High-Dose Irradiation Inhibits Motility and Induces Autophagy in Caenorhabditis elegans
Abstract
1. Introduction
2. Results
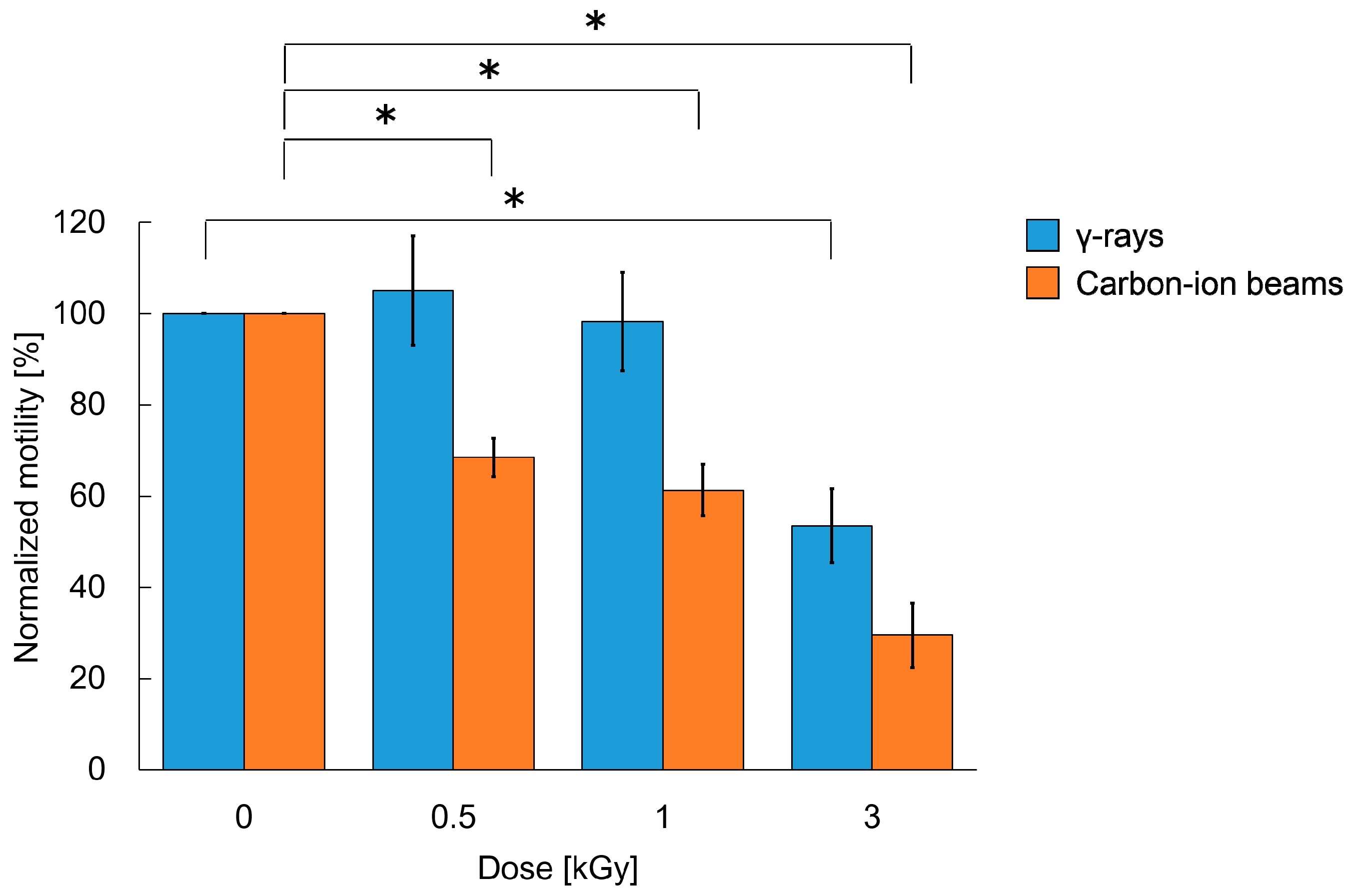
2.1. Motility Was Reduced by Whole-Body Irradiation with High-Dose γ-rays and Carbon-Ion Beams
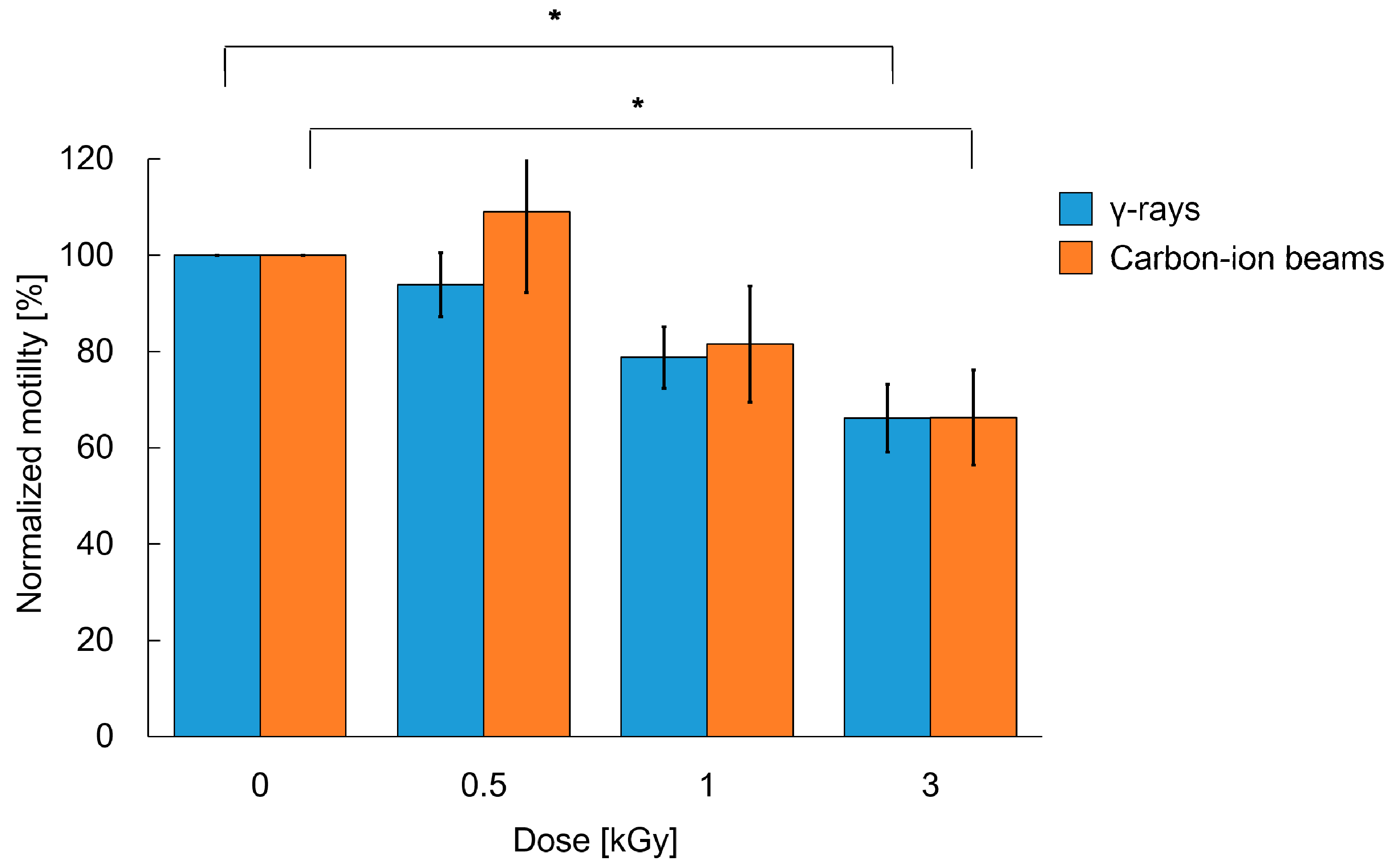
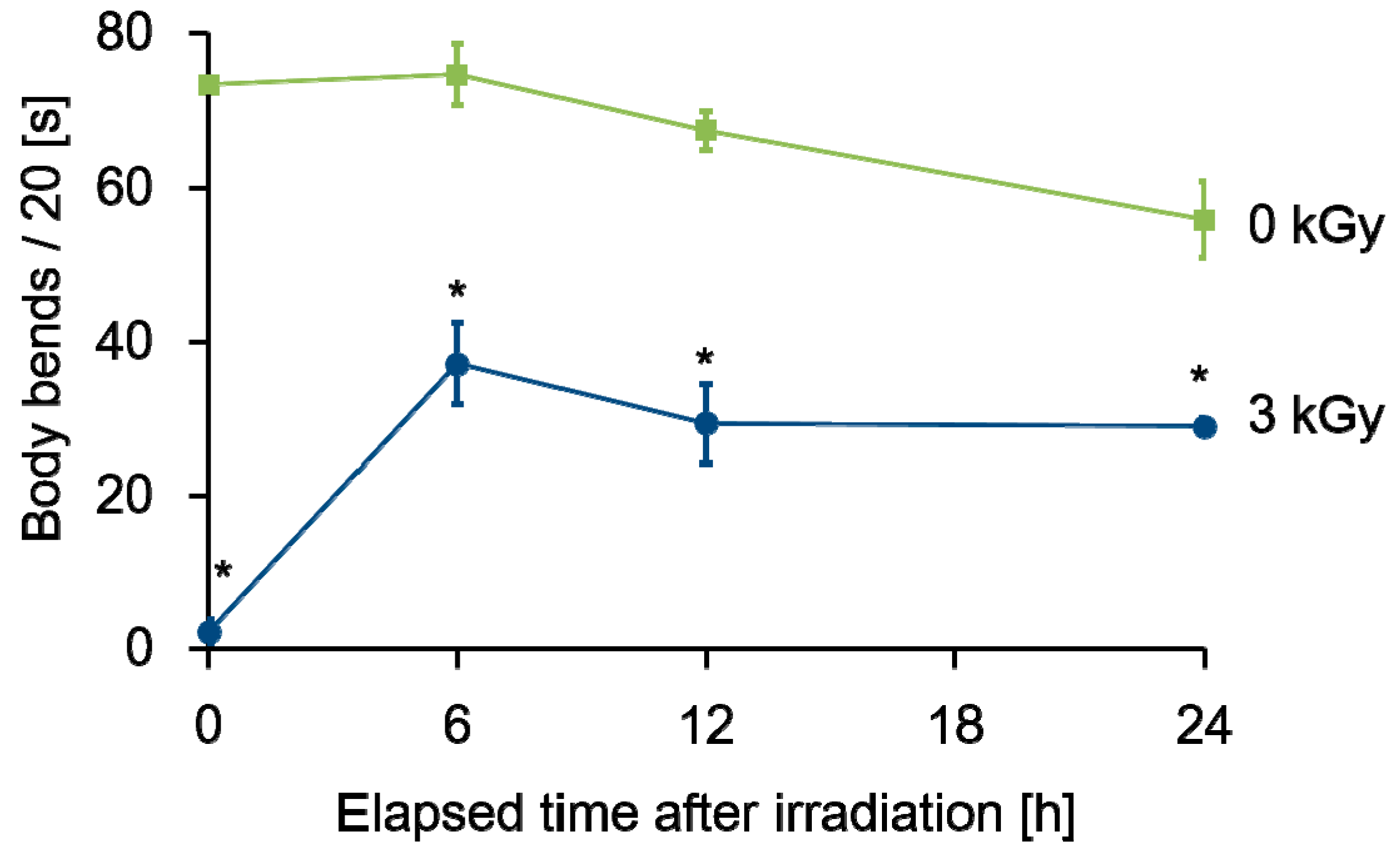
2.2. The Decreased Motility after Whole-Body Irradiation Partially Recovered
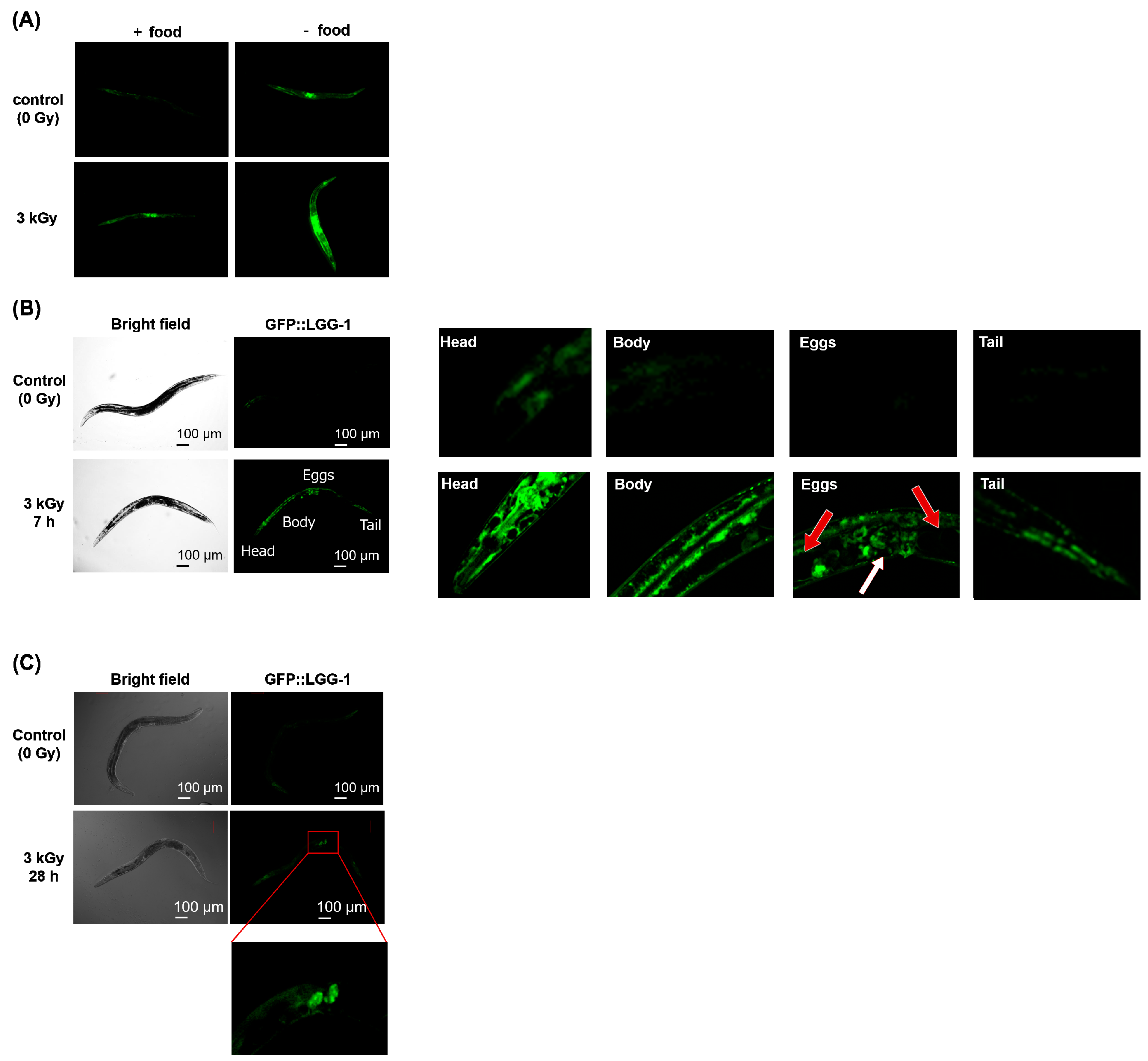
2.3. Autophagy Was Induced during the Recovery of Decreased Motility after Whole-Body Irradiation
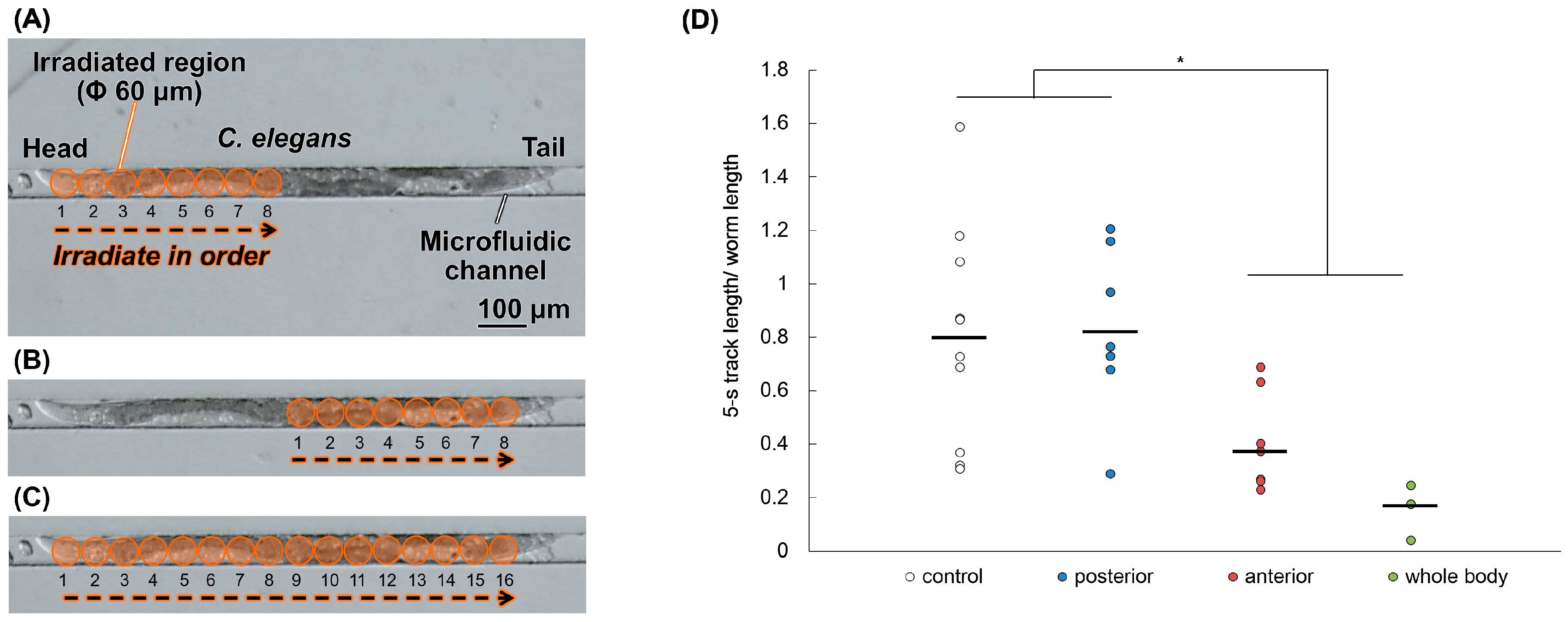
2.4. Anterior Half-Body Irradiation by Carbon ion Microbeams Affects Motility
3. Discussion
4. Materials and Methods
4.1. Strains and Culture
4.2. Whole-Body Irradiation
4.3. Crawling Analysis
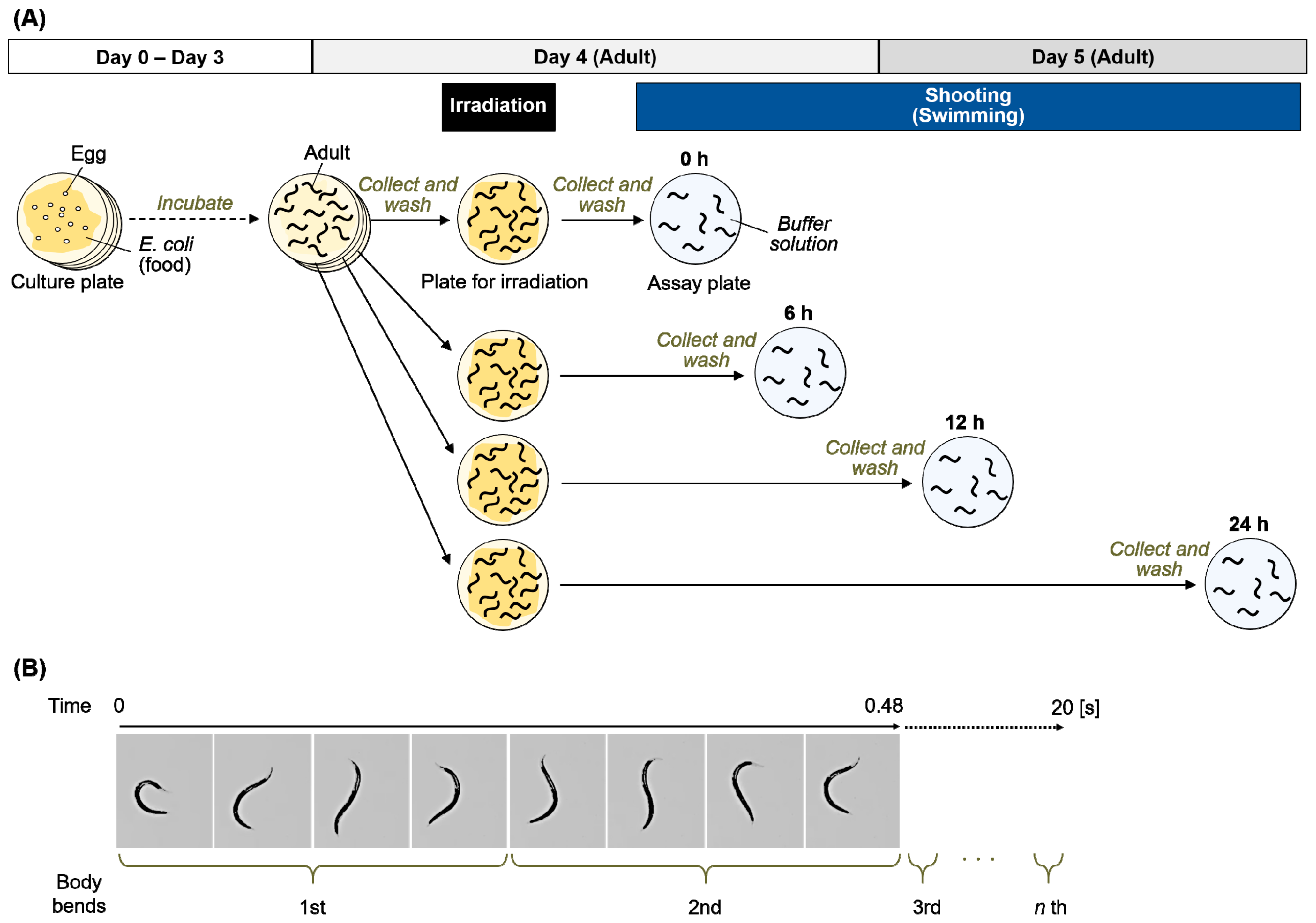
4.4. Swimming Analysis
4.5. Fluorescence Microscopy Observation
4.6. Region-Specific Carbon-Ion Microbeam Irradiation
4.7. Statistical Analysis
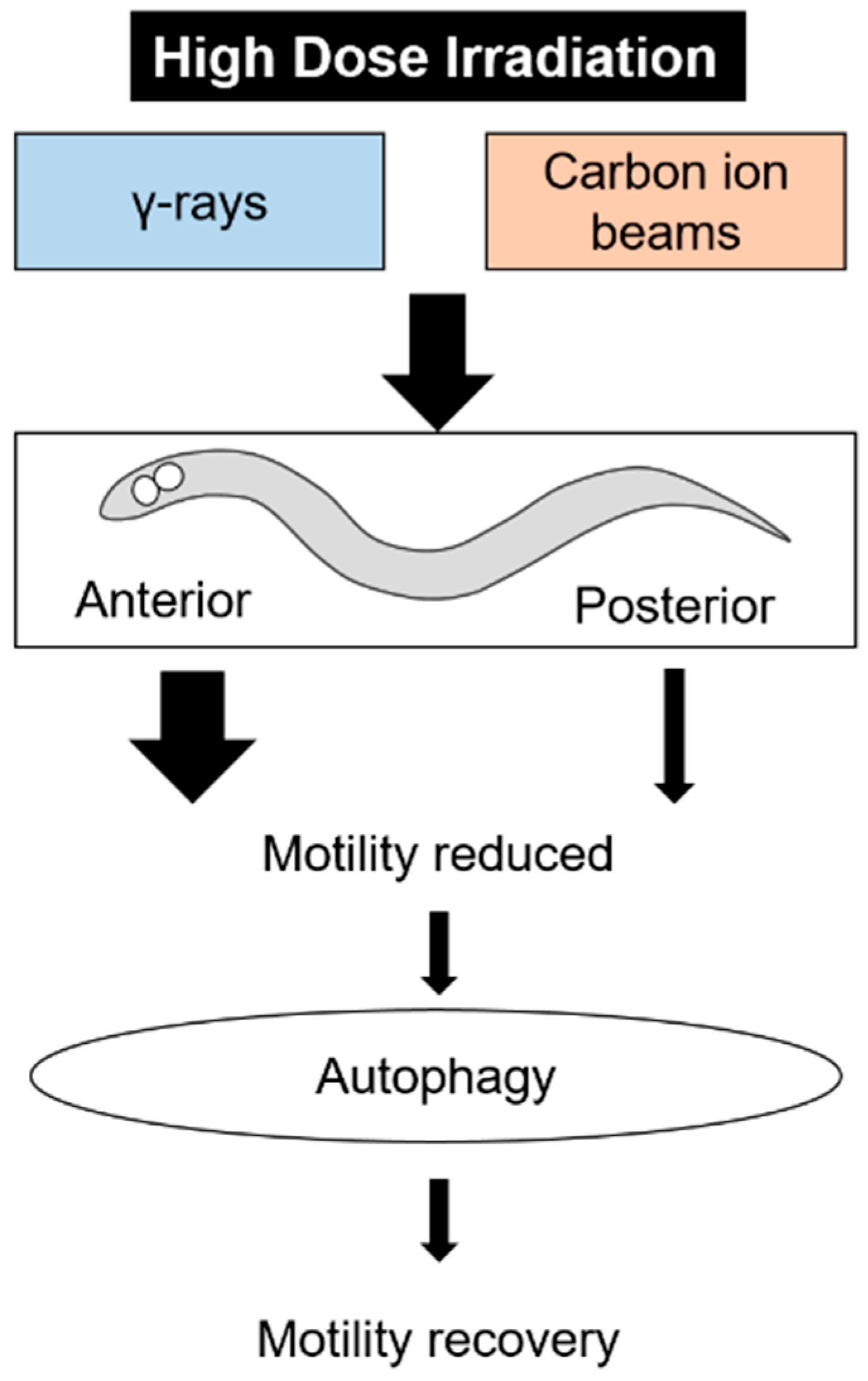
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fry, R.J.M.; Hall, E.J. Radiobiology for the radiologist. Radiat. Res. 1995, 141, 347. [Google Scholar] [CrossRef]
- Krisko, A.; Leroy, M.; Radman, M.; Meselson, M. Extreme anti-oxidant protection against ionizing radiation in bdelloid rotifers. Proc. Natl. Acad. Sci. USA 2012, 109, 2354–2357. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.J. Death by protein damage in irradiated cells. DNA Repair 2012, 11, 12–21. [Google Scholar] [CrossRef]
