants, anti-inflammatory drugs and antibiotics in the treatment of reproductive tract infections and their association with male infertility. Chem. Biol. Lett. 2020, 7, 156–165. [Google Scholar]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Sengupta, P.; Dutta, S. Coenzyme Q10 improves sperm parameters, oxidative stress markers and sperm DNA fragmentation in infertile patients with idiopathic oligoasthenozoospermia. World J. Mens Health 2021, 39, 346. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T.; Sengupta, P.; Dutta, S.; Calogero, A.E. Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin. Exp. Reprod. Med. 2021, 48, 150. [Google Scholar] [CrossRef] [PubMed]
- Cotran, R.S. Acute and chronic inflammation. In Robbins Pathological Basis of Disease; Elsevier Health Sciences: Philadelphia, PA, USA, 1999; pp. 50–88. [Google Scholar]
- Anderson, M.T.; Staal, F.; Gitler, C.; Herzenberg, L.A. Separation of oxidant-initiated and redox-regulated steps in the NF-kappa B signal transduction pathway. Proc. Natl. Acad. Sci. USA 1994, 91, 11527–11531. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Brigelius-Flohé, R.; Saliou, C.; Traber, M.G.; Packer, L. Redox regulation of NF-kappa B activation. Free Radic. Biol. Med. 1997, 22, 1115–1126. [Google Scholar] [CrossRef]
- Dohle, G.R.; Smit, M.; Weber, R.F. Androgens and male fertility. World J. Urol. 2003, 21, 341–345. [Google Scholar] [CrossRef]
- Garolla, A.; Pizzol, D.; Bertoldo, A.; Menegazzo, M.; Barzon, L.; Foresta, C. Sperm viral infection and male infertility: Focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J. Reprod. Immunol. 2013, 100, 20–29. [Google Scholar] [CrossRef]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef]
- Laleman, W.; Claria, J.; Van der Merwe, S.; Moreau, R.; Trebicka, J. Systemic inflammation and acute-on-chronic liver failure: Too much, not enough. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; D’Souza, U.; Alahmar, A. Reproductive tract infection, inflammation and male infertility. Chem. Biol. Lett. 2020, 7, 75–84. [Google Scholar]
- İrez, T.; Karkada, I.R.; Dutta, S.; Sengupta, P. Obestatin in male reproduction and infertility. Chem. Biol. Lett. 2019, 8, 239. [Google Scholar]
- Hammoud, A.O.; Gibson, M.; Peterson, C.M.; Meikle, A.W.; Carrell, D.T. Impact of male obesity on infertility: A critical review of the current literature. Fertil. Steril. 2008, 90, 897–904. [Google Scholar] [CrossRef]
- Irez, T.; Bicer, S.; Sahin, S.; Dutta, S.; Sengupta, P. Cytokines and adipokines in the regulation of spermatogenesis and semen quality. Chem. Biol. Lett. 2020, 7, 131–139. [Google Scholar]
- Dutta, S.; Biswas, A.; Sengupta, P. Obesity, endocrine disruption and male infertility. Asian Pac. J. Reprod. 2019, 8, 195–201. [Google Scholar] [CrossRef]
- Sengupta, P.; Bhattacharya, K.; Dutta, S. Leptin and male reproduction. Asian Pac. J. Reprod. 2019, 8, 220–226. [Google Scholar] [CrossRef]
- Davi, G.; Falco, A. Oxidant stress, inflammation and atherogenesis. Lupus 2005, 14, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Singh, R.; Cannarella, R.; Sengupta, P.; Dutta, S. Coenzyme Q10, oxidative stress, and male infertility: A review. Clin. Exp. Reprod. Med. 2021, 48, 97–104. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Sengupta, P. Impact of Coenzyme Q10 and Selenium on Seminal Fluid Parameters and Antioxidant Status in Men with Idiopathic Infertility. Biol. Trace Elem. Res. 2021, 199, 1246–1252. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Desai, N.R.; Prabakaran, S.; Tavares, A.; Sabanegh, E. Role of oxidative stress in pathogenesis of varicocele and infertility. Urology 2009, 73, 461–469. [Google Scholar] [CrossRef] [PubMed]
