Angiogenesis and Anti-Angiogenic Treatment in Prostate Cancer: Mechanisms of Action and Molecular Targets
Abstract
1. Introduction
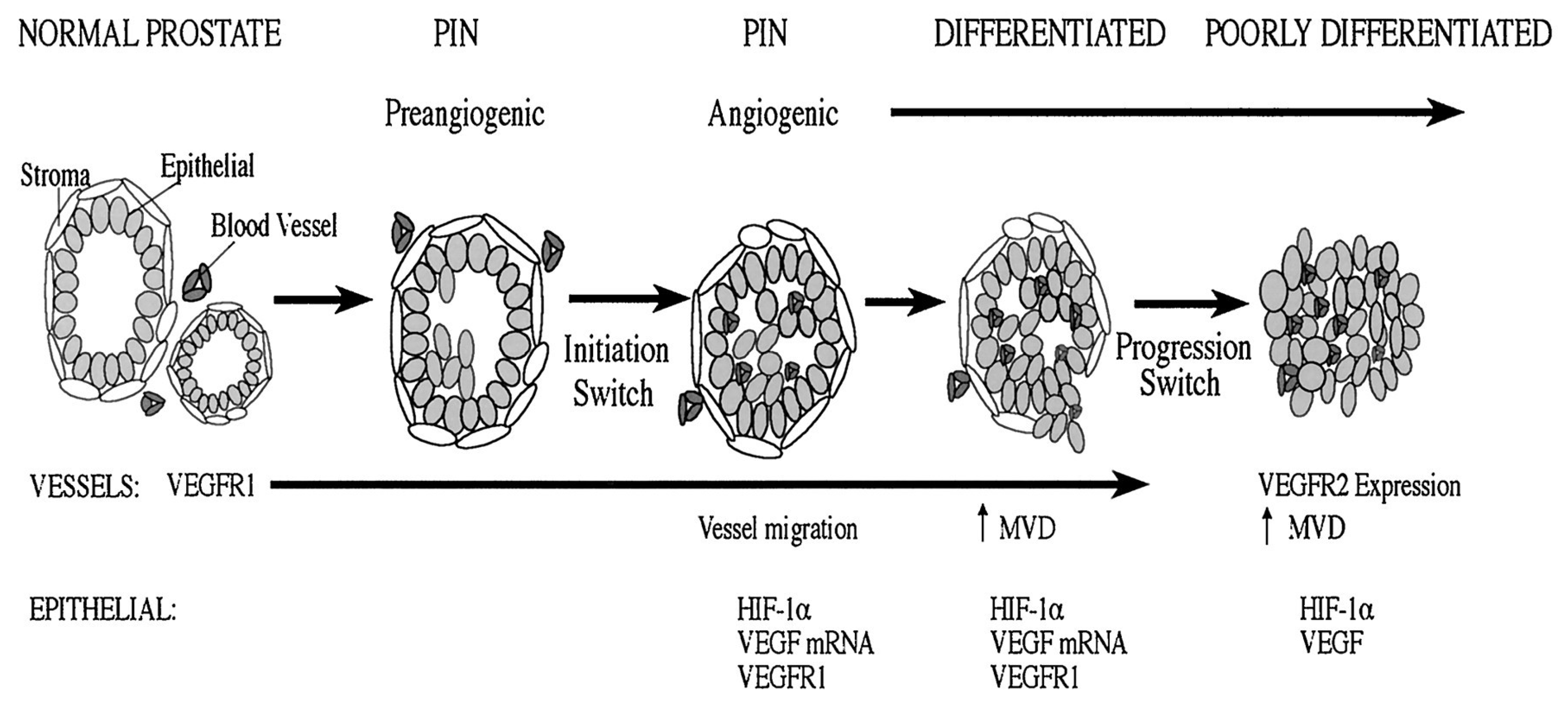
1.1. Pathways Involved in the Angiogenesis of Prostate Cancer
1.1.1. Vascular Endothelial Growth Factors (VEGFs)
1.1.2. Fibroblast Growth Factors (FGFs)
1.1.3. Matrix Metalloproteinases (MMPs)
1.1.4. Transforming Growth Factor β (TGFβ)
1.1.5. Pathways of Hypoxia-Inducible Factors (HIF)
1.1.6. Cyclooxygenases (COXs)
1.1.7. Interleukins (ILs)
1.1.8. microRNAs (miRNAs)
2. Chemotherapeutic Agents with Antiangiogenic Activity
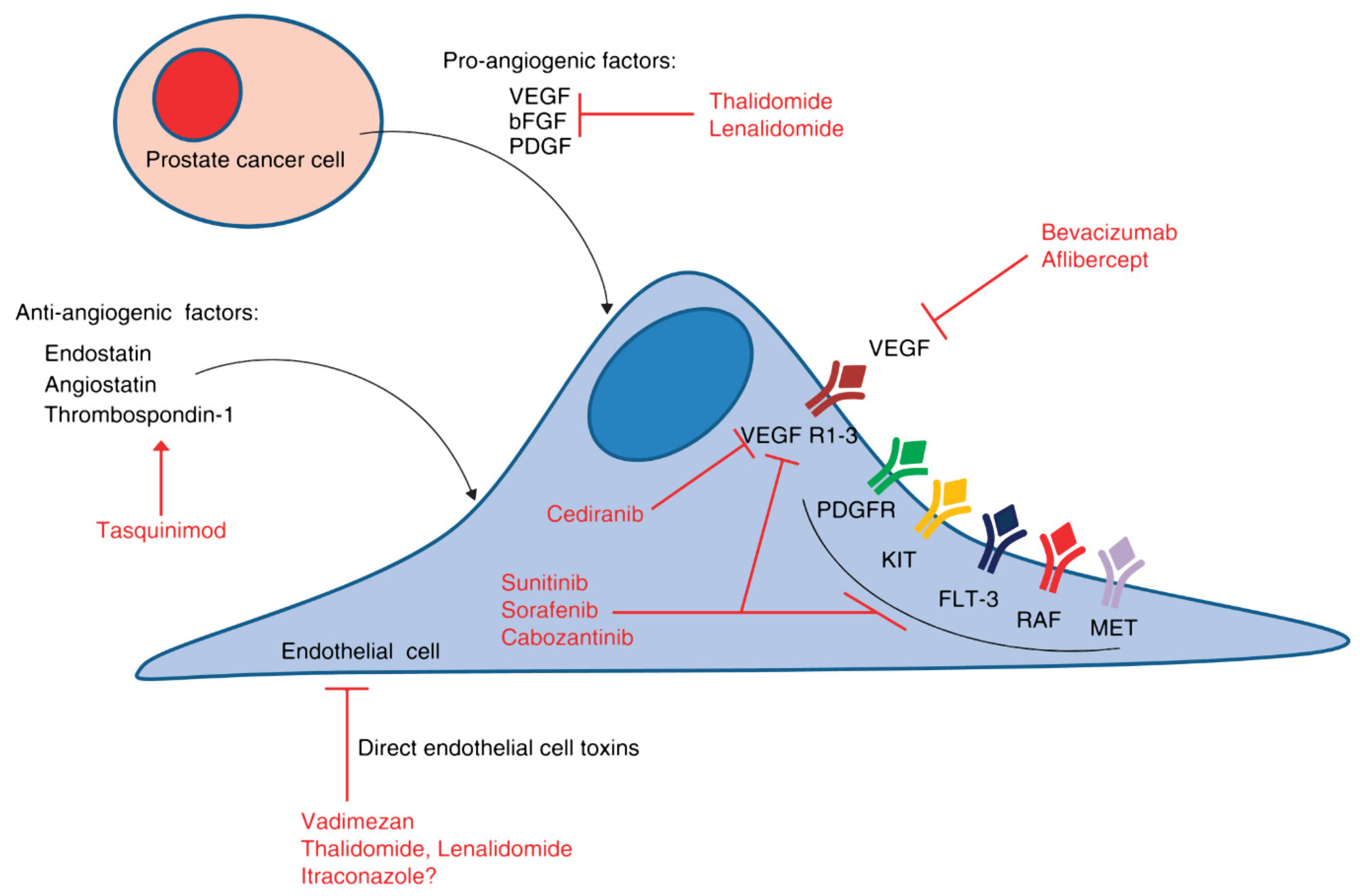
3. Anti-Angiogenic Agents in Prostate Cancer
3.1. VEGF-Directed Agents
3.1.1. Bevacizumab
3.1.2. Aflibercept
3.2. VEGFR Tyrosine Kinase Inhibitors
3.2.1. Sorafenib
3.2.2. Sunitinib
3.2.3. Cediranib
3.2.4. Vandetanib
3.2.5. Cabozantinib
3.3. PDGF-Targeted Therapy
3.4. Antiangiogenic/Immunomodulatory Drugs
3.4.1. Thalidomide
3.4.2. Lenalidomide
3.4.3. Miscellaneous Angiogenesis Inhibitors
- Tasquinimod
- ii.
- Itraconazole
- iii.
- Trebanabib
4. Mechanisms of Resistance
5. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Compérat, E.; Wasinger, G.; Oszwald, A.; Kain, R.; Cancel-Tassin, G.; Cussenot, O. The Genetic Complexity of Prostate Cancer. Genes 2020, 11, 1396. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Nair, M.P.; Sufrin, G.; Mahajan, S.D.; Chadha, K.C.; Chawda, R.P.; Schwartz, S.A. Gene Expression of Angiogenic Factors Correlates with Metastatic Potential of Prostate Cancer Cells. Cancer Res. 2004, 64, 5311–5321. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Ching, J.B.; Madan, R.A.; Dahut, W.L. Angiogenesis Inhibition in Prostate Cancer: Current Uses and Future Promises. J. Oncol. 2010, 2010, 361836. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Roobol, M.J.; Savage, C.J.; Peltola, M.; Pettersson, K.; Scardino, P.T.; Vickers, A.J.; Schröder, F.H.; Lilja, H. A four-kallikrein panel for the prediction of repeat prostate biopsy: Data from the European Randomized Study of Prostate Cancer screening in Rotterdam, Netherlands. Br. J. Cancer 2010, 103, 708–714. [Google Scholar] [CrossRef]
- Scattoni, V.; Lazzeri, M.; Lughezzani, G.; De Luca, S.; Passera, R.; Bollito, E.; Randone, D.; Abdollah, F.; Capitanio, U.; Larcher, A.; et al. Head-to-head comparison of prostate health index and urinary PCA3 for predicting cancer at initial or repeat biopsy. J. Urol. 2013, 190, 496–501. [Google Scholar] [CrossRef]
- Wei, J.T.; Feng, Z.; Partin, A.W.; Brown, E.; Thompson, I.; Sokoll, L.; Chan, D.W.; Lotan, Y.; Kibel, A.S.; Busby, J.E.; et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer? J. Clin. Oncol. 2014, 32, 4066–4072. [Google Scholar] [CrossRef]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Diagnostic Performance of Prostate Imaging Reporting and Data System Version 2 for Detection of Prostate Cancer: A Systematic Review and Diagnostic Meta-analysis. Eur. Urol. 2017, 72, 177–188. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Armstrong, A.J.; Bahnson, R.R.; D’Amico, A.V.; Davis, B.J.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; Horwitz, E.M.; et al. Prostate Cancer, Version 1.2016. J. Natl. Compr. Cancer Netw. 2016, 14, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Sommariva, S.; Tarricone, R.; Lazzeri, M.; Ricciardi, W.; Montorsi, F. Prognostic Value of the Cell Cycle Progression Score in Patients with Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 69, 107–115. [Google Scholar] [CrossRef]
- Lindenberg, M.L.; Turkbey, B.; Mena, E.; Choyke, P.L. Imaging Locally Advanced, Recurrent, and Metastatic Prostate Cancer: A Review. JAMA Oncol. 2017, 3, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Filson, C.P.; Marks, L.S.; Litwin, M.S. Expectant management for men with early stage prostate cancer. CA Cancer J. Clin. 2015, 65, 265–282. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.U.; et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Novara, G.; Ahlering, T.E.; Costello, A.; Eastham, J.A.; Graefen, M.; Guazzoni, G.; Menon, M.; Mottrie, A.; Patel, V.R.; et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Wortel, R.C.; Incrocci, L.; Pos, F.J.; Lebesque, J.V.; Witte, M.G.; van der Heide, U.A.; van Herk, M.; Heemsbergen, W.D. Acute toxicity after image-guided intensity modulated radiation therapy compared to 3D conformal radiation therapy in prostate cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Viani, G.A.; Viana, B.S.; Martin, J.E.; Rossi, B.T.; Zuliani, G.; Stefano, E.J. Intensity-modulated radiotherapy reduces toxicity with similar biochemical control compared with 3-dimensional conformal radiotherapy for prostate cancer: A randomized clinical trial. Cancer 2016, 122, 2004–2011. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021. Epub ahead of Print. [Google Scholar] [CrossRef]
