GABA: A Key Player in Drought Stress Resistance in Plants
Abstract
:1. Introduction
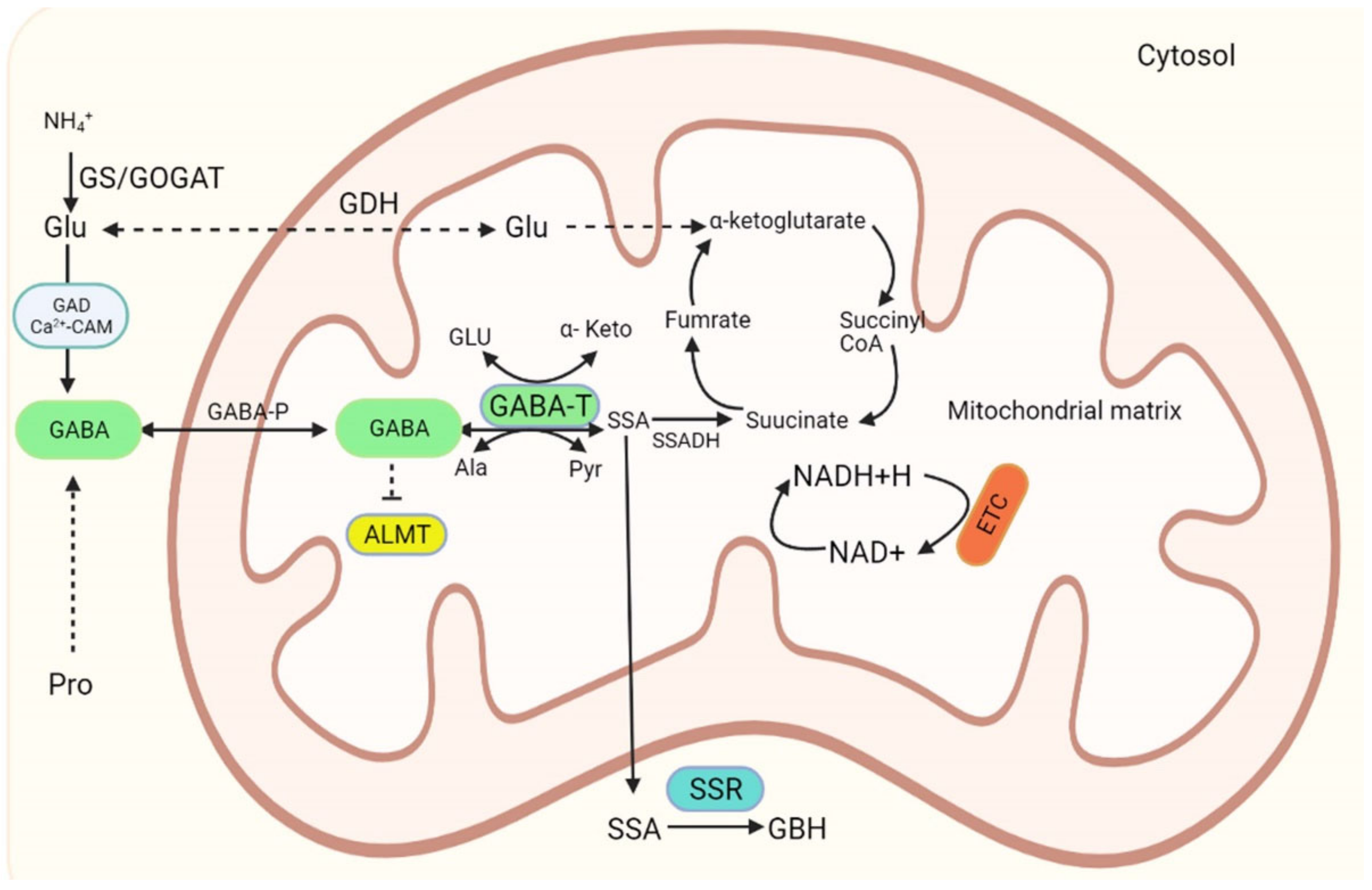
2. GABA Biosynthesis and Metabolism in Plants
3. GABA-Induced Drought Tolerance in Plants
4. GABA and Antioxidant Systems under Drought Stress
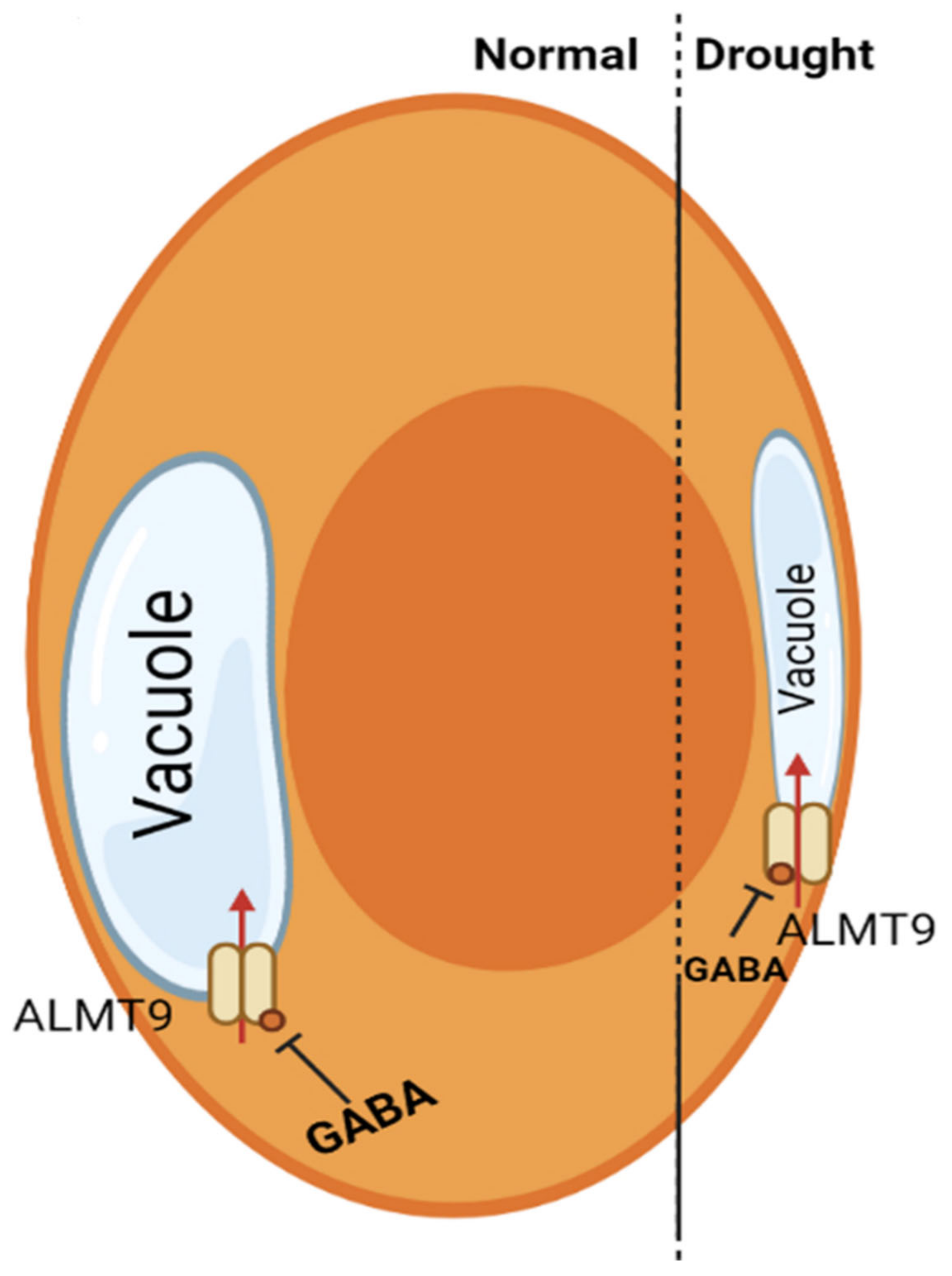
5. GABA-Induced Stomatal Regulations under Drought Stress
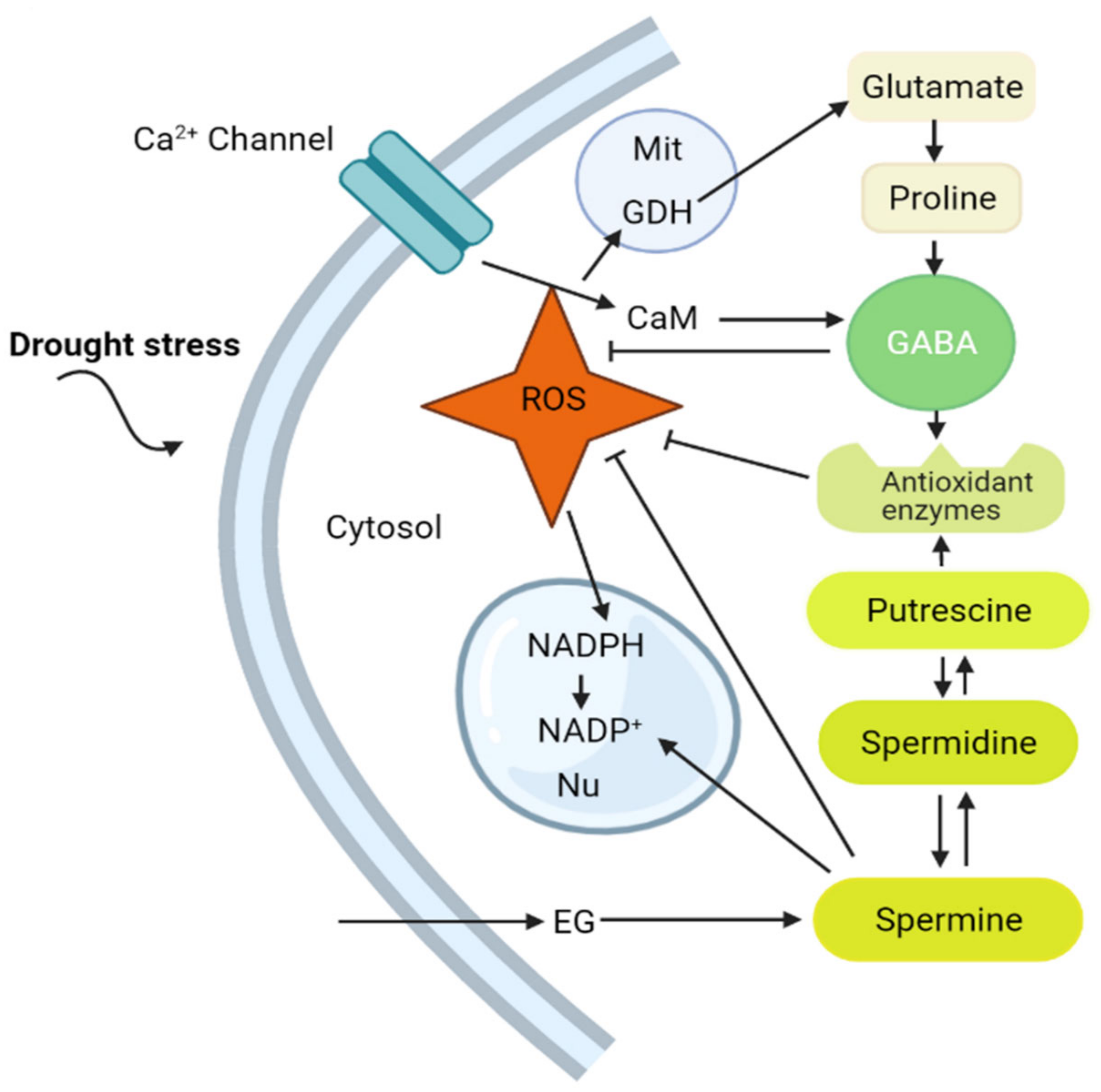
6. GABA and Polyamines Interrelationships under Drought Stress
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steward, F.C. γ-Aminobutyric acid: A constituent of the potato tuber? Science 1949, 110, 439–440. [Google Scholar]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal Behav. 2021, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.R. Does GABA act as a signal in plants? Hints from molecular studies: Hints from molecular studies. Plant Signal. Behav. 2007, 2, 408–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaleel, C.A.; Manivannan, P.; Whid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Agric. 2009, 29, 153–188. [Google Scholar]
- Vanani, F.R.; Shabani, L.; Sabzalian, M.R.; Dehghanian, F.; Winner, L. Comparative physiological and proteomic analysis indicates lower shock response to drought stress conditions in a self-pollinating perennial ryegrass. PLoS ONE 2020, 15, e0234317. [Google Scholar]
- Yao, G.Q.; Li, F.P.; Nie, Z.F.; Bi, M.H.; Jiang, H.; Liu, X.D.; Wei, Y.; Fang, X.W. Ethylene, not ABA, is closely linked to the recovery of gas exchange after drought in four Caragana species. Plant Cell Environ. 2020, 44, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by abscisic acid, salicylic acid, and γ-aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera). Physiol. Plant. 2017, 159, 42–58. [Google Scholar] [CrossRef]
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ. Sci. Pollut. Res. 2016, 23, 21206–21218. [Google Scholar] [CrossRef] [PubMed]
- Razik, E.S.A.; Alharbi, B.M.; Pirzadah, T.B.; Alnusairi, G.S.H.; Soliman, M.H.; Hakeem, K.R. γ-Aminobutyric acid (GABA) mitigates drought and heat stress in sunflower (Helianthus annuus L.) by regulating its physiological, biochemical and molecular pathways. Physiol. Plant. 2020, 172, 505–527. [Google Scholar] [CrossRef]
- Hasan, M.M.; Gong, L.; Nie, Z.; Feng, X.; Ahammed, G.J.; Fang., X.W. ABA-induced stomatal movements in vascular plants during dehydration versus rehydration. Environ. Exp. Bot. 2021, 186, 104436. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F.M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef]
