Growth, Proliferation and Metastasis of Prostate Cancer Cells Is Blocked by Low-Dose Curcumin in Combination with Light Irradiation
Abstract
:1. Introduction
2. Results
2.1. Tumor Cell Growth
2.2. Clonogenic Growth
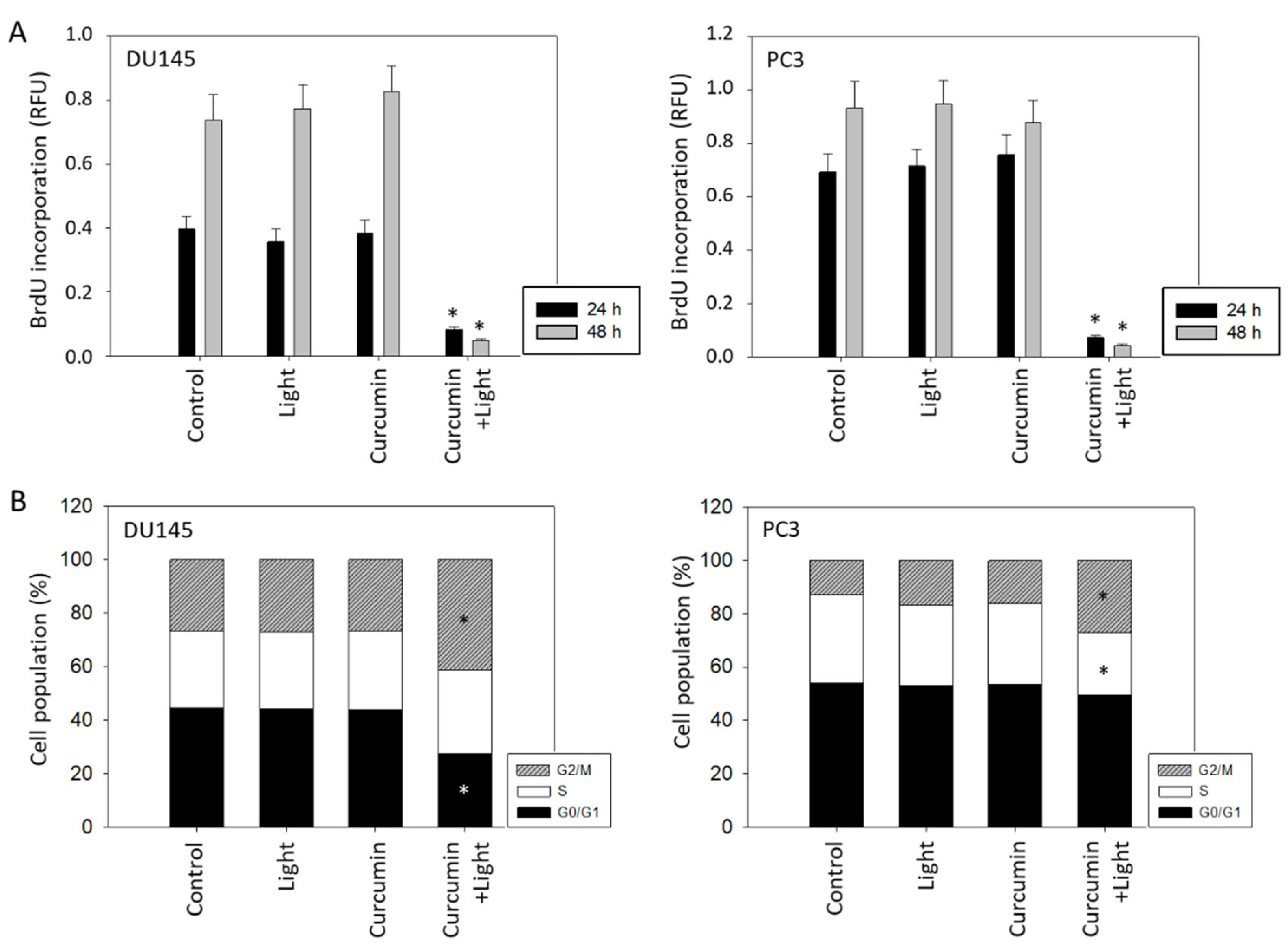
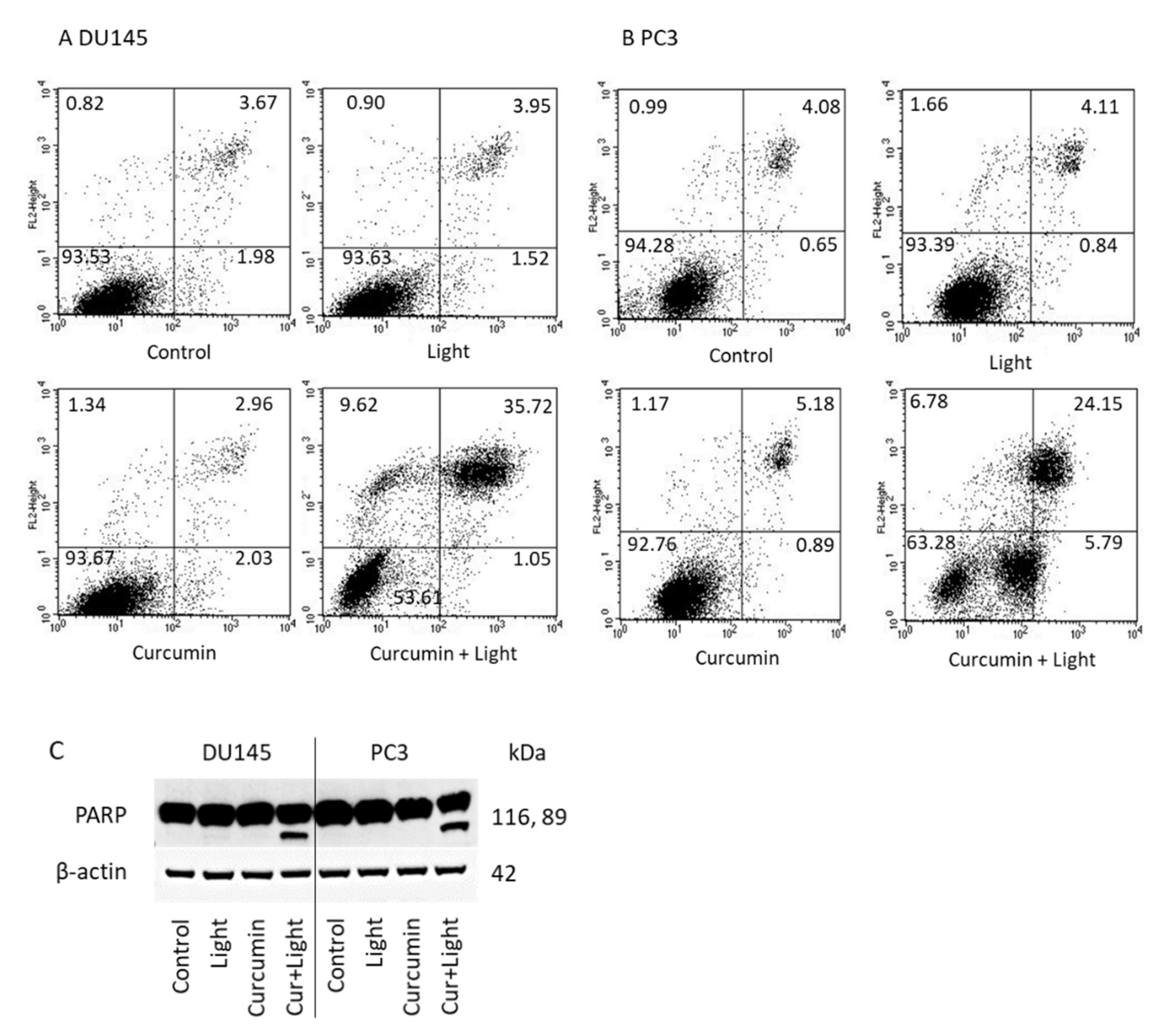
2.3. Tumor Cell Proliferation and Cell Cycling