- Dahut, W.L.; Gulley, J.L.; Arlen, P.M.; Liu, Y.; Fedenko, K.M.; Steinberg, S.M.; Wright, J.J.; Parnes, H.; Chen, C.C.; Jones, E.; et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J. Clin. Oncol. 2004, 22, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Rassy, E.; Shah, S.; Ioannidou, E.; Sheriff, M.; Pavlidis, N. Aberrations of DNA repair pathways in prostate cancer: A cornerstone of precision oncology. Expert Opin. Ther. Targets 2021, 25, 329–333. [Google Scholar] [CrossRef]
- Melegh, Z.; Oltean, S. Targeting Angiogenesis in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 2676. [Google Scholar] [CrossRef]
- Escaff, S.; Fernández, J.M.; González, L.O.; Suárez, A.; González-Reyes, S.; González, J.M.; Vizoso, F.J. Study of matrix metalloproteinases and their inhibitors in prostate cancer. Br. J. Cancer 2010, 102, 922–929. [Google Scholar] [CrossRef]
- Botelho, F.; Pina, F.; Lunet, N. VEGF and prostatic cancer: A systematic review. Eur. J. Cancer Prev. 2010, 19, 385–392. [Google Scholar] [CrossRef]
- Wikström, P.; Damber, J.; Bergh, A. Role of transforming growth factor-beta1 in prostate cancer. Microsc. Res. Tech. 2001, 52, 411–419. [Google Scholar] [CrossRef]
- Hussain, T.; Gupta, S.; Mukhtar, H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003, 191, 125–135. [Google Scholar] [CrossRef]
- Chung, L.W.; Baseman, A.; Assikis, V.; Zhau, H.E. Molecular insights into prostate cancer progression: The missing link of tumor microenvironment. J. Urol. 2005, 173, 10–20. [Google Scholar] [CrossRef]
- Bono, A.V.; Celato, N.; Cova, V.; Salvadore, M.; Chinetti, S.; Novario, R. Microvessel density in prostate carcinoma. Prostate Cancer Prostatic Dis. 2002, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Roberts, E.; Cossigny, D.A.; Quan, G.M. The role of vascular endothelial growth factor in metastatic prostate cancer to the skeleton. Prostate Cancer 2013, 2013, 418340. [Google Scholar] [CrossRef] [PubMed]
- Delongchamps, N.B.; Peyromaure, M. The role of vascular endothelial growth factor in kidney and prostate cancer. Can. J. Urol. 2007, 14, 3669–3677. [Google Scholar]
- Li, R.; Younes, M.; Wheeler, T.M.; Scardino, P.; Ohori, M.; Frolov, A.; Ayala, G. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) in human prostate. Prostate 2004, 58, 193–199. [Google Scholar] [CrossRef]
- Duque, J.L.; Loughlin, K.R.; Adam, R.M.; Kantoff, P.W.; Zurakowski, D.; Freeman, M.R. Plasma levels of vascular endothelial growth factor are increased in patients with metastatic prostate cancer. Urology 1999, 54, 523–527. [Google Scholar] [CrossRef]
- Jennbacken, K.; Vallbo, C.; Wang, W.; Damber, J.E. Expression of vascular endothelial growth factor C (VEGF-C) and VEGF receptor-3 in human prostate cancer is associated with regional lymph node metastasis. Prostate 2005, 65, 110–116. [Google Scholar] [CrossRef] [PubMed]
- West, A.F.; O’Donnell, M.; Charlton, R.G.; Neal, D.E.; Leung, H.Y. Correlation of vascular endothelial growth factor expression with fibroblast growth factor-8 expression and clinico-pathologic parameters in human prostate cancer. Br. J. Cancer 2001, 85, 576–583. [Google Scholar] [CrossRef]
- Cao, S.; Durrani, F.A.; Toth, K.; Rustum, Y.M.; Seshadri, M. Bevacizumab enhances the therapeutic efficacy of Irinotecan against human head and neck squamous cell carcinoma xenografts. Oral Oncol. 2011, 47, 459–466. [Google Scholar] [CrossRef]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 14 July 2021).
- Ribatti, D.; Vacca, A. New Insights in Anti-Angiogenesis in Multiple Myeloma. Int. J. Mol. Sci. 2018, 19, 2031. [Google Scholar] [CrossRef]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Fujisato, T.; Sajiki, T.; Liu, Q.; Ikada, Y. Effect of basic fibroblast growth factor on cartilage regeneration in chondrocyte-seeded collagen sponge scaffold. Biomaterials 1996, 17, 155–162. [Google Scholar] [CrossRef]
- Wang, J.S. Basic fibroblast growth factor for stimulation of bone formation in osteoinductive or conductive implants. Acta Orthop. Scand. 1996, 269, 1–33. [Google Scholar] [CrossRef]
- Matsusaki, M.; Ochi, M.; Uchio, Y.; Shu, N.; Kurioka, H.; Kawasaki, K.; Adachi, N. Effects of Basic Fibroblast Growth Factor on Proliferation and Phenotype Expression of Chondrocytes Embedded in Collagen Gel. Gen. Pharmacol. 1998, 31, 759–764. [Google Scholar] [CrossRef]