- Yang, Y.J.; Bi, M.H.; Nie, Z.F.; Jiang, H.; Liu, X.D.; Fang, X.W.; Brodribb, T.J. Evolution of stomatal closure to optimise water use efficiency in response to dehydration in ferns and seed plants. New Phytol. 2021, 230, 2001–2010. [Google Scholar] [CrossRef]
- Gong, L.; Liu, X.D.; Zeng, Y.Y.; Tian, X.Q.; Li, Y.L.; Turner, N.C.; Fang, X.W. Differences in stomatal morphology and physiology explain differences in stomatal sensitivity to abscisic acid across vascular plant lineages. Plant Physiol. 2021, 186, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Papanatsiou, M.; Petersen, J.; Henderson, L.; Wang, Y.; Christie, J.M.; Blatt, M.R. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 2019, 363, 1456–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. γ-aminobutyric acid (GABA): Biosynthesis, role, commercial production, and applications. Stud. Nat. Prod. Chem. 2018, 57, 413–452. [Google Scholar]
- Bown, A.W.; Shelp, B.J. The metabolism and functions of [gamma]-aminobutyric acid. Plant Physiol. 1997, 115, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Ruiz, R.; Martinez, F.; Knauf-Beiter, G. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Clark, S.M.; Di Leo, R.; Dhanoa, P.K.; Van Cauwenberghe, O.R.; Mullen, R.T.; Shelp, B.J. Biochemical characterization, mitochondrial localization, expression, and potential functions for an Arabidopsis c-aminobutyrate transaminase that utilizes both pyruvate and glyoxylate. J. Exp. Bot. 2009, 60, 1743–1757. [Google Scholar] [CrossRef] [Green Version]
- Akihiro, T.; Koike, S.; Tani, R.; Tominaga, T.; Watanabe, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Ashihara, H.; Matsukura, C.; et al. Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol. 2008, 49, 1378–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelp, B.J.; Mullen, R.T.; Waller, J.C. Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci. 2012, 17, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Trobacher, C.P.; Clark, S.M.; Bozzo, G.G.; Mullen, R.T.; DeEll, J.; Shelp, B.J. Catabolism of GABA in apple fruit: Subcellular localization and biochemical characterization of two c-aminobutyrate transaminases. Postharvest Biol. Tech. 2013, 75, 106–113. [Google Scholar] [CrossRef]
