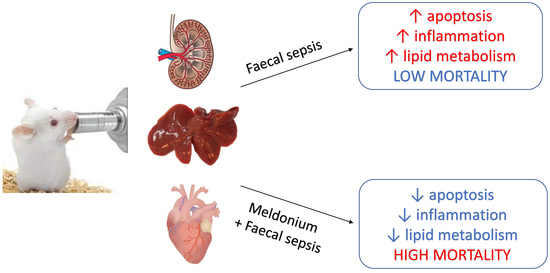
The Effects of a Meldonium Pre-Treatment on the Course of the Faecal-Induced Sepsis in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Survival Analysis
2.2. AST, ALT, ALP and Troponin T Serum Analysis
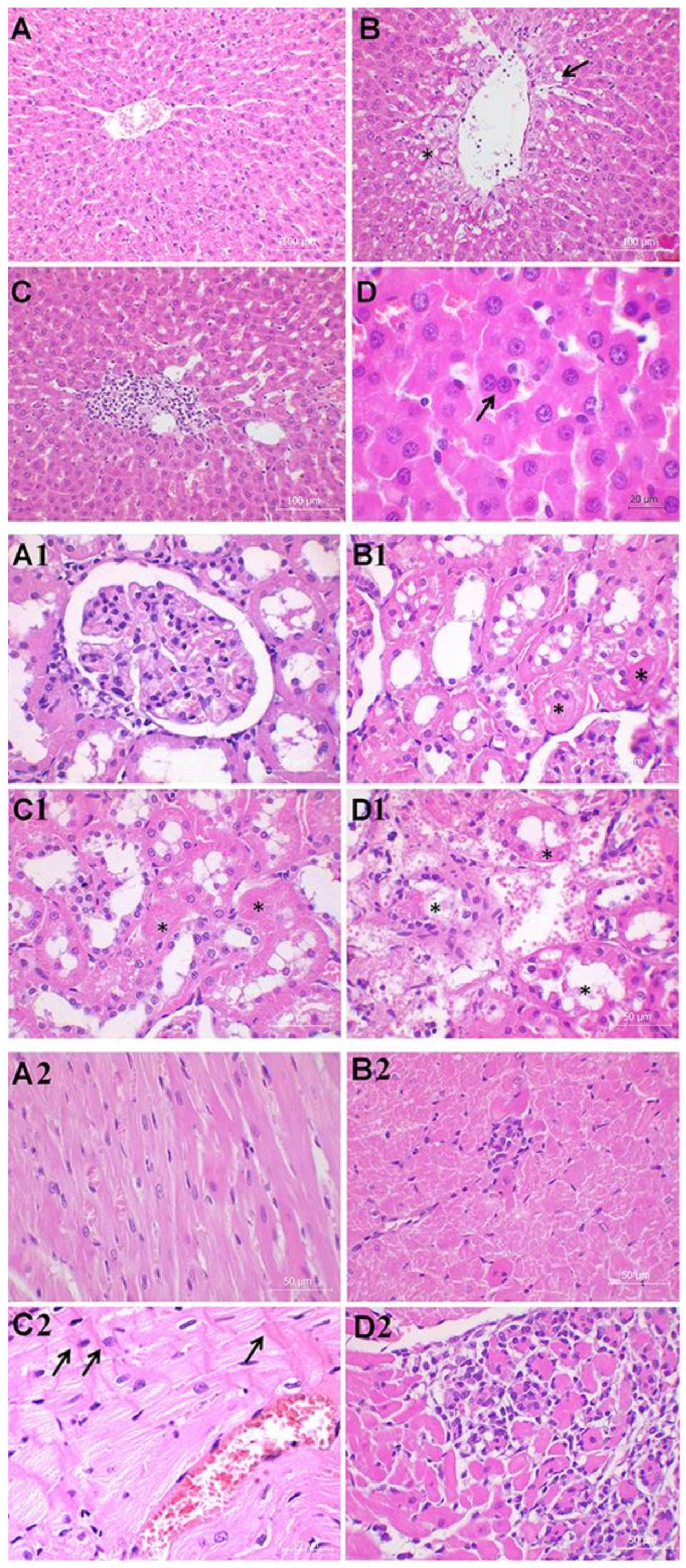
2.3. Histology Analysis
2.4. Assessment of the Inflammatory Status
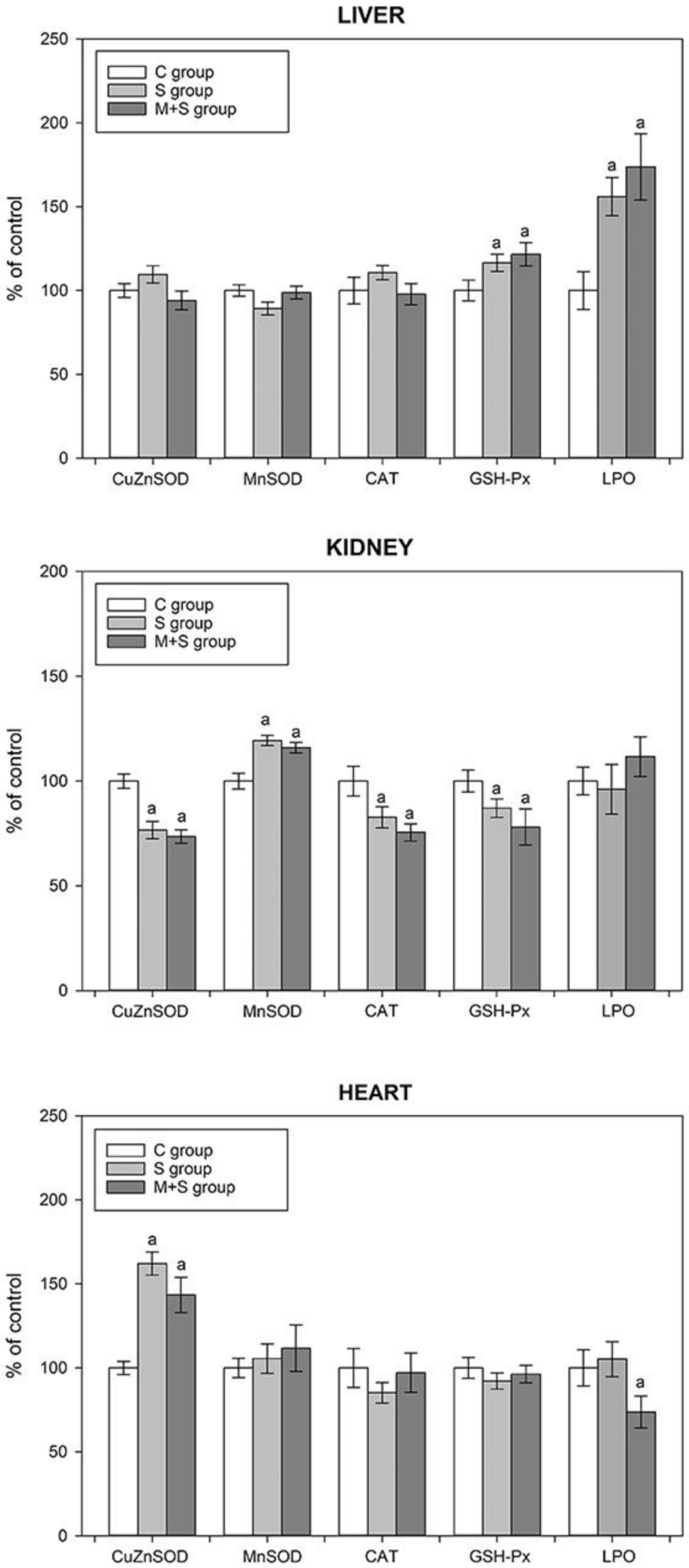
2.5. Assessment of the Oxidative Status
2.6. Lipidomic Analysis
2.7. Sympathoadrenal Activation
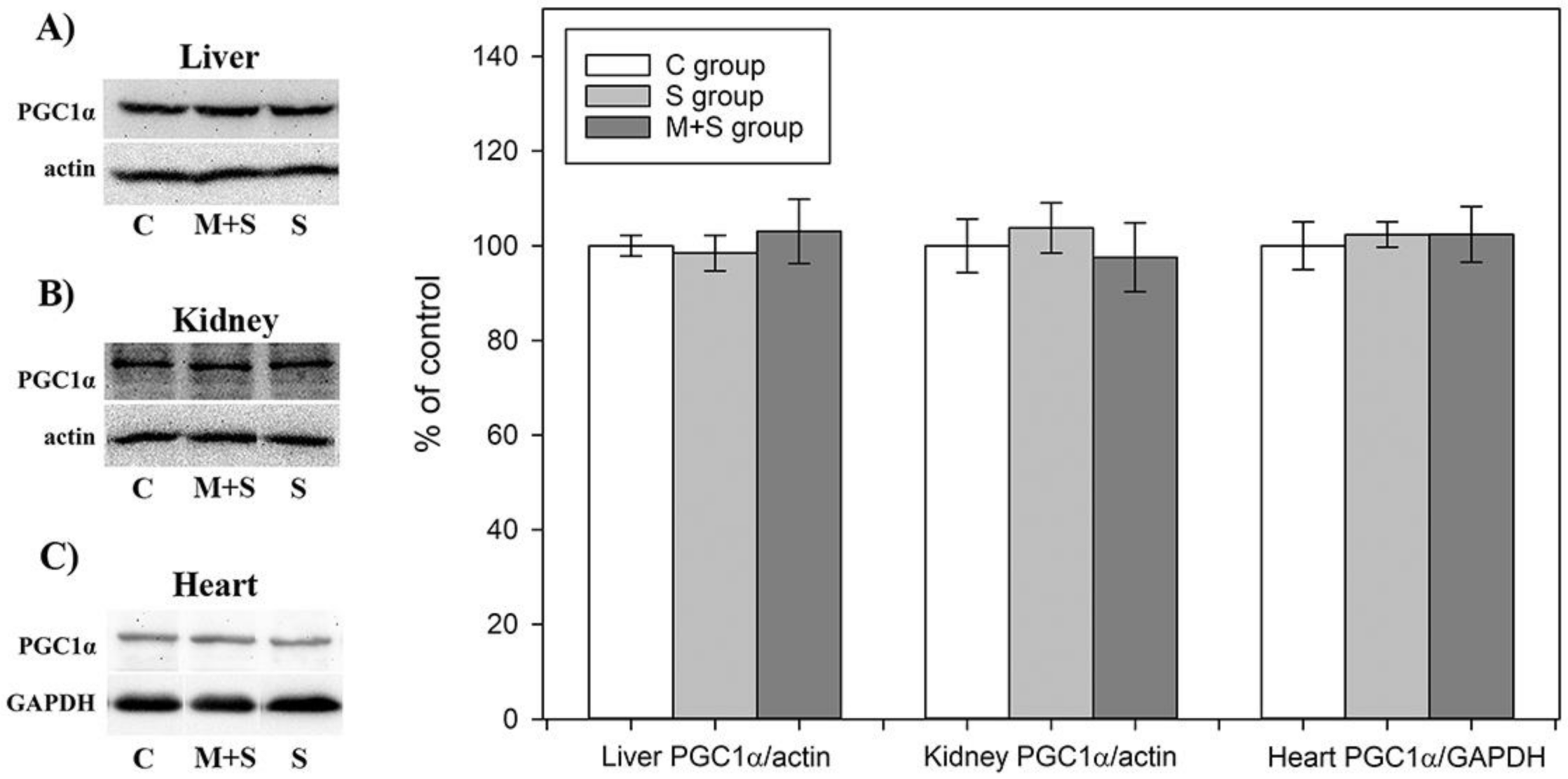
2.8. PGC1-α Protein Level Analysis
3. Materials and Methods
3.1. Animals and Treatments
3.2. Serum and Tissue Collection
3.3. Biochemical Analysis
3.3.1. Serum Analysis
3.3.2. Tissue Analysis of Lactic Acid, L Carnitine, Sugars, and Sugar Alcohols
3.3.3. Lipidomics Tissue Preparation
3.3.4. Tissue Fatty Acids Determination
3.3.5. Tissue Triglycerides Determination
3.4. Determination of Oxidative Stress Biomarkers
3.5. Determination of Adrenaline and Noradrenaline Content
3.5.1. Determination of Adrenaline and Noradrenaline Content in Serum
3.5.2. Determination of Adrenaline and Noradrenaline Content in Adrenal Glands
3.6. Western Immunoblot Analysis
3.7. Histology Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrum, B.; Anantha, R.V.; Xu, S.X.; Donnelly, M.; Haeryfar, S.M.; McCormick, J.K.; Mele, T. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res. Notes 2014, 7, 233. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, J.H. An update on sepsis biomarkers. Infect. Chemother. 2020, 52, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Carre, J.E.; Singer, M. Cellular energetic metabolism in sepsis: The need for a systems approach. Biochim. Biophys. Acta 2008, 1777, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L.; Jones, G.; David, S.; Olariu, E.; Cadwell, K.K. Frequency and mortality of septic shock in Europe and North America: A systematic review and meta-analysis. Crit. Care 2019, 23, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Wasyluk, W.; Zwolak, A. Metabolic alterations in sepsis. J. Clin. Med. 2021, 10, 2412. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, L.; Granfeldt, A.; Secher, N.; Tietze, A.; Iversen, N.K.; Jensen, M.S.; Andersen, K.K.; Nagenthiraja, K.; Gutierrez-Lizardi, P.; Mouridsen, K.; et al. Microcirculatory dysfunction and tissue oxygenation in critical illness. Acta Anaesthesiol. Scand. 2015, 59, 1246–1259. [Google Scholar] [CrossRef]
- Arulkumaran, N.; Deutschman, C.S.; Pinsky, M.R.; Zuckerbraun, B.; Schumacker, P.T.; Gomez, H.; Gomez, A.; Murray, P.; Kellum, J.A.; Workgroup, A.X. Mitochondrial function in sepsis. Shock 2016, 45, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suetrong, B.; Walley, K.R. Lactic acidosis in sepsis: It’s not all anaerobic: Implications for diagnosis and management. Chest 2016, 149, 252–261. [Google Scholar] [CrossRef]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef]