- Harper, M.E.; Glynne-Jones, E.; Goddard, L.; Thurston, V.J.; Griffiths, K. Vascular endothelial growth factor (VEGF) expression in prostatic tumours and its relationship to neuroendocrine cells. Br. J. Cancer 1996, 74, 910–916. [Google Scholar] [CrossRef]
- Liang, W.C.; Wu, X.; Peale, F.V.; Lee, C.V.; Meng, Y.G.; Gutierrez, J.; Fu, L.; Malik, A.K.; Gerber, H.P.; Ferrara, N.; et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J. Biol. Chem. 2006, 281, 951–961. [Google Scholar] [CrossRef]
- Deep, G.; Jain, A.; Kumar, A.; Agarwal, C.; Kim, S.; Leevy, W.M.; Agarwal, R. Exosomes secreted by prostate cancer cells under hypoxia promote matrix metalloproteinases activity at pre-metastatic niches. Mol. Carcinog. 2020, 59, 323–332. [Google Scholar] [CrossRef]
- Katiyar, S.K. Matrix Metalloproteinases in Cancer Metastasis: Molecular Targets for Prostate Cancer Prevention by Green Tea Polyphenols and Grape Seed Proanthocyanidins. Endocr. Metab. Immune Disord. Drug Targets 2006, 6, 17–24. [Google Scholar] [CrossRef]
- Franko, A.; Berti, L.; Hennenlotter, J.; Rausch, S.; Scharpf, M.O.; Angelis, M.H.; Stenzl, A.; Peter, A.; Birkenfeld, A.L.; Lutz, S.Z.; et al. Increased Expressions of Matrix Metalloproteinases (MMPs) in Prostate Cancer Tissues of Men with Type 2 Diabetes. Biomedicines 2020, 8, 507. [Google Scholar] [CrossRef]
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol. 2019, 56, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Ahel, J.; Hudorović, N.; Vičić-Hudorović, V.; Nikles, H. TGF-Beta in the Natural History of Prostate Cancer. Acta Clin. Croat. 2019, 58, 128–138. [Google Scholar] [CrossRef]
- Barrett, C.S.; Millena, A.C.; Khan, S.A. TGF-β Effects on Prostate Cancer Cell Migration and Invasion Require FosB. Prostate 2017, 77, 72–81. [Google Scholar] [CrossRef]
- Fournier, P.G.; Juárez, P.; Jiang, G.; Clines, G.A.; Niewolna, M.; Kim, H.S.; Walton, H.W.; Peng, X.H.; Liu, Y.; Mohammad, K.S.; et al. The TGF-β Signaling Regulator PMEPA1 Suppresses Prostate Cancer Metastases to Bone. Cancer Cell 2015, 27, 809–821. [Google Scholar] [CrossRef]
- Kloss, C.C.; Lee, J.; Zhang, A.; Chen, F.; Melenhorst, J.J.; Lacey, S.F.; Maus, M.V.; Fraietta, J.A.; Zhao, Y.; June, C.H. Dominant-Negative TGF-β Receptor Enhances PSMA-Targeted Human CAR T Cell Proliferation and Augments Prostate Cancer Eradication. Mol. Ther. 2018, 26, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.Q.; He, Y.H.; Wang, S.B.; Yang, S.; Wang, Y.J.; Nan, C.J.; Bao, Y.F.; Xie, Q.P.; Chen, Y.H. MiR-130b/TNF-α/NF-κB/VEGFA loop inhibits prostate cancer angiogenesis. Clin. Transl. Oncol. 2020, 22, 111–121. [Google Scholar] [CrossRef]
- Teixeira, A.F.; Ten Dijke, P.; Zhu, H.J. On-Target Anti-TGF-β Therapies Are Not Succeeding in Clinical Cancer Treatments: What Are Remaining Challenges? Front. Cell Dev. Biol. 2020, 8, 605. [Google Scholar] [CrossRef]
- Semenza, G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010, 29, 625–634. [Google Scholar] [CrossRef]
- Janssen, H.L.; Haustermans, K.M.; Sprong, D.; Blommestijn, G.; Hofland, I.; Hoebers, F.J.; Blijweert, E.; Raleigh, J.A.; Semenza, G.L.; Varia, M.A.; et al. HIF-1A, pimonidazole, and iododeoxyuridine to estimate hypoxia and perfusion in human head-and-neck tumors. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1537–1549. [Google Scholar] [CrossRef]
- Boddy, J.L.; Fox, S.B.; Han, C.; Campo, L.; Turley, H.; Kanga, S.; Malone, P.R.; Harris, A.L. The Androgen Receptor Is Significantly Associated with Vascular Endothelial Growth Factor and Hypoxia Sensing via Hypoxia-Inducible Factors HIF-1a, HIF-2a, and the Prolyl Hydroxylases in Human Prostate Cancer. Clin. Cancer Res. 2005, 11, 7658–7663. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Zhang, Z.; Bai, Z.; Jin, H.; Guo, X.; Huang, X.; Li, M.; Wang, M.; Shu, X.S.; et al. CDCA2 Inhibits Apoptosis and Promotes Cell Proliferation in Prostate Cancer and Is Directly Regulated by HIF-1α Pathway. Front. Oncol. 2020, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Pavlakis, D.; Kampantais, S.; Gkagkalidis, K.; Gourvas, V.; Memmos, D.; Tsionga, A.; Dimitriadis, G.; Vakalopoulos, I. Hypoxia-Inducible Factor 2a Expression Is Positively Correlated with Gleason Score in Prostate Cancer. Technol. Cancer Res. Treat. 2021, 20, 1533033821990010. [Google Scholar] [CrossRef]
- Zha, S.; Yegnasubramanian, V.; Nelson, W.G.; Isaacs, W.B.; De Marzo, A.M. Cyclooxygenases in cancer: Progress and perspective. Cancer Lett. 2004, 215, 1–20. [Google Scholar] [CrossRef]