- Priya, M.; Sharma, L.; Kaur, R.; Bindumadhava, H.; Nair, R.M.; Siddique, K.H.M.; Nayyar, H. GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci. Rep. 2019, 9, 7788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, M.S.; Wang, Y.; Shu, S.; Hasan, M.M.; El-Yazied, A.A.; Alabdallah, N.M.; Hajjar, D.; Altaf, M.A.; Sun, J.S.; Guo, S. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA and GA-mediated pathways. Front. Plant Sci. 2021, 12, 650955. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Nie, N.F.; Yao, G.Q.; Hasan, M.M.; Xiang, Y.; Fang, X.W. Dew absorption by leaf trichomes in Caragana korshinskii: An alternative water acquisition strategy to withstand drought in arid environment. Physiol. Plant. 2021, 172, 528–539. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alharby, H.F.; Hajar, A.S.; Hakeem, K.R.; Alzahrani, Y. The effect of magnetized water on the growth and physiological conditions of Moringa species under drought stress. Pol. J. Environ. Stud. 2019, 28, 1145–1155. [Google Scholar] [CrossRef]
- Hasan, M.M.; Ali, M.A.; Soliman, M.H.; Alqarawi, A.A.; Abd Allah, E.F.; Fang, X.W. Insights into 28-homobrassinolide (HBR)—Mediated redox homeostasis, AsA–GSH cycle, and methylglyoxal detoxification in soybean under drought-induced oxidative stress. J. Plant Inter. 2020, 15, 371–385. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alharby, H.F.; Uddin, M.N.; Ali, M.A.; Anwar, Y.; Fang, X.W.; Hakeem, K.R.; Alzahrani, Y.; Hajar, A.S. Magnetized water confers drought stress tolerance in Moringa biotype via modulation of growth, gas exchange, lipid peroxidation and antioxidant activity. Pol. J. Environ. Stud. 2020, 29, 1625–1636. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hajar, A.S.; Alharby, H.F.; Hakeem, K.R. Effects of magnetized water on phenolic compounds, lipid peroxidation and antioxidant activity of Moringa species under drought stress. J. Anim. Plant Sci. 2018, 28, 803–810. [Google Scholar]
- Khan, A.; Anwar, Y.; Hasan, M.; Iqbal, A.; Ali, M.; Alharby, H.F.; Hakeem, K.R.; Hasanuzzaman, M. Attenuation of drought stress in Brassica seedlings with exogenous application of Ca2+ and H2O2. Plants 2017, 6, 20. [Google Scholar] [CrossRef]
- Zhang, L.; Becker, D.F. Connecting proline metabolism and signaling pathways in plant senescence. Front. Plant Sci. 2015, 6, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Bouche, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Pulido, P.; Domínguez, F.; Cejudo, F.J. A hydrogen peroxide detoxification system in the nucleus of wheat seed cells: Protection or signaling role? Plant Signal. Behav. 2009, 4, 23–25. [Google Scholar] [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Gawad, H.G.; Mukherjee, S.; Farag, R.; Abd Elbar, O.H.; Hikal, M.; Abou El-Yazied, A.; Abd Elhady, S.A.; Helal, N.; ElKelish, A.; El Nahhas, N.; et al. Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal. Behav. 2020, 16, 1853384. [Google Scholar] [CrossRef]