2.4. Apoptosis
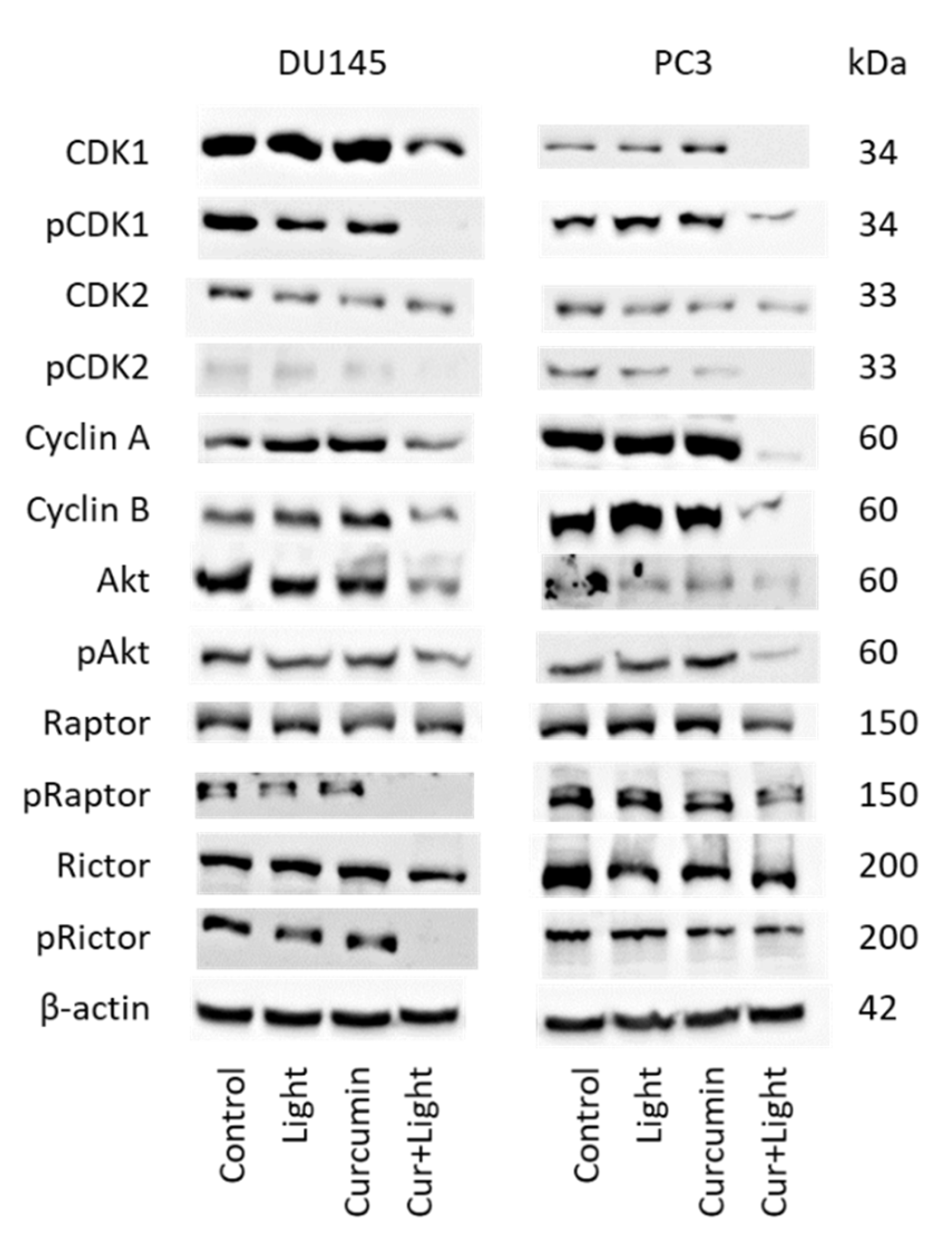
2.5. Cell Cycle Regulating Protein Expression
2.6. Adhesion and Migration Behavior
2.7. Integrin Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Drug Treatment and Light Application
4.3. Measurement of Tumor Cell Growth and Proliferation
4.4. Apoptosis
4.5. Clonogenic Growth
4.6. Cell Cycle Analysis
4.7. Western Blot Analysis
4.8. Adhesion to Immobilized Collagen
4.9. Adhesion to Endothelial Cells
4.10. Chemotaxis
4.11. Integrin Expression
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Prostate Cancer Fact Sheet. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf (accessed on 4 April 2021).