- Mai, S.H.C.; Sharma, N.; Kwong, A.C.; Dwivedi, D.J.; Khan, M.; Grin, P.M.; Fox-Robichaud, A.E.; Liaw, P.C. Body temperature and mouse scoring systems as surrogate markers of death in cecal ligation and puncture sepsis. Intensive Care Med. Exp. 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Feng, Y.W.; Yao, Y.M. Potential therapy strategy: Targeting mitochondrial dysfunction in sepsis. Mil. Med. Res. 2018, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Irahara, T.; Sato, N.; Otake, K.; Matsumura, S.; Inoue, K.; Ishihara, K.; Fushiki, T.; Yokota, H. Alterations in energy substrate metabolism in mice with different degrees of sepsis. J. Surg. Res. 2018, 227, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Van Wyngene, L.; Vandewalle, J.; Libert, C. Reprogramming of basic metabolic pathways in microbial sepsis: Therapeutic targets at last? EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. What is lipotoxicity? Adv. Exp. Med. Biol. 2017, 960, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef]
- Sjakste, N.; Gutcaits, A.; Kalvinsh, I. Mildronate: An antiischemic drug for neurological indications. CNS Drug Rev. 2005, 11, 151–168. [Google Scholar] [CrossRef]
- Durasevic, S.; Stojkovic, M.; Sopta, J.; Pavlovic, S.; Borkovic-Mitic, S.; Ivanovic, A.; Jasnic, N.; Tosti, T.; Durovic, S.; Dordevic, J.; et al. The effects of meldonium on the acute ischemia/reperfusion liver injury in rats. Sci. Rep. 2021, 11, 1305. [Google Scholar] [CrossRef]
- Durasevic, S.; Stojkovic, M.; Bogdanovic, L.; Pavlovic, S.; Borkovic-Mitic, S.; Grigorov, I.; Bogojevic, D.; Jasnic, N.; Tosti, T.; Durovic, S.; et al. The effects of meldonium on the renal acute ischemia/reperfusion injury in rats. Int. J. Mol. Sci. 2019, 20, 5747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlato, D.G.; Bairros, A.V.D. Meldonium: Pharmacological, toxicological, and analytical aspects. Toxicol. Res. Appl. 2020, 4. [Google Scholar] [CrossRef]
- Porter, C.; Constantin-Teodosiu, D.; Constantin, D.; Leighton, B.; Poucher, S.M.; Greenhaff, P.L. Muscle carnitine availability plays a central role in regulating fuel metabolism in the rodent. J. Physiol. 2017, 595, 5765–5780. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Vilskersts, R.; Makarova, E.; Kuka, J.; Liepinsh, E. Pharmacological effects of meldonium: Biochemical mechanisms and biomarkers of cardiometabolic activity. Pharmacol. Res. 2016, 113, 771–780. [Google Scholar] [CrossRef]
- Di Cristo, F.; Finicelli, M.; Digilio, F.A.; Paladino, S.; Valentino, A.; Scialo, F.; D’Apolito, M.; Saturnino, C.; Galderisi, U.; Giordano, A.; et al. Meldonium improves Huntington’s disease mitochondrial dysfunction by restoring peroxisome proliferator-activated receptor γ coactivator 1α expression. J. Cell. Physiol. 2019, 234, 9233–9246. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Diener, K.R.; Al-Dasooqi, N.; Lousberg, E.L.; Hayball, J.D. The multifunctional alarmin HMGB1 with roles in the pathophysiology of sepsis and cancer. Immunol. Cell. Biol. 2013, 91, 443–450. [Google Scholar] [CrossRef]
- Stevens, N.E.; Chapman, M.J.; Fraser, C.K.; Kuchel, T.R.; Hayball, J.D.; Diener, K.R. Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hall, P.; Cash, J. What is the real function of the liver ‘function’ tests? Ulster Med. J. 2012, 81, 30–36. [Google Scholar]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. [Google Scholar] [CrossRef] [Green Version]
- Smeding, L.; Plotz, F.B.; Groeneveld, A.B.; Kneyber, M.C. Structural changes of the heart during severe sepsis or septic shock. Shock 2012, 37, 449–456. [Google Scholar] [CrossRef]