- Filomeni, G.; de Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, H.; Yonekura, S.I.; Nakamura, N.; Sugiyama, H.; Yonei, S.; Zhang-Akiyama, Q.M. Purification and characterization of Caenorhabditis elegans NTH, a homolog of human endonuclease III: Essential role of N-terminal region. DNA Repair 2009, 8, 844–851. [Google Scholar] [CrossRef]
- Oh, S.-I.; Park, J.-K.; Park, S.-K. Lifespan extension and increased resistance to environmental stressors by N-Acetyl-L-Cysteine in Caenorhabditis elegans. Clinics 2015, 70, 380–386. [Google Scholar] [CrossRef]
- Michaelidesová, A.; Konířová, J.; Bartůněk, P.; Zíková, M. Effects of radiation therapy on neural stem cells. Genes 2019, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Kuzmic, M.; Galas, S.; Lecomte-Pradines, C.; Dubois, C.; Dubourg, N.; Frelon, S. Interplay between ionizing radiation effects and aging in C. elegans. Free Radic. Biol. Med. 2019, 134, 657–665. [Google Scholar] [CrossRef]
- Heselich, A.; Frieß, J.L.; Ritter, S.; Benz, N.P.; Layer, P.G.; Thielemann, C. High LET radiation shows no major cellular and functional effects on primary cardiomyocytes in vitro. Life Sci. Space Res. 2018, 16, 93–100. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Kimble, J.; Hirsh, D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 1979, 70, 396–417. [Google Scholar] [CrossRef]
- Sulston, J.E.; Horvitz, H.R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 1977, 56, 110–156. [Google Scholar] [CrossRef]
- Dhakal, R.; Yosofvand, M.; Yavari, M.; Abdulrahman, R.; Schurr, R.; Moustaid-Moussa, N.; Moussa, H. Review of biological effects of acute and chronic radiation exposure on Caenorhabditis elegans. Cells 2021, 10, 1966. [Google Scholar] [CrossRef]