- Ferreira, R.A.; Borella, J.; Hüther, C.M.; do Canto, A.C.B.; da Costa Correa, N.P.; Correia, D.M.; Borges, R.P.; de Pinho, C.F.; Machado, T.B.; Pereira, C.R. Drought induced stress in leaves of Coix lacryma-jobi L. under exogenous application of proline and GABA amino acids. Braz. J. Bot. 2020, 43, 513–521. [Google Scholar] [CrossRef]
- Krishnan, S.; Laskowski, K.; Shukla, V.; Merewitz, E.B. Mitigation of Drought Stress Damage by Exogenous Application of a Non-Protein Amino Acid γ-Aminobutyric Acid on Perennial Ryegrass. J. Am. Soc. Hort. Sci. 2013, 138, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Peng, Y.; Huang, B. Alteration of Transcripts of Stress-Protective Genes and Transcriptional Factors by γ-Aminobutyric Acid (GABA) Associated with Improved Heat and Drought Tolerance in Creeping Bentgrass (Agrostis stolonifera). Int. J. Mol. Sci. 2018, 19, 1623. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 2016, 6, 30338. [Google Scholar] [CrossRef] [Green Version]
- Rezaei-Chiyaneh, E.; Seyyedi, S.M.; Ebrahimian, E.; Moghaddam, S.S.; Damalas, C.A. Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa L.). Ind. Crops Prod. 2018, 112, 741–748. [Google Scholar] [CrossRef]
- Sadaghiani, F.M.; Dehaghi, M.A.; Pirzad, A.; Fotokian, M.H. Variation in yield and biochemical factors of German chamomile (Matricaria recutita L.) under foliar application of osmolytes and drought stress conditions. J. Herbmed Pharmacol. 2019, 8, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumari, K.; Puthur, J.T. γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul. 2016, 78, 57–67. [Google Scholar] [CrossRef]
- Yong, B.; Xie, H.; Li, Z.; Li, Y.P.; Zhang, Y.; Nie, G.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Yan, Y.H.; et al. Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front. Physiol. 2017, 8, 1107. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Alharbi, B.M.; Elhakem, A.H.; Alnusairi, G.S.H.; Mona, H.; Soliman, M.H.; Hakeem, K.R.; Hasan, M.M.; Abdelhamid, M.T. Exogenous application of melatonin alleviates salt stress-induced decline in growth and photosynthesis in Glycine max (L.) seedlings by improving mineral uptake, antioxidant and glyoxalase system. Plant Soil Environ. 2021, 67, 208–220. [Google Scholar] [CrossRef]