- Schmittinger, C.A.; Dunser, M.W.; Torgersen, C.; Luckner, G.; Lorenz, I.; Schmid, S.; Joannidis, M.; Moser, P.; Hasibeder, W.R.; Halabi, M.; et al. Histologic pathologies of the myocardium in septic shock: A prospective observational study. Shock 2013, 39, 329–335. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Zhou, R.; Li, W.; Sama, A.E. Therapeutic potential of HMGB1-targeting agents in sepsis. Expert Rev. Mol. Med. 2008, 10, e32. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Malik, A.B. NF-kB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L622–L645. [Google Scholar] [CrossRef]
- Uskokovic, A.; Dinic, S.; Mihailovic, M.; Grdovic, N.; Arambasic, J.; Vidakovic, M.; Bogojevic, D.; Ivanovic-Matic, S.; Martinovic, V.; Petrovic, M.; et al. STAT3/ NF-kB interactions determine the level of haptoglobin expression in male rats exposed to dietary restriction and/or acute phase stimuli. Mol. Biol. Rep. 2012, 39, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Martinović, V.I.; Arambašić-Jovanović, J.D.; Bogojević, D.B.; Ivanović, A.S.; Otašević, V.M.; Stančić, A.T.; Grigorov, I.I. Contribution of O-GlcNAc modification of NF-kB p65 in attenuation of diabetes-induced haptoglobin expression in rat liver. Arch. Biol. Sci. 2020, 72, 555–565. [Google Scholar] [CrossRef]
- Janz, D.R.; Bastarache, J.A.; Sills, G.; Wickersham, N.; May, A.K.; Bernard, G.R.; Ware, L.B. Association between haptoglobin, hemopexin and mortality in adults with sepsis. Crit. Care 2013, 17, R272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheij, M.W.; Bulder, I.; Wuillemin, W.A.; Voermans, C.; Zeerleder, S.S. Scavengers of hemoproteins as potential biomarkers for severe sepsis and septic shock. Transl. Med. Commun. 2021, 6, 1–5. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Nicholson, D.W. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006, 6, 813–822. [Google Scholar] [CrossRef]
- Lin, B.; Williams-Skipp, C.; Tao, Y.; Schleicher, M.S.; Cano, L.L.; Duke, R.C.; Scheinman, R.I. NF-kB functions as both a proapoptotic and antiapoptotic regulatory factor within a single cell type. Cell Death Differ. 1999, 6, 570–582. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.H.B. Release of cardiac troponin from healthy and damaged myocardium. Front. Lab. Med. 2017, 1, 144–150. [Google Scholar] [CrossRef]
- Hammarsten, O.; Mair, J.; Möckel, M.; Lindahl, B.; Jaffe, A.S. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018, 23, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Xing, C.; Xu, L.; Yao, Y. Beneficial role of oleuropein in sepsis-induced myocardial injury. Possible Involvement of GSK-3β/NF-kB pathway. Acta Cir. Bras. 2021, 36, e360107. [Google Scholar] [CrossRef]
- Nagar, H.; Piao, S.; Kim, C.S. Role of mitochondrial oxidative stress in sepsis. Acute Crit. Care 2018, 33, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.L.; Ping, D.; Boss, J.M. Tumor necrosis factor alpha and interleukin-1beta regulate the murine manganese superoxide dismutase gene through a complex intronic enhancer involving C/EBP-β and NF-kB. Mol. Cell. Biol. 1997, 17, 6970–6981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djavaheri-Mergny, M.; Javelaud, D.; Wietzerbin, J.; Besancon, F. NF-kB activation prevents apoptotic oxidative stress via an increase of both thioredoxin and MnSOD levels in TNFα-treated Ewing sarcoma cells. FEBS Lett. 2004, 578, 111–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo, A.I.; Salinas, M.; Martin, D.; Perona, R.; Cuadrado, A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kB. J. Neurosci. 2004, 24, 7324–7334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.Z.; Johnson, A.P.; Rando, T.A. NF-kB and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic. Biol. Med. 2001, 31, 1405–1416. [Google Scholar] [CrossRef]