- Zheng, Y.; Comaills, V.; Burr, R.; Boulay, G.; Miyamoto, D.T.; Wittner, B.S.; Emmons, E.; Sil, S.; Koulopoulos, M.W.; Broderick, K.T.; et al. COX-2 mediates tumor-stromal prolactin signaling to initiate tumorigenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 5223–5232. [Google Scholar] [CrossRef] [PubMed]
- Doat, S.; Cénée, S.; Trétarre, B.; Rebillard, X.; Lamy, P.J.; Bringer, J.P.; Iborra, F.; Murez, T.; Sanchez, M.; Menegaux, F. Nonsteroidal anti-inflammatory drugs (NSAIDs) and prostate cancer risk: Results from the EPICAP study. Cancer Med. 2017, 6, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Blando, J.M.; Perez, C.J.; Lal, P.; Feldman, M.D.; Smyth, E.M.; Ricciotti, E.; Grosser, T.; Benavides, F.; Kazanietz, M.G. COX-2 mediates pro-tumorigenic effects of PKCε in prostate cancer. Oncogene 2018, 37, 4735–4749. [Google Scholar] [CrossRef]
- Tian, J.; Guo, F.; Chen, Y.; Li, Y.; Yu, B.; Li, Y. Nanoliposomal formulation encapsulating celecoxib and genistein inhibiting COX-2 pathway and Glut-1 receptors to prevent prostate cancer cell proliferation. Cancer Lett. 2019, 448, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; Parajuli, K.R.; Zhang, W.; Zhang, K.; Mo, Z.; Liu, J.; Chen, Z.; Yang, S.; Wang, A.R.; et al. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene 2017, 36, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ho, C.H.; Hu, S.W.; Tzou, K.Y.; Wang, Y.H.; Wu, C.C. Association between interleukin-8 rs4073 polymorphism and prostate cancer: A meta-analysis. J. Formos. Med. Assoc. 2020, 119, 1201–1210. [Google Scholar] [CrossRef]
- Culig, Z.; Puhr, M. Interleukin-6 and prostate cancer: Current developments and unsolved questions. Mol. Cell. Endocrinol. 2018, 462, 25–30. [Google Scholar] [CrossRef]
- Nguyen, D.P.; Li, J.; Tewari, A.K. Inflammation and prostate cancer: The role of interleukin 6 (IL-6): Role of IL-6 in prostate cancer. BJU Int. 2014, 113, 986–992. [Google Scholar] [CrossRef]
- Sakellariou, C.; Elhage, O.; Papaevangelou, E.; Giustarini, G.; Esteves, A.M.; Smolarek, D.; Smith, R.A.; Dasgupta, P.; Galustian, C. Prostate cancer cells enhance interleukin-15-mediated expansion of NK cells. BJU Int. 2020, 125, 89–102. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Boussios, S.; Ozturk, M.A.; Moschetta, M.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Katsanos, K.H.; Christodoulou, D.K.; Pavlidis, N. The Developing Story of Predictive Biomarkers in Colorectal Cancer. J. Pers. Med. 2019, 9, 12. [Google Scholar] [CrossRef]
- Zarkavelis, G.; Boussios, S.; Papadaki, A.; Katsanos, K.H.; Christodoulou, D.K.; Pentheroudakis, G. Current and future biomarkers in colorectal cancer. Ann. Gastroenterol. 2017, 30, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Mikropoulos, C.; Samartzis, E.; Karihtala, P.; Moschetta, M.; Sheriff, M.; Karathanasi, A.; Sadauskaite, A.; Rassy, E.; Pavlidis, N. Wise Management of Ovarian Cancer: On the Cutting Edge. J. Pers. Med. 2020, 10, 41. [Google Scholar] [CrossRef]
- Boussios, S.; Moschetta, M.; Karathanasi, A.; Tsiouris, A.K.; Kanellos, F.S.; Tatsi, K.; Katsanos, K.H.; Christodoulou, D.K. Malignant peritoneal mesothelioma: Clinical aspects, and therapeutic perspectives. Ann. Gastroenterol. 2018, 31, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Revythis, A.; Shah, S.; Kutka, M.; Moschetta, M.; Ozturk, M.A.; Pappas-Gogos, G.; Ioannidou, E.; Sheriff, M.; Rassy, E.; Boussios, S. Unraveling the Wide Spectrum of Melanoma Biomarkers. Diagnostics 2021, 11, 1341. [Google Scholar] [CrossRef]
- Fabris, L.; Ceder, Y.; Chinnaiyan, A.M.; Jenster, G.W.; Sorensen, K.D.; Tomlins, S.; Visakorpi, T.; Calin, G.A. The Potential of MicroRNAs as Prostate Cancer Biomarkers. Eur. Urol. 2016, 70, 312–322. [Google Scholar] [CrossRef]
- Saxby, H.; Mikropoulos, C.; Boussios, S. An Update on the Prognostic and Predictive Serum Biomarkers in Metastatic Prostate Cancer. Diagnostics 2020, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Balacescu, O.; Dumitrescu, R.G.; Marian, C. MicroRNAs Role in Prostate Cancer. Methods Mol. Biol. 2018, 1856, 103–117. [Google Scholar]
- Bryzgunova, O.E.; Konoshenko, M.Y.; Laktionov, P.P. MicroRNA-guided gene expression in prostate cancer: Literature and database overview. J. Gene Med. 2018, 20, e3016. [Google Scholar] [CrossRef]
- Kanwal, R.; Plaga, A.R.; Liu, X.; Shukla, G.C.; Gupta, S. MicroRNAs in prostate cancer: Functional role as biomarkers. Cancer Lett. 2017, 407, 9–20. [Google Scholar] [CrossRef]
- Kasomva, K.; Sen, A.; Paulraj, M.G.; Sailo, S.; Raphael, V.; Puro, K.U.; Assumi, S.R.; Ignacimuthu, S. Roles of microRNA in prostate cancer cell metabolism. Int. J. Biochem. Cell Biol. 2018, 102, 109–116. [Google Scholar] [CrossRef]