- Guler, N.S.; Pehlivan, N. Exogenous low-dose hydrogen peroxide enhances drought tolerance of soybean (Glycine max L.) through inducing antioxidant system. Acta Biol. Hung. 2016, 67, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.M.; Skalicky, M.; Jahan, M.S.; Hossain, M.N.; Anwar, Z.; Nie, Z.F.; Alabdallah, N.M.; Brestic, M.; Hejnak, V.; Fang, X.-W. Spermine: Its Emerging Role in Regulating Drought Stress Responses in Plants. Cells 2021, 10, 261. [Google Scholar] [CrossRef]
- Abedi, T.; Pakniyat, H. Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus). Czech J. Genet. Plant Breed. 2010, 46, 27–34. [Google Scholar] [CrossRef]
- Slabbert, M.M.; Krüger, G.H.J. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 2014, 95, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Capell, T.; Bassie, L.; Christou, P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci. USA 2004, 101, 9909–9914. [Google Scholar] [CrossRef] [Green Version]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Schmid, H.P.; Richardson, A.D. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Sussmilch, F.C.; Schultz, J.; Hedrich, R.; Roelfsema, M.R.G. Acquiring control: The evolution of stomatal signalling pathways. Trends Plant Sci. 2019, 24, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Yao, G.Q.; Nie, Z.F.; Turner, N.C.; Li, F.M.; Gao, T.P.; Fang, X.W.; Scoffoni, C. Combined high leaf hydraulic safety and efficiency provides drought tolerance in Caragana species adapted to low mean annual precipitation. New Phytol. 2021, 229, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Mori, I.C.; Munemasa, S. Diverse stomatal signaling and the signal integration mechanism. Annu. Rev. Plant Biol. 2015, 66, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Renault, H.; El Amrani, A.; Palanivelu, R.; Updegraff, E.P.; Yu, A.; Renou, J.P.; Preuss, D.; Bouchereau, A.; Deleu, C. GABA accumulation causes cell elongation defects and a decrease in expression of genes encoding secreted and cell wall-related proteins in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 894–908. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signaling modulates plant growth by directly regulating the activity of plant-specification transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef] [Green Version]