- Schreiber, J.; Jenner, R.G.; Murray, H.L.; Gerber, G.K.; Gifford, D.K.; Young, R.A. Coordinated binding of NF-kB family members in the response of human cells to lipopolysaccharide. Proc. Natl. Acad. Sci. USA 2006, 103, 5899–5904. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.G.; Cheng, W.H.; McClung, J.P. Metabolic regulation and function of glutathione peroxidase-1. Annu. Rev. Nutr. 2007, 27, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Durasevic, S.; Jasnic, N.; Prokic, M.; Grigorov, I.; Martinovic, V.; Dordevic, J.; Pavlovic, S. The protective role of virgin coconut oil on the alloxan-induced oxidative stress in the liver, kidneys and heart of diabetic rats. Food Funct. 2019, 10, 2114–2124. [Google Scholar] [CrossRef]
- Preau, S.; Vodovar, D.; Jung, B.; Lancel, S.; Zafrani, L.; Flatres, A.; Oualha, M.; Voiriot, G.; Jouan, Y.; Joffre, J.; et al. Energetic dysfunction in sepsis: A narrative review. Ann. Intensive Care 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Cerra, F.B.; Siegel, J.H.; Coleman, B.; Border, J.R.; McMenamy, R.R. Septic autocannibalism. A failure of exogenous nutritional support. Ann. Surg. 1980, 192, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Spanaki, A.M.; Tavladaki, T.; Dimitriou, H.; Kozlov, A.V.; Duvigneau, J.C.; Meleti, E.; Weidinger, A.; Papakonstantinou, E.; Briassoulis, G. Longitudinal profiles of metabolism and bioenergetics associated with innate immune hormonal inflammatory responses and amino-acid kinetics in severe sepsis and systemic inflammatory response syndrome in children. JPEN J. Parenter. Enteral Nutr. 2018, 42, 1061–1074. [Google Scholar] [CrossRef]
- Simkhovich, B.Z.; Shutenko, Z.V.; Meirena, D.V.; Khagi, K.B.; Mezapuke, R.J.; Molodchina, T.N.; Kalvins, I.J.; Lukevics, E. 3-(2,2,2-Trimethylhydrazinium) propionate (THP)—A novel γ-butyrobetaine hydroxylase inhibitor with cardioprotective properties. Biochem. Pharmacol. 1988, 37, 195–202. [Google Scholar] [CrossRef]
- Kuwajima, M.; Harashima, H.; Hayashi, M.; Ise, S.; Sei, M.; Lu, K.M.; Kiwada, H.; Sugiyama, Y.; Shima, K. Pharmacokinetic analysis of the cardioprotective effect of 3-(2, 2, 2-trimethylhydrazinium) propionate in mice: Inhibition of carnitine transport in kidney. J. Pharmacol. Exp. Ther. 1999, 289, 93–102. [Google Scholar]
- Mecatti, G.C.; Messias, M.C.F.; de Oliveira Carvalho, P. Lipidomic profile and candidate biomarkers in septic patients. Lipids Health Dis. 2020, 19, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Li, H.; Xu, H.; Halim, V.; Zhang, W.; Wang, H.; Ong, K.T.; Woo, S.L.; Walzem, R.L.; Mashek, D.G.; et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS ONE 2012, 7, e39286. [Google Scholar] [CrossRef]