- Sugimoto, T.; Dazai, K.; Sakashita, T.; Funayama, T.; Wada, S.; Hamada, N.; Kakizaki, T.; Kobayashi, Y.; Higashitani, A. Cell cycle arrest and apoptosis in Caenorhabditis elegans germline cells following heavy-ion microbeam irradiation. Int. J. Radiat. Biol. 2006, 82, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, T.; Suzuki, M.; Hamada, N.; Shimozawa, Y.; Shirai-Fukamoto, K.; Yokota, Y.; Hamada-Sora, S.; Kakizaki, T.; Wada, S.; Funayama, T.; et al. Behavioral resistance of Caenorhabditis elegans against high-LET radiation exposure. Biol. Sci. Space 2012, 26, 7–11. [Google Scholar] [CrossRef][Green Version]
- Ishii, N.; Suzuki, K. X-ray inactivation of Caenorhabditis elegans embryos or larvae. Int. J. Radiat. Biol. 1990, 58, 827–833. [Google Scholar] [CrossRef]
- Johnson, T.E.; Hartman, P.S. Radiation effects on life span in Caenorhabditis elegans. J. Gerontol. Biol. Sci. 1988, 43, B137–B141. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, T.; Hamada, N.; Ikeda, D.D.; Suzuki, M.; Yanase, S.; Ishii, N.; Kobayashi, Y. Locomotion—Learning behavior relationship in Caenorhabditis elegans following γ-ray irradiation. J. Radiat. Res. 2008, 49, 285–291. [Google Scholar] [CrossRef][Green Version]
- Suzuki, M.; Soh, Z.; Yamashita, H.; Tsuji, T.; Funayama, T. Targeted central nervous system irradiation of Caenorhabditis elegans induces a limited effect on motility. Biology 2020, 9, 289. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakashita, T.; Yanase, S.; Kikuchi, M.; Ohba, H.; Higashitani, A.; Hamada, N.; Funayama, T.; Fukamoto, K.; Tsuji, T.; et al. Effects of ionizing radiation on locomotory behavior and mechanosensation in Caenorhabditis elegans. J. Radiat. Res. 2009, 50, 119–125. [Google Scholar] [CrossRef][Green Version]