- French, D.B.; Desai, N.R.; Agarwal, A. Varicocele repair: Does it still have a role in infertility treatment? Curr. Opin. Obstet. Gynecol. 2008, 20, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, F.F.; Sundaram, A.; Sharma, R.K.; Borges, E., Jr.; Pasqualotto, E.B.; Agarwal, A. Semen quality and oxidative stress scores in fertile and infertile patients with varicocele. Fertil. Steril. 2008, 89, 602–607. [Google Scholar] [CrossRef]
- Ozbek, E.; Turkoz, Y.; Gokdeniz, R.; Davarci, M.; Ozugurlu, F. Increased nitric oxide production in the spermatic vein of patients with varicocele. Eur. Urol. 2000, 37, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Sultana, T.; Svechnikov, K.; Weber, G.; Söder, O. Molecular cloning and expression of a functionally different alternative splice variant of prointerleukin-1alpha from the rat testis. Endocrinology 2000, 141, 4413–4418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeinali, M.; Hadian Amree, A.; Khorramdelazad, H.; Karami, H.; Abedinzadeh, M. Inflammatory and anti-inflammatory cytokines in the seminal plasma of infertile men suffering from varicocele. Andrologia 2017, 49, 1–4. [Google Scholar] [CrossRef]
- Allen, J.D.; Gow, A.J. Nitrite, NO and hypoxic vasodilation. Br. J. Pharmacol. 2009, 158, 1653–1654. [Google Scholar] [CrossRef]
- Romeo, C.; Ientile, R.; Impellizzeri, P.; Turiaco, N.; Teletta, M.; Antonuccio, P.; Basile, M.; Gentile, C. Preliminary report on nitric oxide-mediated oxidative damage in adolescent varicocele. Hum. Reprod. 2003, 18, 26–29. [Google Scholar] [CrossRef]
- Benoff, S.; Goodwin, L.O.; Millan, C.; Hurley, I.R.; Pergolizzi, R.G.; Marmar, J.L. Deletions in L-type calcium channel alpha1 subunit testicular transcripts correlate with testicular cadmium and apoptosis in infertile men with varicoceles. Fertil. Steril. 2005, 83, 622–634. [Google Scholar] [CrossRef]
- Coban, S.; Keles, I.; Biyik, İ.; Guzelsoy, M.; Turkoglu, A.R.; Ocak, N. Does varicocele correction lead to normalization of preoperatively elevated mean platelet volume levels? Can. Urol. Assoc. J. 2015, 9, E5–E9. [Google Scholar] [CrossRef]
- Nazari, A.; Hassanshahi, G.; Khorramdelazad, H. Elevated levels of epithelial neutrophil activating peptide-78 (ENA-78)(CXCL5) and Interleukin-1β is correlated with varicocele-caused infertility: A novel finding. Middle East Fertil. Soc. J. 2017, 22, 333–335. [Google Scholar] [CrossRef]
- Hirik, E.; Suleyman, B.; Mammadov, R.; Yapanoglu, T.; Cimen, F.K.; Cetin, N.; Kurt, N. Effect of anakinra, an interleukin one beta antagonist, on oxidative testicular damage induced in rats with ischemia reperfusion. Rev. Int. Androl. 2018, 16, 87–94. [Google Scholar] [CrossRef]
- Plante, M.; de Lamirande, E.; Gagnon, C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil. Steril. 1994, 62, 387–393. [Google Scholar] [CrossRef]
- Henkel, R.; Maass, G.; Jung, A.; Haidl, G.; Schill, W.B.; Schuppe, H.C. Age-related changes in seminal polymorphonuclear elastase in men with asymptomatic inflammation of the genital tract. Asian J. Androl. 2007, 9, 299–304. [Google Scholar] [CrossRef]
- Wolff, H. The biologic significance of white blood cells in semen. Fertil. Steril. 1995, 63, 1143–1157. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Comhaire, F.; Bosmans, E.; Ombelet, W.; Punjabi, U.; Schoonjans, F. Cytokines in semen of normal men and of patients with andrological diseases. Am. J. Reprod. Immunol. 1994, 31, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, J.S.; Raburn, D.; Muasher, S. Leukocytospermia: Overview of diagnosis, implications, and management of a controversial finding. Middle East Fertil. Soc. J. 2013, 18, 129–134. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Aziz, N.; Agarwal, A.; Lewis-Jones, I.; Sharma, R.K.; Thomas, A.J., Jr. Novel associations between specific sperm morphological defects and leukocytospermia. Fertil. Steril. 2004, 82, 621–627. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Kandirali, E.; Sharma, R.K.; Thomas, A.J.; Nada, E.A.; Evenson, D.P.; Alvarez, J.G. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil. Steril. 2002, 78, 1215–1224. [Google Scholar] [CrossRef]