- Yang, Z.H.; Miyahara, H.; Hatanaka, A. Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-Ay Mice with genetic type 2 diabetes. Lipids Health Dis. 2011, 10, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolsoni-Lopes, A.; Festuccia, W.T.; Farias, T.S.; Chimin, P.; Torres-Leal, F.L.; Derogis, P.B.; de Andrade, P.B.; Miyamoto, S.; Lima, F.B.; Curi, R.; et al. Palmitoleic acid (n-7) increases white adipocyte lipolysis and lipase content in a PPARalpha-dependent manner. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1093–E1102. [Google Scholar] [CrossRef] [Green Version]
- Cherkaoui-Malki, M.; Surapureddi, S.; El-Hajj, H.I.; Vamecq, J.; Andreoletti, P. Hepatic steatosis and peroxisomal fatty acid beta-oxidation. Curr. Drug Metab. 2012, 13, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.J.; Seyssel, K.; Chiu, S.; Pan, P.H.; Lin, S.Y.; Stanley, E.; Ament, Z.; West, J.A.; Summerhill, K.; Griffin, J.L.; et al. Odd chain fatty acids; New insights of the relationship between the gut microbiota, dietary intake, biosynthesis and glucose intolerance. Sci. Rep. 2017, 7, 44845. [Google Scholar] [CrossRef] [Green Version]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kröger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Khaw, K.T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef] [Green Version]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Ford, D.A. Elaidic acid increases hepatic lipogenesis by mediating sterol regulatory element binding protein-1c activity in HuH-7 cells. Lipids 2014, 49, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Duann, P.; Lin, P.H. Mitochondria damage and kidney disease. Adv. Exp. Med. Biol. 2017, 982, 529–551. [Google Scholar] [CrossRef] [PubMed]
- De Montmollin, E.; Aboab, J.; Mansart, A.; Annane, D. Bench-to-bedside review: Beta-adrenergic modulation in sepsis. Crit. Care 2009, 13, 230. [Google Scholar] [CrossRef] [Green Version]
- Orso, D.; Copetti, R. Adrenergic overstimulation: A neglected mechanism of sepsis-related cardiomyopathy. Chest 2019, 155, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Suzuki, Y.; Okuda, J.; Kurazumi, T.; Suhara, T.; Ueda, T.; Nagata, H.; Morisaki, H. Sepsis-induced cardiac dysfunction and beta-adrenergic blockade therapy for sepsis. J. Intensive Care 2017, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, S.R.; Berg, R.M.; Windeløv, N.A.; Meyer, M.A.; Plovsing, R.R.; Møller, K.; Johansson, P.I. Coagulopathy, catecholamines, and biomarkers of endothelial damage in experimental human endotoxemia and in patients with severe sepsis: A prospective study. J. Crit. Care 2013, 28, 586–596. [Google Scholar] [CrossRef]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Drosatos, K.; Khan, R.S.; Trent, C.M.; Jiang, H.; Son, N.-H.; Blaner, W.S.; Homma, S.; Schulze, P.C.; Goldberg, I.J. Peroxisome proliferator–activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ. Heart Fail. 2013, 6, 550–562. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Ma, Z.; Guo, A.; Wu, H.; Yang, X. Salvianolic acid B protects against sepsis-induced liver injury via activation of SIRT1/PGC-1α signaling. Exp. Ther. Med. 2020, 20, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Huang, A.; Jin, Y.; Lin, M.; Xia, X.; Chen, X.; Huang, A. Protective effects of exogenous NaHS against sepsis-induced myocardial mitochondrial injury by enhancing the PGC-1α/NRF2 pathway and mitochondrial biosynthesis in mice. Am. J. Transl. Res. 2018, 10, 1422. [Google Scholar] [PubMed]