- Meyer, S.; Mumm, P.; Imes, D.; Endler, A.; Weder, B.; Al-Rasheid, K.A.; Geiger, D.; Marten, I.; Martinoia, E.; Hedrich, R. AtALMT12 represents an R-type anion channel required for stomata movement in Arabidopsis guard cells. Plant J. 2010, 63, 1054–1062. [Google Scholar] [CrossRef] [Green Version]
- Mekonnen, D.W.; Flügge, U.I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef]
- Meyer, S.; Scholz-Starke, J.; De Angeli, A.; Kovermann, P.; Burla, B.; Gambale, F.; Martinoia, E. Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J. 2011, 67, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Xu, Z.; Xu, W.; Li, J.; Zhao, N.; Zhou, Y. Application of γ-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca (NO3)2. Plant Physiol. Biochem. 2015, 92, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, L.; Gao, H.; Wu, X.; Li, J.; Lv, G.; Gong, B. Polyamine biosynthesis and degradation are modulated by exogenous gamma-aminobutyric acid in root-zone hypoxia-stressed melon roots. Plant Physiol. Biochem. 2014, 82, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.K.; Minocha, S.C.; Minocha, R.; Handa, A.K. Polyamines and cellular metabolism in plants: Transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids 2010, 38, 405–413. [Google Scholar] [CrossRef]
- Silveira, V.; de Vita, A.M.; Macedo, A.F.; Dias, M.F.R.; Floh, E.I.S.; Santa-Catarina, C. Morphological and polyamine content changes in embryogenic and non-embryogenic callus of sugarcane. Plant Cell Tissue Organ Cult. 2013, 114, 351–364. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, B.S.; Hwang, B.K. Pepper arginine decarboxylase is required for polyamine and γ-aminobutyric acid signaling in cell death and defense response. Plant Physiol. 2013, 162, 2067–2083. [Google Scholar] [CrossRef] [Green Version]
- Gil-Amado, J.A.; Gomez-Jimenez, M.C. Regulation of polyamine metabolism and biosynthetic gene expression during olive mature-fruit abscission. Planta 2012, 235, 1221–1237. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Guo, Q.; Gu, Z. GABA shunt and polyamine degradation pathway on γ-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chem. 2013, 136, 152–159. [Google Scholar] [CrossRef]
- AL-Quraan, N.A. GABA shunt deficiencies and accumulation of reactive oxygen species under UV treatments: Insight from Arabidopsis thaliana calmodulin mutants. Acta Physiol. Plant. 2015, 37, 86. [Google Scholar] [CrossRef]