- Hagan, S.; Khurana, N.; Chandra, S.; Abdel-Mageed, A.B.; Mondal, D.; Hellstrom, W.J.; Sikka, S.C. Differential expression of novel biomarkers (TLR-2, TLR-4, COX-2, and Nrf-2) of inflammation and oxidative stress in semen of leukocytospermia patients. Andrology 2015, 3, 848–855. [Google Scholar] [CrossRef]
- Mazzoli, S.; Cai, T.; Addonisio, P.; Bechi, A.; Mondaini, N.; Bartoletti, R. Chlamydia trachomatis infection is related to poor semen quality in young prostatitis patients. Eur. Urol. 2010, 57, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Ouzounova-Raykova, V.; Rangelov, S.; Ouzounova, I.; Mitov, I. Detection of Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma hominis in infertile Bulgarian men with multiplex real-time polymerase chain reaction. Apmis 2015, 123, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, F.; Sommese, L.; Gorga, F.; Galdiero, E.; Rizzo, A.; Ajello, M. Toxic effect on human spermatozoa by Chlamydia trachomatis purified lipopolysaccharide. FEMS Microbiol. Lett. 1994, 115, 197–200. [Google Scholar] [CrossRef][Green Version]
- Nunez-Calonge, R.; Caballero, P.; Redondo, C.; Baquero, F.; Martinez-Ferrer, M.; Meseguer, M. Ureaplasma urealyticum reduces motility and induces membrane alterations in human spermatozoa. Hum. Reprod. 1998, 13, 2756–2761. [Google Scholar] [CrossRef]
- Jarecki-Black, J.; Lushbaugh, W.; Golosov, L.; Glassman, A. Trichomonas vaginalis: Preliminary characterization of a sperm motility inhibiting factor. Ann. Clin. Lab. Sci. 1988, 18, 484–489. [Google Scholar] [PubMed]
- Alshahrani, S.; McGill, J.; Agarwal, A. Prostatitis and male infertility. J. Reprod. Immunol. 2013, 100, 30–36. [Google Scholar] [CrossRef]
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Martínez, P.; Proverbio, F.; Camejo, M.I. Sperm lipid peroxidation and pro-inflammatory cytokines. Asian J. Androl. 2007, 9, 102–107. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Male infertility; evidences, risk factors, causes, diagnosis and management in human. Ann. Clin. Lab. Res. 2017, 5, 188. [Google Scholar] [CrossRef]
- Schuppe, H.C.; Meinhardt, A.; Allam, J.; Bergmann, M.; Weidner, W.; Haidl, G. Chronic orchitis: A neglected cause of male infertility? Andrologia 2008, 40, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Byren, I.; Hoey, C.T. Treatment of bacterial prostatitis. Clin. Infect. Dis. 2010, 50, 1641–1652. [Google Scholar] [CrossRef]
- Krupp, K.; Madhivanan, P. Antibiotic resistance in prevalent bacterial and protozoan sexually transmitted infections. Indian J. Sex. Transm. Dis. AIDS 2015, 36, 3. [Google Scholar] [CrossRef] [PubMed]
- Magri, V.; Trinchieri, A.; Pozzi, G.; Restelli, A.; Garlaschi, M.C.; Torresani, E.; Zirpoli, P.; Marras, E.; Perletti, G. Efficacy of repeated cycles of combination therapy for the eradication of infecting organisms in chronic bacterial prostatitis. Int. J. Antimicrob. Agents 2007, 29, 549–556. [Google Scholar] [CrossRef]
- Oliphant, C.M.; Green, G. Quinolones: A comprehensive review. Am. Fam. Physician 2002, 65, 455. [Google Scholar]
- Costello, L.; Feng, P.; Milon, B.; Tan, M.; Franklin, R. Role of zinc in the pathogenesis and treatment of prostate cancer: Critical issues to resolve. Prostate Cancer Prostatic Dis. 2004, 7, 111–117. [Google Scholar] [CrossRef]
- Krause, W.; Bohring, C. Male infertility and genital chlamydial infection: Victim or perpetrator? Andrologia 2003, 35, 209–216. [Google Scholar] [CrossRef]
- Workowski, K.A.; Berman, S.M. Sexually Transmitted Diseases Treatment Guidelines; Department of Health and Human Services: Washington, DC, USA, 2010; Volume 59.