- An, H.; Hu, Z.; Chen, Y.; Cheng, L.; Shi, J.; Han, L. Angiotensin II-mediated improvement of renal mitochondrial function via the AMPK/PGC-1α/NRF-2 pathway is superior to norepinephrine in a rat model of septic shock associated with acute renal injury. Ann. Transl. Med. 2021, 9. [Google Scholar] [CrossRef]
- Alvarez-Guardia, D.; Palomer, X.; Coll, T.; Davidson, M.M.; Chan, T.O.; Feldman, A.M.; Laguna, J.C.; Vázquez-Carrera, M. The p65 subunit of NF-κB binds to PGC-1α, linking inflammation and metabolic disturbances in cardiac cells. Cardiovasc. Res. 2010, 87, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomer, X.; Álvarez-Guardia, D.; Rodríguez-Calvo, R.; Coll, T.; Laguna, J.C.; Davidson, M.M.; Chan, T.O.; Feldman, A.M.; Vázquez-Carrera, M. TNF-α reduces PGC-1 α expression through NF-κB and p38 MAPK leading to increased glucose oxidation in a human cardiac cell model. Cardiovasc. Res. 2009, 81, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Massart, J.; Begriche, K.; Buron, N.; Porceddu, M.; Borgne-Sanchez, A.; Fromenty, B. Drug-induced inhibition of mitochondrial fatty acid oxidation and steatosis. Curr. Pathobiol. Rep. 2013, 1, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Khazaee, R.; Vinokurtseva, A.; McCaig, L.A.; Yamashita, C.; Hardy, D.B.; Arany, E.; Veldhuizen, R.A.W. The impact of maternal protein restriction during perinatal life on the response to a septic insult in adult rats. J. Dev. Orig. Health Dis. 2020, 1–8. [Google Scholar] [CrossRef]
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard operating procedures for serum and plasma collection: Early detection research network consensus statement standard operating procedure integration working group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1984; pp. 283–284. [Google Scholar]
- Tamura, M.; Oshino, N.; Chance, B. Some characteristics of hydrogen- and alkylhydroperoxides metabolizing systems in cardiac tissue. J. Biochem. 1982, 92, 1019–1031. [Google Scholar] [CrossRef]
- Rehncrona, S.; Smith, D.S.; Akesson, B.; Westerberg, E.; Siesjo, B.K. Peroxidative changes in brain cortical fatty acids and phospholipids, as characterized during Fe2+- and ascorbic acid-stimulated lipid peroxidation in vitro. J. Neurochem. 1980, 34, 1630–1638. [Google Scholar] [CrossRef]
- Aretz, H.T. Myocarditis: The Dallas criteria. Hum. Pathol. 1987, 18, 619–624. [Google Scholar] [CrossRef]
- Suzuki, S.; Toledo-Pereyra, L.H.; Rodriguez, F.J.; Cejalvo, D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 1993, 55, 1265–1272. [Google Scholar] [CrossRef]
- Gregorini, M.; Corradetti, V.; Pattonieri, E.F.; Rocca, C.; Milanesi, S.; Peloso, A.; Canevari, S.; De Cecco, L.; Dugo, M.; Avanzini, M.A.; et al. Perfusion of isolated rat kidney with mesenchymal stromal cells/extracellular vesicles prevents ischaemic injury. J. Cell. Mol. Med. 2017, 21, 3381–3393. [Google Scholar] [CrossRef]


| C Group | S Group | M + S Group | |
|---|---|---|---|
| AST | 266.88 ± 19.34 | 345.17 ± 25.31 a | 243.50 ± 21.79 b |
| ALT | 102.13 ± 2.57 | 172.00 ± 12.00 a | 103.00 ± 5.59 b |
| ALP | 150.50 ± 5.67 | 227.83 ± 23.92 a | 244.50 ± 18.22 a |
| Troponin T | 53.00 ± 5.79 | 141.17 ± 3.07 a | 91.67 ± 5.19 a,b |
| Liver | C Group | S Group | M + S Group |
| Congestion | 1 | 1.333 | 1.125 |
| Vacuolization | 0.375 | 1.333 | 0.625 |