- Xing, S.G.; Jun, Y.B.; Hau, Z.W.; Liang, L.Y. Higher accumulation of γ-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol. Biochem. 2007, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Hu, L.; Xu, W.; Zhen, A.; Zhang, L.; Hu, X. Exogenous γ-aminobutyric acid improves the structure and function of photosystem II in muskmelon seedlings exposed to salinity-alkalinity stress. PLoS ONE 2016, 11, e0164847. [Google Scholar] [CrossRef] [PubMed]

| Species | Stress | GABA Treatment | Effect | Outcome | References |
|---|---|---|---|---|---|
| Phaseolus vulgaris L | Drought (semiarid conditions) | 0.5, 1.0, and 2.0 mM (foliar application) | Increased leaf area, fresh and dry shoot weight, and improved osmotic adjustment, membrane permeability, uptake of nutrients, and antioxidant defense | Increased drought tolerance of Phaseolus vulgaris L. | [37] |
| Coix lacryma-jobi L. | Withholding water | 20.0 mM (foliar application) | Preserved the electron transport chain and minimized oxidative damage caused by reactive oxygen species | Mitigated the deleterious effects of drought in C. lacryma-jobi plant leaves | [38] |
| Ryegrass (Lolium perenne) | Withholding water | 50.0 or 70.0 mM (foliar application) | Reduced lipid peroxidation and electrolyte leakage and improved relative water content (RWC) and antioxidant activity | Alleviated drought stress in ryegrass seedlings | [39] |
| Creeping bentgrass (Agrostis stolonifera) | Withholding water (soil volumetric water content declined to 7%) | 0.5 mM (foliar application) | Increased turf quality, leaf water content, cell membrane permeability, photosynthetic pigments, and expression of CDPK26, MAPK1, ABF3, WRKY75, MYB13, HSP70, MT1, 14-3-3 | Significantly improved plant tolerance to drought stress | [40] |
| Creeping bentgrass (Agrostis stolonifera) | Withholding water | 0.5 mM (foliar application) | Increased amino acid (GABA, glycine, valine, proline, 5-oxoproline, serine, threonine, aspartic acid, and glutamic acid) and organic acid (malic acid, lactic acid, gluconic acid, malonic acid, and ribonic acid) accumulation | Enhanced drought tolerance | [41] |
| Sunflower (Helianthus annuus L.) | 50% field capacity of drought stress | 2.0 mg L−1 (foliar application) | Increased plant height, fresh and dry weight of shoot and root; improved osmolyte metabolism, expression of genes, and antioxidant enzyme activity | Effectively alleviated drought-induced oxidative stress | [10] |
| Cumin (Nigella sativa L.) | Three irrigation treatments (irrigation after 50, 100, and 150 mm evaporation based on evaporation from class A pan) | 0, 0.5, 1.0, and 2.0 mg L−1 (foliar application) | Significantly improved chlorophyll content and antioxidant activity | Improved growth and productivity | [42] |