- Moriwaki, T.; Yamasaki, A.; Zhang-Akiyama, Q.M. ATM induces cell death with autophagy in response to H2O2 specifically in Caenorhabditis elegans nondividing cells. Oxid. Med. Cell. Longev. 2018, 2018, 3862070. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Kaminskyy, V.O.; Zhivotovsky, B. Autophagy in toxicology: Cause or consequence? Annu. Rev. Pharmacol. Toxicol. 2013, 53, 275–297. [Google Scholar] [CrossRef]
- Morishita, H.; Mizushima, N. Diverse cellular roles of autophagy. Annu. Rev. Pharmacol. Toxicol. 2019, 35, 453–475. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Levine, B.; Kroemer, G. Metabolic control of autophagy. Cell 2014, 159, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Vermezovic, J.; Stergiou, L.; Hengartner, M.O.; d’Adda di Fagagna, F. Differential regulation of DNA damage response activation between somatic and germline cells in Caenorhabditis elegans. Cell Death Differ. 2012, 19, 1847–1855. [Google Scholar] [CrossRef]
- Hou, J.; Han, Z.P.; Jing, Y.Y.; Yang, X.; Zhang, S.S.; Sun, K.; Hao, C.; Meng, Y.; Yu, F.H.; Liu, X.Q.; et al. Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Funayama, T. Heavy-ion microbeams for biological science: Development of system and utilization for biological experiments in QST-takasaki. Quantum Beam Sci. 2019, 3, 13. [Google Scholar] [CrossRef]