- Raz, R.; Colodner, R.; Kunin, C.M. Who are you—Staphylococcus saprophyticus? Clin. Infect. Dis. 2005, 40, 896–898. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Izuka, E.; Menuba, I.; Jegasothy, R.; Nwagha, U. Staphylococcal infections and infertility: Mechanisms and management. Mol. Cell Biochem. 2020, 474, 57–72. [Google Scholar] [CrossRef]
- Garolla, A.; Torino, M.; Sartini, B.; Cosci, I.; Patassini, C.; Carraro, U.; Foresta, C. Seminal and molecular evidence that sauna exposure affects human spermatogenesis. Hum. Reprod. 2013, 28, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Kehl, S.; Weigel, M.; Müller, D.; Gentili, M.; Hornemann, A.; Sütterlin, M. HIV-infection and modern antiretroviral therapy impair sperm quality. Arch. Gynecol. Obstet. 2011, 284, 229–233. [Google Scholar] [CrossRef]
- Pavili, L.; Daudin, M.; Moinard, N.; Walschaerts, M.; Cuzin, L.; Massip, P.; Pasquier, C.; Bujan, L. Decrease of mitochondrial DNA level in sperm from patients infected with human immunodeficiency virus-1 linked to nucleoside analogue reverse transcriptase inhibitors. Fertil. Steril. 2010, 94, 2151–2156. [Google Scholar] [CrossRef]
- Frapsauce, C.; Grabar, S.; Leruez-Ville, M.; Launay, O.; Sogni, P.; Gayet, V.; Viard, J.; De Almeida, M.; Jouannet, P.; Dulioust, E. Impaired sperm motility in HIV-infected men: An unexpected adverse effect of efavirenz? Hum. Reprod. 2015, 30, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Vicari, E.; Condorelli, R.; d’Agata, R.; Calogero, A. Male accessory gland infection and sperm parameters. Int. J. Androl. 2011, 34, e330–e347. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Ma, H.X.; Shi, X.X.; Liu, Y. Ureaplasma spp. in male infertility and its relationship with semen quality and seminal plasma components. J. Microbiol. Immunol. Infect. 2018, 51, 778–783. [Google Scholar] [CrossRef]
- Kang, X.; Xie, Q.; Zhou, X.; Li, F.; Huang, J.; Liu, D.; Huang, T. Effects of hepatitis B virus S protein exposure on sperm membrane integrity and functions. PLoS ONE 2012, 7, e33471. [Google Scholar] [CrossRef]
- Qian, L.; Li, Q.; Li, H. Effect of hepatitis B virus infection on sperm quality and oxidative stress state of the semen of infertile males. Am. J. Reprod. Immunol. 2016, 76, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Sacks-Davis, R.; McBryde, E.; Grebely, J.; Hellard, M.; Vickerman, P. Many hepatitis C reinfections that spontaneously clear may be undetected: Markov-chain Monte Carlo analysis of observational study data. J. R. Soc. Interface 2015, 12, 20141197. [Google Scholar] [CrossRef]
- Benova, L.; Mohamoud, Y.A.; Calvert, C.; Abu-Raddad, L.J. Vertical transmission of hepatitis C virus: Systematic review and meta-analysis. Clin. Infect. Dis. 2014, 59, 765–773. [Google Scholar] [CrossRef]
- Machida, K.; Cheng, K.T.-H.; Lai, C.-K.; Jeng, K.-S.; Sung, V.M.-H.; Lai, M.M. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J. Virol. 2006, 80, 7199–7207. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.; Vicari, E.; D’agata, R.; Calogero, A. High frequency of sexual dysfunction in patients with male accessory gland infections. Andrologia 2012, 44, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Leisegang, K.; Agarwal, A. The impact of COVID-19 on the male reproductive tract and fertility: A systematic review. Arab. J. Urol. 2021, 19, 423–436. [Google Scholar] [CrossRef]
- Xu, J.; Qi, L.; Chi, X.; Yang, J.; Wei, X.; Gong, E.; Peh, S.; Gu, J. Orchitis: A complication of severe acute respiratory syndrome (SARS). Biol. Reprod. 2006, 74, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Donato, F.; Bianco, P.M.; Lettieri, G.; Guglielmino, A.; Motta, O.; Bonapace, I.M.; Piscopo, M. Air Pollution and COVID-19: A Possible Dangerous Synergy for Male Fertility. Int. J. Environ. Res. Public Health 2021, 18, 1–21. [Google Scholar] [CrossRef]