- Khanmi, K.; Ignacimuthu, S.; Paulraj, M.G. MicroRNA in prostate cancer. Clin. Chim. Acta 2015, 451, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Baruah, M.M. The microRNA signatures: Aberrantly expressed miRNAs in prostate cancer. Clin. Transl. Oncol. 2019, 21, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Cassinello, J.; Carballido Rodríguez, J.; Antón Aparicio, L. Role of taxanes in advanced prostate cancer. Clin. Transl. Oncol. 2016, 18, 972–980. [Google Scholar] [CrossRef]
- Thienger, P.; Rubin, M.A. Prostate cancer hijacks the microenvironment. Nat. Cell Biol. 2021, 23, 3–5. [Google Scholar] [CrossRef]
- Luz Flores, M.; Sáez, C. Protocols for the Study of Taxanes Chemosensitivity in Prostate Cancer. Methods Mol. Biol. 2018, 1786, 153–173. [Google Scholar]
- Petrylak, D.P. Docetaxel (Taxotere) in hormone-refractory prostate cancer. Semin. Oncol. 2000, 27, 24–29. [Google Scholar]
- Stein, C.A. Mechanisms of action of taxanes in prostate cancer. Semin. Oncol. 1999, 26, 3–7. [Google Scholar]
- Small, E.J.; Srinivas, S.; Egan, B.; McMillan, A.; Rearden, T.P. Doxorubicin and dose-escalated cyclophosphamide with granulocyte colony-stimulating factor for the treatment of hormone-resistant prostate cancer. J. Clin. Oncol. 1996, 14, 1617–1625. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 Signaling and its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Reese, D.M.; Fratesi, P.; Corry, M.; Novotny, W.; Holmgren, E.; Small, E.J. A Phase II Trial of Humanized Anti-Vascular Endothelial Growth Factor Antibody for the Treatment of Androgen-Independent Prostate Cancer. Prostate J. 2001, 3, 65–70. [Google Scholar] [CrossRef]
- Iacobelli, S. Hormone-refractory prostate cancer responding to bevacizumab. Int. J. Urol. 2008, 15, 754. [Google Scholar] [CrossRef] [PubMed]
- Picus, J.; Halabi, S.; Kelly, W.K.; Vogelzang, N.J.; Whang, Y.E.; Kaplan, E.B.; Stadler, W.M.; Small, E.J.; Cancer and Leukemia Group B. A phase 2 study of estramustine, docetaxel, and bevacizumab in men with castrate-resistant prostate cancer: Results from Cancer and Leukemia Group B Study 90006. Cancer 2011, 117, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Figg, W.D.; Fossa, S.D.; Mirone, V.; Autorino, R.; Longo, N.; Imbimbo, C.; Perdonà, S.; Giordano, A.; Giuliano, M.; et al. Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: A phase 2 study. Eur. Urol. 2008, 54, 1089–1094. [Google Scholar] [CrossRef]
- Kelly, W.K.; Halabi, S.; Carducci, M.; George, D.; Mahoney, J.F.; Stadler, W.M.; Morris, M.; Kantoff, P.; Monk, J.P.; Kaplan, E.; et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J. Clin. Oncol. 2012, 30, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 2002, 99, 11393–11398. [Google Scholar] [CrossRef]
- Tannock, I.F.; Fizazi, K.; Ivanov, S.; Karlsson, C.T.; Fléchon, A.; Skoneczna, I.; Orlandi, F.; Gravis, G.; Matveev, V.; Bavbek, S.; et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): A phase 3, double-blind randomised trial. Lancet Oncol. 2013, 14, 760–768. [Google Scholar] [CrossRef]
- Chi, K.N.; Ellard, S.L.; Hotte, S.J.; Czaykowski, P.; Moore, M.; Ruether, J.D.; Schell, A.J.; Taylor, S.; Hansen, C.; Gauthier, I.; et al. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Ann. Oncol. 2008, 19, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Dahut, W.L.; Scripture, C.; Posadas, E.; Jain, L.; Gulley, J.L.; Arlen, P.M.; Wright, J.J.; Yu, Y.; Cao, L.; Steinberg, S.M.; et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin. Cancer Res. 2008, 14, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Steinbild, S.; Mross, K.; Frost, A.; Morant, R.; Gillessen, S.; Dittrich, C.; Strumberg, D.; Hochhaus, A.; Hanauske, A.R.; Edler, L.; et al. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: A study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Br. J. Cancer 2007, 97, 1480–1485. [Google Scholar] [CrossRef]
- Rini, B.I.; Garcia, J.A.; Cooney, M.M.; Elson, P.; Tyler, A.; Beatty, K.; Bokar, J.; Mekhail, T.; Bukowski, R.M.; Budd, G.T.; et al. A phase I study of sunitinib plus bevacizumab in advanced solid tumors. Clin. Cancer Res. 2009, 15, 6277–6283. [Google Scholar] [CrossRef]
- Dror Michaelson, M.; Regan, M.M.; Oh, W.K.; Kaufman, D.S.; Olivier, K.; Michaelson, S.Z.; Spicer, B.; Gurski, C.; Kantoff, P.W.; Smith, M.R. Phase II study of sunitinib in men with advanced prostate cancer. Ann. Oncol. 2009, 20, 913–920. [Google Scholar] [CrossRef]
- Payton, S. Targeting prostate tumour angiogenesis with cediranib. Nat. Rev. Urol. 2013, 10, 187. [Google Scholar] [CrossRef]