| Necrosis | 0.250 | 1.417 | 1.125 |
| Kidney | C group | S group | M + S group |
| TF | 2.875 | 7.333 | 8.250 |
| BBL | 2.250 | 6.583 | 9.500 |
| BF | 2.125 | 6.500 | 15.500 |
| TN | 1.375 | 7.000 | 14.750 |
| TO | 1.375 | 6.000 | 14.500 |
| Heart | C group | S group | M + S group |
| II | 0.250 | 0.583 | 1.750 |
| Distribution | 0 | 0.333 | 1.250 |
| Adrenal Glands | C Group | S Group | M + S Group |
| Adrenaline | 100 ± 4.945 | 60.586 ± 2.946 a | 29.058 ± 0.964 a,b |
| Noradrenaline | 100 ± 3.221 | 98.356 ± 2.389 | 83.698 ± 3.232 a,b |
| Serum | C group | S group | M + S group |
| Adrenaline | 1651.983 ± 66.585 | 1238.067 ± 53.207 a | 1354.9 ± 17.081 a |
| Noradrenaline | 1297.667 ± 21.243 | 1475.2 ± 24.198 a | 957.233 ± 46.875 a,b |
| Liver | C group | S group | M + S group |
| L carnitine | 6.600 ± 0.024 | 17.256 ± 0.922 a | 4.631 ± 0.052 a,b |
| Glycerol | 0.071 ± 0.001 | 1.231 ± 0.019 a | 0.879 ± 0.014 a,b |
| Triglycerides | 1.372 ± 0.085 | 5.750 ± 0.078 a | 2.052 ± 0.070 a,b |
| Glucose | 2.353 ± 0.021 | 3.459 ± 0.045 a | 2.588 ± 0.024 b |
| Lactate | 1.936 ± 0.011 | 4.194 ± 0.146 a | 2.469 ± 0.017 a,b |
| Fructose | 0.024 ± 0.001 | 0.888 ± 0.006 a | 0.081 ± 0.001 a,b |
| Sucrose | 0.021 ± 0.001 | 0.108 ± 0.005 a | 0.061 ± 0.001 a,b |
| C14:0 | 8.333 ± 0.078 | 13.358 ± 0.400 a | 6.393 ± 0.062 a,b |
| C15:0 | 6.363 ± 0.041 | 11.768 ± 0.326 a | 7.248 ± 0.029 b |
| C16:0 | 5.145 ± 0.024 | 10.205 ± 0.304 a | 4.681 ± 0.034 b |
| C16:1 | 0.461 ± 0.011 | 0.650 ± 0.009 a | 0.307 ± 0.012 a,b |
| C17:0 | 0.346 ± 0.036 | 0.723 ± 0.003 a | 0.309 ± 0.012 b |
| C18:0 | 5.121 ± 0.008 | 12.429 ± 0.127 a | 4.779 ± 0.032 b |
| C18:1, c + t | 2.175 ± 0.025 | 3.030 ± 0.024 a | 1.519 ± 0.030 a,b |
| C18:2, c + t | 5.013 ± 0.043 | 9.461 ± 0.145 a | 4.265 ± 0.027 b |
| C20:4 | 5.484 ± 0.112 | 12.267 ± 0.122 a | 5.633 ± 0.093 b |
| C22:0 | 1.505 ± 0.037 | 1.340 ± 0.056 a | 0.832 ± 0.010 a,b |
| C22:1 | 0.230 ± 0.008 | 0.395 ± 0.027 a | 0.313 ± 0.007 a,b |
| C22:6 | 0.825 ± 0.005 | 1.565 ± 0.104 a | 0.845 ± 0.012 b |
| The FFAs sum | 40.951 ± 0.263 | 77.189 ± 0.917 a | 37.124 ± 0.191 b |
| Kidney | C group | S group | M + S group |
| L carnitine | 1.757 ± 0.017 | 8.512 ± 0.664 a | 1.551 ± 0.179 b |
| Glycerol | 0.372 ± 0.007 | 0.671 ± 0.018 a | 0.317 ± 0.015 b |
| Triglycerides | 1.624 ± 0.049 | 1.107 ± 0.013 a | 1.278 ± 0.022 a |
| Glucose | 1.275 ± 0.007 | 3.309 ± 0.098 a | 0.700 ± 0.012 a,b |
| Lactate | 1.932 ± 0.128 | 3.473 ± 0.179 a | 2.719 ± 0.158 a,b |
| Fructose | 0.117 ± 0.055 | 0.573 ± 0.113 a | 0.104 ± 0.056 b |
| Sucrose | 0.051 ± 0.001 | 0.103 ± 0.010 a | 0.060 ± 0.001 b |
| C14:0 | 6.054 ± 0.123 | 37.325 ± 1.935 a | 16.685 ± 0.260 a,b |
| C15:0 | 82.960 ± 2.172 | 3.288 ± 0.861 a | 0.127 ± 0.006 a,b |
| C16:0 | 39.500 ± 1.617 | 489.563 ± 25.487 a | 143.866 ± 3.856 a,b |
| C16:1 | 56.845 ± 1.250 | 11.740 ± 0.928 | 0.095 ± 0.002 a,b |
| C17:0 | 59.144 ± 1.534 | 24.153 ± 3.155 a | 0.098 ± 0.002 a,b |
| C18:0 | 44.908 ± 1.154 | 172.758 ± 17.153 a | 50.237 ± 1.445 b |
| C18:1 cis + trans | 18.642 ± 0.852 | 103.390 ± 14.797 a | 0.260 ± 0.011 a,b |
| C18:2 cis + trans | 24.550 ± 0.601 | 82.404 ± 14.128 a | 20.061 ± 0.664 b |
| C20:4 | 46.280 ± 1.108 | 0.499 ± 0.0267 a | 0.523 ± 0.025 a |
| C22:0 | 20.489 ± 0.699 | 0.825 ± 0.105 a | 0.836 ± 0.018 a |