- Itoh, M.; Hiramine, C.; Tokunaga, Y.; Mukasa, A.; Hojo, K. A new murine model of autoimmune orchitis induced by immunization with viable syngeneic testicular germ cells alone. II. Immunohistochemical findings of fully-developed inflammatory lesion. Autoimmunity 1991, 10, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Mukhopadhyay, L.D.; Goswami, R.; Dutta, S.; Sengupta, P.; Irez, T.; Hamid, H.A.; Syamal, A.K. SARS-CoV-2 infection and human semen: Possible modes of contamination and transmission. Middle East Fertil. Soc. J. 2021, 26, 18. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. SARS-CoV-2 and Male Infertility: Possible Multifaceted Pathology. Reprod. Sci. 2021, 28, 23–26. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. SARS-CoV-2 infection, oxidative stress and male reproductive hormones: Can testicular-adrenal crosstalk be ruled-out? J. Basic Clin. Physiol. Pharmacol. 2020, 31, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative stress and anxiety: Relationship and cellular pathways. Oxid. Med. Cell. Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Dutta, S. Does SARS-CoV-2 infection cause sperm DNA fragmentation? Possible link with oxidative stress. Eur. J. Contracept. Reprod. Health Care 2020, 25, 405–406. [Google Scholar] [CrossRef] [PubMed]
- DePalma, A.F.; Rothman, R.H.; Lewinnek, G.E.; Canale, S.T. Anterior interbody fusion for severe cervical disc degeneration. Surg. Gynecol. Obstet. 1972, 134, 755–758. [Google Scholar] [PubMed]
- Purvis, K.; Christiansen, E. Infection in the male reproductive tract. Impact, diagnosis and treatment in relation to male infertility. Int. J. Androl. 1993, 16, 1–13. [Google Scholar] [CrossRef]
- Comhaire, F.H.; Mahmoud, A.M.; Depuydt, C.E.; Zalata, A.A.; Christophe, A.B. Mechanisms and effects of male genital tract infection on sperm quality and fertilizing potential: The andrologist’s viewpoint. Hum. Reprod. Update 1999, 5, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Hales, D.B.; Diemer, T.; Hales, K.H. Role of cytokines in testicular function. Endocrine 1999, 10, 201–217. [Google Scholar] [CrossRef]
- Söder, O.; Sultana, T.; Jonsson, C.; Wahlgren, A.; Petersen, C.; Holst, M. The interleukin-1 system in the testis. Andrologia 2000, 32, 52–55. [Google Scholar]
- Diemer, T.; Hales, D.B.; Weidner, W. Immune-endocrine interactions and Leydig cell function: The role of cytokines. Andrologia 2003, 35, 55–63. [Google Scholar] [CrossRef]
- Maegawa, M.; Kamada, M.; Irahara, M.; Yamamoto, S.; Yoshikawa, S.; Kasai, Y.; Ohmoto, Y.; Gima, H.; Thaler, C.J.; Aono, T. A repertoire of cytokines in human seminal plasma. J. Reprod. Immunol. 2002, 54, 33–42. [Google Scholar] [CrossRef]
- Cudicini, C.; Lejeune, H.; Gomez, E.; Bosmans, E.; Ballet, F.; Saez, J.; Jégou, B. Human Leydig cells and Sertoli cells are producers of interleukins-1 and -6. J. Clin. Endocrinol. Metab. 1997, 82, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32. [Google Scholar] [CrossRef]
- Keck, C.; Gerber-Schäfer, C.; Clad, A.; Wilhelm, C.; Breckwoldt, M. Seminal tract infections: Impact on male fertility and treatment options. Hum. Reprod. Update 1998, 4, 891–903. [Google Scholar] [CrossRef]
- Potts, J.M.; Sharma, R.; Pasqualotto, F.; Nelson, D.; Hall, G.; Agarwal, A. Association of ureaplasma urealyticum with abnormal reactive oxygen species levels and absence of leukocytospermia. J. Urol. 2000, 163, 1775–1778. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Selvam, M.K.P.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C. Sperm DNA fragmentation: A new guideline for clinicians. World J. Mens Health 2020, 38, 412. [Google Scholar] [CrossRef]
- Agarwal, A.; Sekhon, L.H. The role of antioxidant therapy in the treatment of male infertility. Hum. Fertil. 2010, 13, 217–225. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prabakaran, S.A. Oxidative stress and antioxidants in male infertility: A difficult balance. Int. J. Reprod. Med. 2005, 3, 1–8. [Google Scholar]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia 2019, 51, e13162. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Baskaran, S.; Sengupta, P.; Dutta, S.; Mokarram, P.; Saliminejad, K.; Sadeghi, M.R. Oxidative stress-induced alterations in seminal plasma antioxidants: Is there any association with keap1 gene methylation in human spermatozoa? Andrologia 2019, 51, e13159. [Google Scholar] [CrossRef]