| Matricaria recutita L. | Two levels of 100 (mild stress) and 150 mm (severe stress) evaporation from class A pan | 50.0 mM (foliar application) | Positive proline response to severe and mild stress in the presence of GABA | Improved drought tolerance | [43] |
| Black pepper (Piper nigrum L.) | PEG (polyethylene glycol 6000; 10% w/v) | 2.0 mM (GABA-primed black pepper) | Reduced wilting percentage Increased leaf RWC and antioxidant enzyme activity; more rapidly decreased cell osmotic potential; reduced lipid peroxidation rate; significantly decreased inhibition of photosynthetic and mitochondrial activity | Enhanced drought stress tolerance | [44] |
| White clover (Trifolium repens) | 15% PEG-induced drought stress | 8.0 mM (pretreated plants with GABA in roots) | Increased activities of GABA transaminase and alpha ketone glutarate dehydrogenase; potential GABA-promoted production of polyamines (PAs) and inhibition of their metabolism | Improved drought tolerance of white clover | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.M.; Alabdallah, N.M.; Alharbi, B.M.; Waseem, M.; Yao, G.; Liu, X.-D.; Abd El-Gawad, H.G.; El-Yazied, A.A.; Ibrahim, M.F.M.; Jahan, M.S.; et al. GABA: A Key Player in Drought Stress Resistance in Plants. Int. J. Mol. Sci. 2021, 22, 10136. https://doi.org/10.3390/ijms221810136
Hasan MM, Alabdallah NM, Alharbi BM, Waseem M, Yao G, Liu X-D, Abd El-Gawad HG, El-Yazied AA, Ibrahim MFM, Jahan MS, et al. GABA: A Key Player in Drought Stress Resistance in Plants. International Journal of Molecular Sciences. 2021; 22(18):10136. https://doi.org/10.3390/ijms221810136
Chicago/Turabian StyleHasan, Md. Mahadi, Nadiyah M. Alabdallah, Basmah M. Alharbi, Muhammad Waseem, Guangqian Yao, Xu-Dong Liu, Hany G. Abd El-Gawad, Ahmed Abou El-Yazied, Mohamed F. M. Ibrahim, Mohammad Shah Jahan, and et al. 2021. "GABA: A Key Player in Drought Stress Resistance in Plants" International Journal of Molecular Sciences 22, no. 18: 10136. https://doi.org/10.3390/ijms221810136
APA StyleHasan, M. M., Alabdallah, N. M., Alharbi, B. M., Waseem, M., Yao, G., Liu, X.-D., Abd El-Gawad, H. G., El-Yazied, A. A., Ibrahim, M. F. M., Jahan, M. S., & Fang, X.-W. (2021). GABA: A Key Player in Drought Stress Resistance in Plants. International Journal of Molecular Sciences, 22(18), 10136. https://doi.org/10.3390/ijms221810136