- Horneber, M.; Bueschel, G.; Dennert, G.; Less, D.; Ritter, E.; Zwahlen, M. How many cancer patients use complementary and alternative medicine: A systematic review and metaanalysis. Integr. Cancer Ther. 2012, 11, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Pawlik, J.; Czekawy, I.; Kozłowski, M.; Cymbaluk-Płoska, A. Complementary Methods in Cancer Treatment-Cure or Curse? Int. J. Environ. Res. Public Health 2021, 18, 356. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Mandal, D.; Sen, G.S.; Pal, S.; Banerjee, S.; Lahiry, L.; Finke, J.H.; Tannenbaum, C.S.; Das, T.; Sa, G. Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: Protection by curcumin. Cancer Res. 2007, 67, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Choudhuri, T.; Pal, S.; Das, T.; Sa, G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J. Biol. Chem. 2005, 280, 20059–20068. [Google Scholar] [CrossRef] [Green Version]
- Campbell, F.C.; Collett, G.P. Chemopreventive properties of curcumin. Future Oncol. 2005, 1, 405–414. [Google Scholar] [CrossRef]
- Sandur, S.K.; Deorukhkar, A.; Pandey, M.K.; Pabón, A.M.; Shentu, S.; Guha, S.; Aggarwal, B.B.; Krishnan, S. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Mehta, H.J.; Patel, V.; Sadikot, R.T. Curcumin and lung cancer—A review. Target. Oncol. 2014, 9, 295–310. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [Green Version]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Askari, G.; Alikiaii, B.; Abbasi, S.; Soleimani, D.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Curcumin for the Treatment of Prostate Diseases: A Systematic Review of Controlled Clinical Trials. Adv. Exp. Med. Biol. 2021, 1291, 345–362. [Google Scholar]
- Passildas-Jahanmohan, J.; Eymard, J.-C.; Pouget, M.; Kwiatkowski, F.; van Praagh, I.; Savareux, L.; Atger, M.; Durando, X.; Abrial, C.; Richard, D.; et al. Multicenter randomized phase II study comparing docetaxel plus curcumin versus docetaxel plus placebo in first-line treatment of metastatic castration-resistant prostate cancer. Cancer Med. 2021, 10, 2332–2340. [Google Scholar] [CrossRef] [PubMed]
- Bernd, A. Visible light and/or UVA offer a strong amplification of the anti-tumor effect of curcumin. Phytochem. Rev. 2014, 13, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Dujic, J.; Kippenberger, S.; Ramirez-Bosca, A.; Diaz-Alperi, J.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A.; Hofmann, M. Curcumin in combination with visible light inhibits tumor growth in a xenograft tumor model. Int. J. Cancer 2009, 124, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Deng, Q.; Liu, Y.; Zhao, P. Curcumin Inhibits Gastric Carcinoma Cell Growth and Induces Apoptosis by Suppressing the Wnt/β-Catenin Signaling Pathway. Med. Sci. Monit. 2017, 23, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghtaderi, H.; Sepehri, H.; Delphi, L.; Attari, F. Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231. BioImpacts 2018, 8, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namkaew, J.; Jaroonwitchawan, T.; Rujanapun, N.; Saelee, J.; Noisa, P. Combined effects of curcumin and doxorubicin on cell death and cell migration of SH-SY5Y human neuroblastoma cells. Vitro Cell. Dev. Biol. Anim. 2018, 54, 629–639. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Zhang, R.; Dong, L.; Chen, H.; Bo, J.; Xue, W.; Huang, Y. Curcumin inhibits cell proliferation and motility via suppression of TROP2 in bladder cancer cells. Int. J. Oncol. 2018, 53, 515–526. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Srivastava, R.K. Involvement of Bcl-2 family members, phosphatidylinositol 3’-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int. J. Oncol. 2007, 30, 905–918. [Google Scholar] [CrossRef]
- Dujic, J.; Kippenberger, S.; Hoffmann, S.; Ramirez-Bosca, A.; Miquel, J.; Diaz-Alperi, J.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A. Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. J. Investig. Dermatol. 2007, 127, 1992–2000. [Google Scholar] [CrossRef]