- Yu, B.; Huang, Z. Variations in Antioxidant Genes and Male Infertility. BioMed Res. Int. 2015, 2015, 513196. [Google Scholar] [CrossRef]
- Carrell, D.T.; Aston, K.I. The search for SNPs, CNVs, and epigenetic variants associated with the complex disease of male infertility. Syst. Biol. Reprod. Med. 2011, 57, 17–26. [Google Scholar] [CrossRef][Green Version]
- Kemal Duru, N.; Morshedi, M.; Oehninger, S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil. Steril. 2000, 74, 1200–1207. [Google Scholar] [CrossRef]
- Naz, R.K.; Evans, L. Presence and modulation of interleukin-12 in seminal plasma of fertile and infertile men. J. Androl. 1998, 19, 302–307. [Google Scholar]
- Gruschwitz, M.S.; Brezinschek, R.; Brezinschek, H.P. Cytokine levels in the seminal plasma of infertile males. J. Androl. 1996, 17, 158–163. [Google Scholar]
- Agarwal, A.; Parekh, N.; Selvam, M.K.P.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S. Male oxidative stress infertility (MOSI): Proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J. Mens Health 2019, 37, 296. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Clarkson, J.S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. Reproduction 1987, 81, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Pasqualotto, F.F.; Nelson, D.R.; Agarwal, A. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J. Androl. 2001, 22, 575–583. [Google Scholar]
- Koppers, A.J.; Garg, M.L.; Aitken, R.J. Stimulation of mitochondrial reactive oxygen species production by unesterified, unsaturated fatty acids in defective human spermatozoa. Free Radic. Biol. Med. 2010, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Gagnon, C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil. Steril. 1992, 57, 409–416. [Google Scholar] [CrossRef]
- Williams, A.; Ford, W. Functional significance of the pentose phosphate pathway and glutathione reductase in the antioxidant defenses of human sperm. Biol. Reprod. 2004, 71, 1309–1316. [Google Scholar] [CrossRef]
- Bui, A.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef]
- Sengupta, P.; Durairajanayagam, D.; Agarwal, A. Fuel/energy sources of spermatozoa. In Male Infertility; Springer: Berlin/Heidelberg, Germany, 2020; pp. 323–335. [Google Scholar]
- Aitken, J.; Fisher, H. Reactive oxygen species generation and human spermatozoa: The balance of benefit and risk. Bioessays 1994, 16, 259–267. [Google Scholar] [CrossRef]
- Tavilani, H.; Goodarzi, M.T.; Vaisi-Raygani, A.; Salimi, S.; Hassanzadeh, T. Activity of antioxidant enzymes in seminal plasma and their relationship with lipid peroxidation of spermatozoa. Int. Braz. J. Urol. 2008, 34, 485–491. [Google Scholar] [CrossRef]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Aitken, R.; Harkiss, D.; Buckingham, D. Analysis of lipid peroxidation mechanisms in human spermatozoa. Mol. Reprod. Dev. 1993, 35, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Arafa, M.; Elbardisi, H. Hormonal regulation of spermatogenesis. In Molecular Signaling in Spermatogenesis and Male Infertility; CRC Press: Boca Raton, FL, USA, 2019; pp. 41–49. [Google Scholar]
- Wells, D.; Bermudez, M.; Steuerwald, N.; Thornhill, A.; Walker, D.; Malter, H.; Delhanty, J.; Cohen, J. Expression of genes regulating chromosome segregation, the cell cycle and apoptosis during human preimplantation development. Hum. Reprod. 2005, 20, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Greco, E.; Mendoza, C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum. Reprod. 2004, 19, 611–615. [Google Scholar] [CrossRef]
- Tesařík, J.; Kopečný, V.; Plachot, M.; Mandelbaum, J. Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. Reproduction 1986, 78, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Takeshima, T.; Takeshima, K.; Usui, K.; Yasuda, K.; Sanjo, H.; Kawahara, T.; Uemura, H.; Murase, M.; Yumura, Y. Early and late paternal effects of reactive oxygen species in semen on embryo development after intracytoplasmic sperm injection. Syst. Biol. Reprod. Med. 2020, 66, 122–128. [Google Scholar] [CrossRef]