- Karakunnel, J.J.; Gulley, J.L.; Arlen, P.M.; Mulquin, M.; Wright, J.J.; Turkbey, I.B.; Choyke, P.; Ahlers, C.M.; Figg, W.D.; Dahut, W.L. Phase II trial of cediranib (AZD2171) in docetaxel-resistant, castrate-resistant prostate cancer (CRPC). J. Clin. Oncol. 2008, 26, 283s. [Google Scholar] [CrossRef]
- Guérin, O.; Etienne-Grimaldi, M.C.; Monteverde, M.; Sudaka, A.; Brunstein, M.C.; Formento, P.; Lattanzio, L.; Maffi, M.; Tonissi, F.; Ortholan, C.; et al. Contrasted effects of the multitarget TKi vandetanib on docetaxel-sensitive and docetaxel-resistant prostate cancer cell lines. Urol. Oncol. 2013, 31, 1567–1575. [Google Scholar] [CrossRef]
- Horti, J.; Widmark, A.; Stenzl, A.; Federico, M.H.; Abratt, R.P.; Sanders, N.; Pover, G.M.; Bodrogi, I. A Randomized, Double-Blind, Placebo-Controlled Phase II Study of Vandetanib Plus Docetaxel/Prednisolone in Patients with Hormone-Refractory Prostate Cancer. Cancer Biother. Radiopharm. 2009, 24, 175–180. [Google Scholar] [CrossRef]
- Azad, A.A.; Beardsley, E.K.; Hotte, S.J.; Ellard, S.L.; Klotz, L.; Chin, J.; Kollmannsberger, C.; Mukherjee, S.D.; Chi, K.N. A randomized phase II efficacy and safety study of vandetanib (ZD6474) in combination with bicalutamide versus bicalutamide alone in patients with chemotherapy naïve castration-resistant prostate cancer. Investig. New Drugs 2014, 32, 746–752. [Google Scholar] [CrossRef]
- Cochin, V.; Gross-Goupil, M.; Ravaud, A.; Godbert, Y.; Le Moulec, S. Cabozantinib: Mechanism of action, efficacy and indications. Bull. Cancer 2017, 104, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Hessel, C.; Halabi, S.; Sanford, B.; Michaelson, M.D.; Hahn, O.; Walsh, M.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur. J. Cancer 2018, 94, 115–125. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Tannir, N.M.; Mainwaring, P.N.; Rini, B.I.; Hammers, H.J.; Donskov, F.; Roth, B.J.; Peltola, K.; et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 917–927. [Google Scholar] [CrossRef]
- Brose, M.S.; Robinson, B.; Sherman, S.I.; Krajewska, J.; Lin, C.C.; Vaisman, F.; Hoff, A.O.; Hitre, E.; Bowles, D.W.; Hernando, J.; et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1126–1138. [Google Scholar] [CrossRef]
- Chott, A.; Sun, Z.; Morganstern, D.; Pan, J.; Li, T.; Susani, M.; Mosberger, I.; Upton, M.P.; Bubley, G.J.; Balk, S.P. Tyrosine kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am. J. Pathol. 1999, 155, 1271–1279. [Google Scholar] [CrossRef]
- Patnaik, A.; Swanson, K.D.; Csizmadia, E.; Solanki, A.; Landon-Brace, N.; Gehring, M.P.; Helenius, K.; Olson, B.M.; Pyzer, A.R.; Wang, L.C.; et al. Cabozantinib Eradicates Advanced Murine Prostate Cancer by Activating Antitumor Innate Immunity. Cancer Discov. 2017, 7, 750–765. [Google Scholar] [CrossRef]
- Sidaway, P. Cabozantinib activates innate immunity. Nat. Rev. Urol. 2017, 14, 327. [Google Scholar] [CrossRef]
- Basch, E.M.; Scholz, M.; de Bono, J.S.; Vogelzang, N.; de Souza, P.; Marx, G.; Vaishampayan, U.; George, S.; Schwarz, J.K.; Antonarakis, E.S.; et al. Cabozantinib Versus Mitoxantrone-prednisone in Symptomatic Metastatic Castration-resistant Prostate Cancer: A Randomized Phase 3 Trial with a Primary Pain Endpoint. Eur. Urol. 2019, 75, 929–937. [Google Scholar] [CrossRef]
- Agarwal, N.; Loriot, Y.; McGregor, B.A.; Dreicer, R.; Dorff, T.B.; Maughan, B.L.; Kelly, W.K.; Pagliaro, L.C.; Srinivas, S.; Squillante, C.M.; et al. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: Results of cohort 6 of the COSMIC-021 study. J. Clin. Oncol. 2020, 38, 5564. [Google Scholar] [CrossRef]
- Lin, A.M.; Rini, B.I.; Weinberg, V.; Fong, K.; Ryan, C.J.; Rosenberg, J.E.; Fong, L.; Small, E.J. A phase II trial of imatinib mesylate in patients with biochemical relapse of prostate cancer after definitive local therapy. BJU Int. 2006, 98, 763–769. [Google Scholar] [CrossRef]
- Rao, K.; Goodin, S.; Levitt, M.J.; Dave, N.; Shih, W.J.; Lin, Y.; Capanna, T.; Doyle-Lindrud, S.; Juvidian, P.; DiPaola, R.S. A phase II trial of imatinib mesylate in patients with prostate specific antigen progression after local therapy for prostate cancer. Prostate 2005, 62, 115–122. [Google Scholar] [CrossRef]
- Bajaj, G.K.; Zhang, Z.; Garrett-Mayer, E.; Drew, R.; Sinibaldi, V.; Pili, R.; Denmeade, S.R.; Carducci, M.A.; Eisenberger, M.A.; DeWeese, T.L. Phase II study of imatinib mesylate in patients with prostate cancer with evidence of biochemical relapse after definitive radical retropubic prostatectomy or radiotherapy. Urology 2007, 69, 526–531. [Google Scholar] [CrossRef]