| C22:1 | 26.175 ± 1.109 | 7.219 ± 0.315 a | 11.429 ± 0.483 a,b |
| C22:6 | 17.587 ± 1.102 | 5.534 ± 0.295 a | 13.175 ± 0.481 b |
| The FFAs sum | 443.134 ± 11.702 | 938.697 ± 66.007 a | 257.395 ± 4.710 a,b |
| Heart | C group | S group | M + S group |
| L carnitine | 0.990 ± 0.012 | 3.976 ± 0.042 a | 0.760 ± 0.008 b |
| Glycerol | 0.074 ± 0.018 | 0.307 ± 0.014 a | 0.088 ± 0.011 b |
| Triglycerides | 0.856 ± 0.033 | 1.959 ± 0.024 a | 0.952 ± 0.017 b |
| Glucose | 0.793 ± 0.006 | 0.554 ± 0.005 a | 0.129 ± 0.002 a,b |
| Lactate | 0.309 ± 0.001 | 1.316 ± 0.014 a | 0.305 ± 0.001 b |
| Fructose | 0.012 ± 0.001 | 0.012 ± 0.001 | 0.0085 ± 0.0001 a,b |
| Sucrose | 0.0012 ± 0.0001 | 0.0065 ± 0.0002 a | 0.0041 ± 0.0001 a,b |
| C14:0 | 7.194 ± 0.074 | 25.342 ± 0.874 a | 11.539 ± 0.130 a,b |
| C15:0 | 44.661 ± 1.097 | 7.528 ± 0.409 a | 3.688 ± 0.0142 a,b |
| C16:0 | 22.322 ± 0.809 | 249.884 ± 12.715 a | 74.273 ± 1.922 a,b |
| C16:1 | 28.653 ± 0.626 | 6.195 ± 0.462 a | 0.201 ± 0.005 a,b |
| C17:0 | 29.745 ± 0.774 | 12.438 ± 1.578 a | 0.204 ± 0.006 a,b |
| C18:0 | 25.015 ± 0.580 | 92.593 ± 8.586 a | 27.508 ± 0.718 b |
| C18:1 cis + trans | 10.408 ± 0.421 | 53.210 ± 7.404 a | 0.890 ± 0.014 a,b |
| C18:2 cis + trans | 14.782 ± 0.290 | 45.933 ± 7.043 a | 12.163 ± 0.324 b |
| C20:4 | 25.882 ± 0.558 | 6.383 ± 0.056 a | 3.078 ± 0.041 a,b |
| C22:0 | 10.997 ± 0.357 | 1.083 ± 0.044 a | 0.835 ± 0.008 a |
| C22:1 | 13.202 ± 0.557 | 3.807 ± 0.150 a | 5.871 ± 0.239 a,b |
| C22:6 | 9.205 ± 0.550 | 3.549 ± 0.144 a | 7.010 ± 0.241 a,b |
| The FFAs sum | 242.067 ± 5.894 | 507.943 ± 32.945 a | 147.260 ± 2.356 a,b |
| Groups | C Group | S Group | M + S Group |
|---|---|---|---|
| Application of meldonium in water | none | none | + |
| Intraperitoneal application of saline | + | none | none |
| Intraperitoneal application of faeces | none | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đurašević, S.; Ružičić, A.; Lakić, I.; Tosti, T.; Đurović, S.; Glumac, S.; Pavlović, S.; Borković-Mitić, S.; Grigorov, I.; Stanković, S.; et al. The Effects of a Meldonium Pre-Treatment on the Course of the Faecal-Induced Sepsis in Rats. Int. J. Mol. Sci. 2021, 22, 9698. https://doi.org/10.3390/ijms22189698
Đurašević S, Ružičić A, Lakić I, Tosti T, Đurović S, Glumac S, Pavlović S, Borković-Mitić S, Grigorov I, Stanković S, et al. The Effects of a Meldonium Pre-Treatment on the Course of the Faecal-Induced Sepsis in Rats. International Journal of Molecular Sciences. 2021; 22(18):9698. https://doi.org/10.3390/ijms22189698
Chicago/Turabian StyleĐurašević, Siniša, Aleksandra Ružičić, Iva Lakić, Tomislav Tosti, Saša Đurović, Sofija Glumac, Slađan Pavlović, Slavica Borković-Mitić, Ilijana Grigorov, Sanja Stanković, and et al. 2021. "The Effects of a Meldonium Pre-Treatment on the Course of the Faecal-Induced Sepsis in Rats" International Journal of Molecular Sciences 22, no. 18: 9698. https://doi.org/10.3390/ijms22189698
APA StyleĐurašević, S., Ružičić, A., Lakić, I., Tosti, T., Đurović, S., Glumac, S., Pavlović, S., Borković-Mitić, S., Grigorov, I., Stanković, S., Jasnić, N., Đorđević, J., & Todorović, Z. (2021). The Effects of a Meldonium Pre-Treatment on the Course of the Faecal-Induced Sepsis in Rats. International Journal of Molecular Sciences, 22(18), 9698. https://doi.org/10.3390/ijms22189698