- Guerin, P.; Matillon, C.; Bleau, G.; Levy, R.; Menezo, Y. Impact of sperm DNA fragmentation on ART outcome. Gynecol. Obstet. Fertil. Senol. 2005, 33, 665–668. [Google Scholar] [CrossRef]
- Simon, L.; Zini, A.; Dyachenko, A.; Ciampi, A.; Carrell, D.T. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J. Androl. 2017, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil. Steril. 2014, 102, 998–1005.e8. [Google Scholar] [CrossRef]
- Trevelyan, S.J.; Brewster, J.L.; Burgess, A.E.; Crowther, J.M.; Cadell, A.L.; Parker, B.L.; Croucher, D.R.; Dobson, R.C.; Murphy, J.M.; Mace, P.D. Structure-based mechanism of preferential complex formation by apoptosis signal–regulating kinases. Sci. Signal. 2020, 13, 1–26. [Google Scholar] [CrossRef]
- Shukla, K.K.; Mahdi, A.A.; Rajender, S. Apoptosis, spermatogenesis and male infertility. Front. Biosci. 2012, 4, 746–754. [Google Scholar] [CrossRef]
- Latchoumycandane, C.; Vaithinathan, S.; D’Cruz, S.; Mathur, P.P. Apoptosis and male infertility. In Male Infertility; Springer: Berlin/Heidelberg, Germany, 2020; pp. 479–486. [Google Scholar]
- O’Bryan, M.; Schlatt, S.; Gerdprasert, O.; Phillips, D.J.; de Kretser, D.M.; Hedger, M.P. Inducible nitric oxide synthase in the rat testis: Evidence for potential roles in both normal function and inflammation-mediated infertility. Biol. Reprod. 2000, 63, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Gow, R.M.; O’Bryan, M.; Canny, B.; Ooi, G.T.; Hedger, M. Differential effects of dexamethasone treatment on lipopolysaccharide-induced testicular inflammation and reproductive hormone inhibition in adult rats. J. Endocrinol. 2001, 168, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, K.M.; Held Hales, K.; Roberts, M.E.; Buchanan Hales, D.; Rivier, C. The inhibitory effect of intracerebroventricularly injected interleukin 1β on testosterone secretion in the rat: Role of steroidogenic acute regulatory protein. Biol. Reprod. 1999, 60, 527–533. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Hassan, M.F.; Biswas, A. Role of toll-like receptors in the reproductive tract inflammation and male infertility. Chem. Biol. Lett. 2020, 7, 113–123. [Google Scholar]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Riccioli, A.; Starace, D.; Galli, R.; Fuso, A.; Scarpa, S.; Palombi, F.; De Cesaris, P.; Ziparo, E.; Filippini, A. Sertoli cells initiate testicular innate immune responses through TLR activation. J. Immunol. 2006, 177, 7122–7130. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Tchatalbachev, S.; Klug, J.; Fijak, M.; Pineau, C.; Chakraborty, T.; Meinhardt, A. Uropathogenic Escherichia coli block MyD88-dependent and activate MyD88-independent signaling pathways in rat testicular cells. J. Immunol. 2008, 180, 5537–5547. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, H.; Xiong, W.; Chen, S.; Tang, H.; Han, D. Expression patterns and functions of toll-like receptors in mouse sertoli cells. Endocrinology 2008, 149, 4402–4412. [Google Scholar] [CrossRef]
- Winnall, W.R.; Muir, J.A.; Hedger, M.P. Differential responses of epithelial Sertoli cells of the rat testis to Toll-like receptor 2 and 4 ligands: Implications for studies of testicular inflammation using bacterial lipopolysaccharides. Innate Immun. 2011, 17, 123–136. [Google Scholar] [CrossRef]
- Lui, W.-Y.; Wong, C.-H.; Mruk, D.D.; Cheng, C.Y. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: An in vivo study. Endocrinology 2003, 144, 1139–1142. [Google Scholar] [CrossRef]
- Okuma, Y.; Saito, K.; O’connor, A.; Phillips, D.J.; de Kretser, D.M.; Hedger, M.P. Reciprocal regulation of activin A and inhibin B by interleukin-1 (IL-1) and follicle-stimulating hormone (FSH) in rat Sertoli cells in vitro. J. Endocrinol. 2005, 185, 99–110. [Google Scholar] [CrossRef]