- Hail, N., Jr.; Chen, P.; Bushman, L.R. Teriflunomide (Leflunomide) Promotes Cytostatic, Antioxidant, and Apoptotic Effects in Transformed Prostate Epithelial Cells: Evidence Supporting a Role for Teriflunomide in Prostate Cancer Chemoprevention. Neoplasia 2010, 12, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Figg, W.D.; Li, H.; Sissung, T.; Retter, A.; Wu, S.; Gulley, J.L.; Arlen, P.; Wright, J.J.; Parnes, H.; Fedenko, K.; et al. Pre-clinical and clinical evaluation of estramustine, docetaxel and thalidomide combination in androgen-independent prostate cancer. BJU Int. 2007, 99, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Pytel, D.; Sliwinski, T.; Poplawski, T.; Ferriola, D.; Majsterek, I. Tyrosine Kinase Blockers: New Hope for Successful Cancer Therapy. Anticancer Agents. Med. Chem. 2009, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.J.; Loughnan, M.S.; Flynn, E.; Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 4082–4085. [Google Scholar] [CrossRef]
- Dixon, S.C.; Kruger, E.A.; Bauer, K.S.; Figg, W.D. Thalidomide up-regulates prostate-specific antigen secretion from LNCaP cells. Cancer Chemother. Pharmacol. 1999, 43, S78–S84. [Google Scholar] [CrossRef]
- Figg, W.D.; Dahut, W.; Duray, P.; Hamilton, M.; Tompkins, A.; Steinberg, S.M.; Jones, E.; Premkumar, A.; Linehan, W.M.; Floeter, M.K.; et al. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin. Cancer Res. 2001, 7, 1888–1893. [Google Scholar]
- Figg, W.D.; Arlen, P.; Gulley, J.; Fernandez, P.; Noone, M.; Fedenko, K.; Hamilton, M.; Parker, C.; Kruger, E.A.; Pluda, J.; et al. A randomized phase II trial of docetaxel (taxotere) plus thalidomide in androgen-independent prostate cancer. Semin. Oncol. 2001, 28, 62–66. [Google Scholar] [CrossRef]
- Ning, Y.M.; Gulley, J.L.; Arlen, P.M.; Woo, S.; Steinberg, S.M.; Wright, J.J.; Parnes, H.L.; Trepel, J.B.; Lee, M.J.; Kim, Y.S.; et al. Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2010, 28, 2070–2076. [Google Scholar] [CrossRef]
- Xing, D.L.; Song, D.K.; Zhang, L.R. Lenalidomide in Treating Patients with Castration-Resistant Prostate Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 3969–3972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sinibaldi, V.J.; Carducci, M.A.; Moore-Cooper, S.; George, B.; Denmeade, S.; Drake, C.G.; Walczak, J.; Pili, R.; Zahurak, M.L.; Eisenberger, M.A. A randomized double blind phase I-II study to determine the tolerability/efficacy of two different doses of lenalidomide (L), CC- 5013, in biochemically relapsed (BR) prostate cancer (PC) patients (pts) (M0) after local treatment (LT). J. Clin. Oncol. 2009, 27, 5130. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Vogelzang, N.J.; Budnik, N.; Wiechno, P.J.; Sternberg, C.N.; Doner, K.; Bellmunt, J.; Burke, J.M.; de Olza, M.O.; Choudhury, A.; et al. Docetaxel and prednisone with or without lenalidomide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (MAINSAIL): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2015, 16, 417–425. [Google Scholar] [CrossRef]
- Dalrymple, S.L.; Becker, R.E.; Isaacs, J.T. The quinoline-3-carboxamide anti-angiogenic agent, tasquinimod, enhances the anti-prostate cancer efficacy of androgen ablation and taxotere without effecting serum PSA directly in human xenografts. Prostate 2007, 67, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Bratt, O.; Häggman, M.; Ahlgren, G.; Nordle, O.; Björk, A.; Damber, J.E. Open-label, clinical phase I studies of tasquinimod in patients with castration-resistant prostate cancer. Br. J. Cancer 2009, 101, 1233–1240. [Google Scholar] [CrossRef]
- Pili, R.; Häggman, M.; Stadler, W.M.; Gingrich, J.R.; Assikis, V.J.; Björk, A.; Nordle, O.; Forsberg, G.; Carducci, M.A.; Armstrong, A.J. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J. Clin. Oncol. 2011, 29, 4022–4028. [Google Scholar] [CrossRef]
- Pantziarka, P.; Sukhatme, V.; Bouche, G.; Meheus, L.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)-itraconazole as an anti-cancer agent. Ecancermedicalscience 2015, 9, 521. [Google Scholar] [CrossRef]
- Lee, M.; Hong, H.; Kim, W.; Zhang, L.; Friedlander, T.W.; Fong, L.; Lin, A.M.; Small, E.J.; Wei, X.X.; Rodvelt, T.J.; et al. Itraconazole as a Noncastrating Treatment for Biochemically Recurrent Prostate Cancer: A Phase 2 Study. Clin. Genitourin. Cancer 2019, 17, e92–e96. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Heath, E.I.; Smith, D.C.; Rathkopf, D.; Blackford, A.L.; Danila, D.C.; King, S.; Frost, A.; Ajiboye, A.S.; Zhao, M.; et al. Repurposing Itraconazole as a Treatment for Advanced Prostate Cancer: A Noncomparative Randomized Phase II Trial in Men With Metastatic Castration-Resistant Prostate Cancer. Oncologist 2013, 18, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bilusic, M.; Wong, Y.N. Anti-angiogenesis in prostate cancer: Knocked down but not out. Asian J. Androl. 2014, 16, 372–377. [Google Scholar] [PubMed]