- Suzuki, M.; Hattori, Y.; Sakashita, T.; Yokota, Y.; Kobayashi, Y.; Funayama, T. Region-specific irradiation system with heavy-ion microbeam for active individuals of Caenorhabditis elegans. J. Radiat. Res. 2017, 58, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Sakashita, T.; Hattori, Y.; Yokota, Y.; Kobayashi, Y.; Funayama, T. Development of ultra-thin chips for immobilization of Caenorhabditis elegans in microfluidic channels during irradiation and selection of buffer solution to prevent dehydration. J. Neurosci. Methods 2018, 306, 32–37. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakashita, T.; Funayama, T. Immobilization of live Caenorhabditis elegans individuals using an ultra-thin polydimethylsiloxane microfluidic chip with water retention. J. Vis. Exp. 2019, 145. [Google Scholar] [CrossRef] [PubMed]
- Roussel, N.; Sprenger, J.; Tappan, S.J.; Glaser, J.R. Robust tracking and quantification of C. elegans body shape and locomotion through coiling, entanglement, and omega bends. Worm 2014, 3, e982437. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, T.; Kato, S.; Kato, Y.; Hosoki, A.; Zhang-Akiyama, Q.-M. Extension of lifespan and protection against oxidative stress by an antioxidant herb mixture complex (KPG-7) in Caenorhabditis elegans. J. Clin. Biochem. Nutr. 2013, 53, 81–88. [Google Scholar] [CrossRef]
- Gaffney, C.J.; Bass, J.J.; Barratt, T.F.; Szewczyk, N.J. Methods to assess subcellular compartments of muscle in C. elegans. J. Vis. Exp. 2014, 93, e52043. [Google Scholar] [CrossRef]
- Hosoki, A.; Yonekura, S.-I.; Zhao, Q.-L.; Wei, Z.-L.; Takasaki, I.; Tabuchi, Y.; Wang, L.-L.; Hasuike, S.; Nomura, T.; Tachibana, A.; et al. Mitochondria-targeted superoxide dismutase (SOD2) regulates radiation resistance and radiation stress response in HeLa Cells. J. Radiat. Res. 2012, 53, 58–71. [Google Scholar] [CrossRef]