- Hedger, M.P. Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation—A perspective. J. Reprod. Immunol. 2011, 88, 130–141. [Google Scholar] [CrossRef]
- O’Bryan, M.K.; Hedger, M.P. Inflammatory networks in the control of spermatogenesis. In Molecular Mechanisms in Spermatogenesis; Springer: Berlin/Heidelberg, Germany, 2009; pp. 92–114. [Google Scholar]
- Shang, T.; Zhang, X.; Wang, T.; Sun, B.; Deng, T.; Han, D. Toll-like receptor-initiated testicular innate immune responses in mouse Leydig cells. Endocrinology 2011, 152, 2827–2836. [Google Scholar] [CrossRef]
- Samir, M.S.; Glister, C.; Mattar, D.; Laird, M.; Knight, P.G. Follicular expression of pro-inflammatory cytokines tumour necrosis factor-α (TNFα), interleukin 6 (IL6) and their receptors in cattle: TNFα, IL6 and macrophages suppress thecal androgen production in vitro. Reproduction 2017, 154, 35–49. [Google Scholar] [CrossRef]
- Ding, D.-C.; Liu, H.-W.; Chu, T.-Y. Interleukin-6 from ovarian mesenchymal stem cells promotes proliferation, sphere and colony formation and tumorigenesis of an ovarian cancer cell line SKOV3. J. Cancer 2016, 7, 1815. [Google Scholar] [CrossRef]
- Frungieri, M.B.; Calandra, R.S.; Lustig, L.; Meineke, V.; Köhn, F.M.; Vogt, H.-J.; Mayerhofer, A. Number, distribution pattern, and identification of macrophages in the testes of infertile men. Fertil. Steril. 2002, 78, 298–306. [Google Scholar] [CrossRef]
- Fehervari, Z. Testicular macrophage origin. Nat. Immunol. 2017, 18, 1067. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.; Schuppe, H.-C.; Guazzone, V.A.; Bhushan, S.; Wahle, E.; Lochnit, G.; Lustig, L.; Meinhardt, A.; Fijak, M. Targeting high mobility group box protein 1 ameliorates testicular inflammation in experimental autoimmune orchitis. Hum. Reprod. 2015, 30, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.A.; Diemer, T.; Janus, P.; Hales, K.H.; Hales, D.B. Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit Leydig cell steroidogenesis via perturbation of mitochondria. Endocrine 2004, 25, 265–275. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, M.H.; Han, J.M.; Hong, J.; Lee, T.-H.; Kim, S.-H.; Yang, W.M. The anti-inflammatory potential of Cortex Phellodendron in vivo and in vitro: Down-regulation of NO and iNOS through suppression of NF-κB and MAPK activation. Int. Immunopharmacol. 2014, 19, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jeong, J.-M.; Kim, S.J.; Seo, W.; Kim, M.-H.; Choi, W.-M.; Yoo, W.; Lee, J.-H.; Shim, Y.-R.; Yi, H.-S. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4–MD2 complex. Nat. Commun. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Aguirre, L.; Bacsi, A.; Radak, Z.; Hazra, T.K.; Mitra, S.; Sur, S.; Brasier, A.R.; Ba, X.; Boldogh, I. Innate inflammation induced by the 8-oxoguanine DNA glycosylase-1-KRAS-NF-κB pathway. J. Immunol. 2014, 193, 4643–4653. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Accardi, C.J.; Ziegler, T.R.; Blanco, R.A.; Ritzenthaler, J.D.; Rojas, M.; Roman, J.; Jones, D.P. Cysteine redox potential determines pro-inflammatory IL-1beta levels. PLoS ONE 2009, 4, e5017. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Jones, D.P. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation 2005, 111, 2973–2980. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. https://doi.org/10.3390/ijms221810043
Dutta S, Sengupta P, Slama P, Roychoudhury S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. International Journal of Molecular Sciences. 2021; 22(18):10043. https://doi.org/10.3390/ijms221810043
Chicago/Turabian StyleDutta, Sulagna, Pallav Sengupta, Petr Slama, and Shubhadeep Roychoudhury. 2021. "Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction" International Journal of Molecular Sciences 22, no. 18: 10043. https://doi.org/10.3390/ijms221810043
APA StyleDutta, S., Sengupta, P., Slama, P., & Roychoudhury, S. (2021). Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. International Journal of Molecular Sciences, 22(18), 10043. https://doi.org/10.3390/ijms221810043