- Leary, S.E.S.; Park, J.R.; Reid, J.M.; Ralya, A.T.; Baruchel, S.; Wu, B.; Roberts, T.P.L.; Liu, X.; Minard, C.G.; Fox, E.; et al. Pediatric Phase I Trial and Pharmacokinetic Study of Trebananib in Relapsed Solid Tumors, Including Primary Tumors of the Central Nervous System ADVL1115: A Children’s Oncology Group Phase I Consortium Report. Clin. Cancer. Res. 2017, 23, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zang, L.; Zhang, Y.; Hong, J.; Yao, Y.; Zou, C.; Zhang, L.; Chen, Y. Early changes in apparent diffusion coefficients predict radiosensitivity of human nasopharyngeal carcinoma xenografts. Laryngoscope 2012, 122, 839–843. [Google Scholar] [CrossRef]
- Gule, M.K.; Chen, Y.; Sano, D.; Frederick, M.J.; Zhou, G.; Zhao, M.; Milas, Z.L.; Galer, C.E.; Henderson, Y.C.; Jasser, S.A.; et al. Targeted therapy of VEGFR2 and EGFR significantly inhibits growth of anaplastic thyroid cancer in an orthotopic murine model. Clin. Cancer Res. 2011, 17, 2281–2291. [Google Scholar] [CrossRef]
- Isayeva, T.; Chanda, D.; Kallman, L.; Eltoum, I.E.; Ponnazhagan, S. Effects of sustained antiangiogenic therapy in multistage prostate cancer in TRAMP model. Cancer Res. 2007, 67, 5789–5797. [Google Scholar] [CrossRef][Green Version]
| Pathway | Factor | Clinical Impact | References |
|---|---|---|---|
| Vascular Endothelial Growth factors (VEGFs) | VEGFR 1, -2 and -3 | Stimulators | [36,37,38,39,40,41,42,43,44,45] |
| Fibroblast growth factors (FGFs) | FGF-1 and -2, bFGF | [46,47,48,49,50,51] | |
| Matrix metalloproteinases (MMPs) | MMP-2, -7 and -9 | [52,53,54,55] | |
| Transforming growth factor β (TGFβ) | TGFβ1 | [56,57,58,59,60,61,62] | |
| Hypoxia-inducible factors (HIF) | HIF-1a | [63,64,65,66,67] | |
| Cyclooxygenases (COXs) | COX2 | [68,69,70,71,72] | |
| Interleukins (ILs) | IL8 | Stimulator | [74,76,77] |
| IL10 and IL27 | Inhibitors | [73,76,78] | |
| microRNAs (miRNAs) | miR-296, miR-30d, miR-323, miR-21, miR-182 and miR-130b | Stimulators | [88] |
| miR-195, miR-218 and miR-146a | Inhibitors | [87] |
| Agent | Mechanism of Action | Phase | Primary Endpoint | Identifier |
|---|---|---|---|---|
| Bevacizumab | Recombinant humanized monoclonal antibody that blocks VEGF-A | II | ORR | NCT01083368 |
| II | PSA rFS | NCT00776594 | ||
| Aflibercept | Binds to circulating VEGF-A | III | OS | NCT00519285 |
| Sunitinib | Receptor tyrosine kinase inhibitor | II | ≥30% PSA decline | NCT00879619 |
| II | PFS | NCT00734851 | ||
| Sorafenib | II | Overall response rate | NCT00414388 | |
| II | ≥50% PSA decline | NCT00589420 | ||
| Cediranib | Inhibitor of VEGFR-1, -2, and -3 | II | PFS | NCT00527124 |
| II | PFS | NCT01260688 | ||
| Cabozantinib | Inhibits VEGFRs, MET, and RET | II | PFS | NCT01428219 |
| Thalidomide | Inhibition of VEGF, PI3K/Akt/NF-kappaB, and mTOR pathways | II | ORR | NCT00307294 |
| Lenalidomide | Inhibition of VEGF-induced PI3K/Akt pathway signalling | II | OS | NCT00942578 |
| Tasquinimod | S100A9 protein that inhibits VEGF | III | PFS | NCT01234311 |
| Itraconazole | Inhibition of the Hedgehog pathway | II | ≥30% PSA decline | NCT01450683 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidou, E.; Moschetta, M.; Shah, S.; Parker, J.S.; Ozturk, M.A.; Pappas-Gogos, G.; Sheriff, M.; Rassy, E.; Boussios, S. Angiogenesis and Anti-Angiogenic Treatment in Prostate Cancer: Mechanisms of Action and Molecular Targets. Int. J. Mol. Sci. 2021, 22, 9926. https://doi.org/10.3390/ijms22189926
Ioannidou E, Moschetta M, Shah S, Parker JS, Ozturk MA, Pappas-Gogos G, Sheriff M, Rassy E, Boussios S. Angiogenesis and Anti-Angiogenic Treatment in Prostate Cancer: Mechanisms of Action and Molecular Targets. International Journal of Molecular Sciences. 2021; 22(18):9926. https://doi.org/10.3390/ijms22189926
Chicago/Turabian StyleIoannidou, Evangelia, Michele Moschetta, Sidrah Shah, Jack Steven Parker, Mehmet Akif Ozturk, George Pappas-Gogos, Matin Sheriff, Elie Rassy, and Stergios Boussios. 2021. "Angiogenesis and Anti-Angiogenic Treatment in Prostate Cancer: Mechanisms of Action and Molecular Targets" International Journal of Molecular Sciences 22, no. 18: 9926. https://doi.org/10.3390/ijms22189926
APA StyleIoannidou, E., Moschetta, M., Shah, S., Parker, J. S., Ozturk, M. A., Pappas-Gogos, G., Sheriff, M., Rassy, E., & Boussios, S. (2021). Angiogenesis and Anti-Angiogenic Treatment in Prostate Cancer: Mechanisms of Action and Molecular Targets. International Journal of Molecular Sciences, 22(18), 9926. https://doi.org/10.3390/ijms22189926