- Yamamori, T.; Ike, S.; Bo, T.; Sasagawa, T.; Sakai, Y.; Suzuki, M.; Yamamoto, K.; Nagane, M.; Yasui, H.; Inanami, O. Inhibition of the mitochondrial fission protein dynamin-related protein 1 (Drp1) impairs mitochondrial fission and mitotic catastrophe after x-irradiation. Mol. Biol. Cell 2015, 26, 4607–4617. [Google Scholar] [CrossRef]
- Kam, W.W.; Banati, R.B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.V.; Bredesen, D.E. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr. Opin. Cell Biol. 2004, 16, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- Kirstein-Miles, J.; Morimoto, R.I. Caenorhabditis elegans as a model system to study intercompartmental proteostasis: Interrelation of mitochondrial function, longevity, and neurodegenerative diseases. Dev. Dyn. 2010, 239, 1529–1538. [Google Scholar] [CrossRef]
- Taferner, A.; Pircher, H.; Koziel, R.; von Grafenstein, S.; Baraldo, G.; Palikaras, K.; Liedl, K.R.; Tavernarakis, N.; Jansen-Dürr, P. FAH domain containing protein 1 (FAHD-1) is required for mitochondrial function and locomotion activity in C. elegans. PLoS ONE 2015, 10, e0134161. [Google Scholar] [CrossRef]
- Qi, Y.; Qiu, Q.; Gu, X.; Tian, Y.; Zhang, Y. ATM mediates spermidine-induced mitophagy via PINK1 and Parkin regulation in human fibroblasts. Sci. Rep. 2016, 6, 24700. [Google Scholar] [CrossRef] [PubMed]
- Meesungnoen, J.; Benrahmoune, M.; Filali-Mouhim, A.; Mankhetkorn, S.; Jay-Gerin, J.P. Monte Carlo calculation of the primary radical and molecular yields of liquid water radiolysis in the linear energy transfer range 0.3–6.5 keV/μm: Application to 137Cs Gamma rays. Radiat. Res. 2001, 155, 269–278. [Google Scholar] [CrossRef]
- Meesungnoen, J.; Jay-Gerin, J.P. High-LET radiolysis of liquid water with 1H+, 4He2+, 12C6+, and 20Ne9+ ions: Effects of multiple ionization. J Phys. Chem. A 2005, 109, 6406–6419. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, T.; Kato, Y.; Nakamura, C.; Ishikawa, S.; Zhang-Akiyama, Q.M. A novel DNA damage response mediated by DNA mismatch repair in Caenorhabditis elegans: Induction of programmed autophagic cell death in non-dividing cells. Genes Cancer 2015, 6, 341–355. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, J.T.; Guo, B.; Hansen, M.; Jia, K.; Ková, A.L.; Kumsta, C.; Lapierre, L.R.; Legouis, R.; Lin, L.; et al. Guidelines for monitoring autophagy in Caenorhabditis elegans. Autophagy 2015, 11, 9–27. [Google Scholar] [CrossRef]
- Palmisano, N.J.; Meléndez, A. Detection of autophagy in Caenorhabditis elegans using GFP::LGG-1 as an autophagy marker. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Laranjeiro, R.; Harinath, G.; Burke, D.; Braeckman, B.P.; Driscoll, M. Single swim sessions in C. elegans induce key features of mammalian exercise. BMC Biol. 2017, 15, 30. [Google Scholar] [CrossRef]
- Hirayama, R. Mechanism of oxygen effect for photon and heavy-ion beams. Jpn. J. Med Phys. 2014, 34, 65–69. [Google Scholar] [CrossRef]
- Hada, M.; Alexandros, G.G. Formation of clustered DNA damage after high-LET irradiation: A review. J. Radiat. Res. 2008, 49, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Terato, H.; Ide, H. Clustered DNA damage induced by heavy ion particles. Biol. Sci. Space 2004, 18, 206–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takanami, T.; Zhang, Y.; Aoki, H.; Abe, T.; Yoshida, S.; Takahashi, H.; Horiuchi, S.; Higashitani, A. Efficient repair of DNA damage induced by heavy ion particles in meiotic prophase I nuclei of Caenorhabditis elegans. J. Radiat. Res. 2003, 44, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; You, N.J.; Avery, L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev. 2007, 21, 2161–2171. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamasaki, A.; Suzuki, M.; Funayama, T.; Moriwaki, T.; Sakashita, T.; Kobayashi, Y.; Zhang-Akiyama, Q.-M. High-Dose Irradiation Inhibits Motility and Induces Autophagy in Caenorhabditis elegans. Int. J. Mol. Sci. 2021, 22, 9810. https://doi.org/10.3390/ijms22189810
Yamasaki A, Suzuki M, Funayama T, Moriwaki T, Sakashita T, Kobayashi Y, Zhang-Akiyama Q-M. High-Dose Irradiation Inhibits Motility and Induces Autophagy in Caenorhabditis elegans. International Journal of Molecular Sciences. 2021; 22(18):9810. https://doi.org/10.3390/ijms22189810
Chicago/Turabian StyleYamasaki, Akira, Michiyo Suzuki, Tomoo Funayama, Takahito Moriwaki, Tetsuya Sakashita, Yasuhiko Kobayashi, and Qiu-Mei Zhang-Akiyama. 2021. "High-Dose Irradiation Inhibits Motility and Induces Autophagy in Caenorhabditis elegans" International Journal of Molecular Sciences 22, no. 18: 9810. https://doi.org/10.3390/ijms22189810
APA StyleYamasaki, A., Suzuki, M., Funayama, T., Moriwaki, T., Sakashita, T., Kobayashi, Y., & Zhang-Akiyama, Q.-M. (2021). High-Dose Irradiation Inhibits Motility and Induces Autophagy in Caenorhabditis elegans. International Journal of Molecular Sciences, 22(18), 9810. https://doi.org/10.3390/ijms22189810