- Rutz, J.; Maxeiner, S.; Justin, S.; Bachmeier, B.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Blaheta, R.A. Low Dosed Curcumin Combined with Visible Light Exposure Inhibits Renal Cell Carcinoma Metastatic Behavior In Vitro. Cancers 2020, 12, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, F.; Binder, K.; Rutz, J.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Juengel, E.; Blaheta, R.A. The Antitumor Effect of Curcumin in Urothelial Cancer Cells Is Enhanced by Light Exposure In Vitro. Evid. Based Complement. Altern. Med. 2019, 2019, 6374940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Rohe, A.; Platzer, C.; Najjar, A.; Erdmann, F.; Sippl, W. Regulation of G2/M Transition by Inhibition of WEE1 and PKMYT1 Kinases. Molecules 2017, 22, 2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teiten, M.-H.; Gaascht, F.; Cronauer, M.; Henry, E.; Dicato, M.; Diederich, M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int. J. Oncol. 2011, 38, 603–611. [Google Scholar]
- Yang, C.; Ma, X.; Wang, Z.; Zeng, X.; Hu, Z.; Ye, Z.; Shen, G. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. DrugDes. Dev. Ther. 2017, 11, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, B.; Guo, C.; Neeb, A.; Paschalis, A.; Sandhu, S.; de Bono, J.S. Prostate-specific Membrane Antigen Biology in Lethal Prostate Cancer and its Therapeutic Implications. Eur. Urol. Focus 2021. [Google Scholar] [CrossRef]
- Westaby, D.; Viscuse, P.V.; Ravilla, R.; de la Maza, M.L.D.F.; Hahn, A.; Sharp, A.; de Bono, J.; Aparicio, A.; Fleming, M.T. Beyond the Androgen Receptor: The Sequence, the Mutants, and New Avengers in the Treatment of Castrate-Resistant Metastatic Prostate Cancer. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e190–e202. [Google Scholar] [CrossRef]
- Cheng, T.-S.; Chen, W.-C.; Lin, Y.-Y.; Tsai, C.-H.; Liao, C.-I.; Shyu, H.-Y.; Ko, C.-J.; Tzeng, S.-F.; Huang, C.-Y.; Yang, P.-C.; et al. Curcumin-targeting pericellular serine protease matriptase role in suppression of prostate cancer cell invasion, tumor growth, and metastasis. Cancer Prev. Res. 2013, 6, 495–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, J.G.; Stadelman, H.L.; Roselli, C.E. Curcumin blocks CCL2 induced adhesion, motility and invasion, in part, through down-regulation of CCL2 expression and proteolytic activity. Int. J. Oncol. 2009, 34, 1319–1327. [Google Scholar] [PubMed]
- Nollet, E.A.; Cardo-Vila, M.; Ganguly, S.S.; Tran, J.D.; Schulz, V.V.; Cress, A.; Corey, E.; Miranti, C.K. Androgen receptor-induced integrin α6β1 and Bnip3 promote survival and resistance to PI3K inhibitors in castration-resistant prostate cancer. Oncogene 2020, 39, 5390–5404. [Google Scholar] [CrossRef]
- Connell, B.; Kopach, P.; Ren, W.; Joshi, R.; Naber, S.; Zhou, M.; Mathew, P. Aberrant integrin αv and α5 expression in prostate adenocarcinomas and bone-metastases is consistent with a bone-colonizing phenotype. Transl. Androl. Urol. 2020, 9, 1630–1638. [Google Scholar] [CrossRef]
- Juan-Rivera, M.C.; Martínez-Ferrer, M. Integrin Inhibitors in Prostate Cancer. Cancers 2018, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.; Ren, W.; Mathew, P. A Bispecific Antibody Targeting the αv and α5β1 Integrins Induces Integrin Degradation in Prostate Cancer Cells and Is Superior to Monospecific Antibodies. Mol. Cancer Res. 2020, 18, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Juengel, E.; Meyer dos Santos, S.; Schneider, T.; Makarevic, J.; Hudak, L.; Bartsch, G.; Haferkamp, A.; Wiesner, C.; Blaheta, R.A. HDAC inhibition suppresses bladder cancer cell adhesion to collagen under flow conditions. Exp. Biol. Med. 2013, 238, 1297–1304. [Google Scholar] [CrossRef]
- Neuschmelting, V.; Kim, K.; Malekzadeh-Najafabadi, J.; Jebiwott, S.; Prakash, J.; Scherz, A.; Coleman, J.A.; Kircher, M.F.; Ntziachristos, V. WST11 Vascular Targeted Photodynamic Therapy Effect Monitoring by Multispectral Optoacoustic Tomography (MSOT) in Mice. Theranostics 2018, 8, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, S.G.C.; Grimbergen, M.C.M.; Rehmann, H.; Bosch, J.L.H.R.; Jans, J.J.M. Photodynamic therapy as novel nephron sparing treatment option for small renal masses. J. Urol. 2012, 187, 289–295. [Google Scholar] [CrossRef]
- Ellerkamp, V.; Bortel, N.; Schmid, E.; Kirchner, B.; Armeanu-Ebinger, S.; Fuchs, J. Photodynamic Therapy Potentiates the Effects of Curcumin on Pediatric Epithelial Liver Tumor Cells. Anticancer Res. 2016, 36, 3363–3372. [Google Scholar] [PubMed]
- Betrouni, N.; Boukris, S.; Benzaghou, F. Vascular targeted photodynamic therapy with TOOKAD® Soluble (WST11) in localized prostate cancer: Efficiency of automatic pre-treatment planning. Lasers Med. Sci. 2017, 32, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Flegar, L.; Buerk, B.; Proschmann, R.; Propping, S.; Groeben, C.; Baunacke, M.; Herout, R.; Huber, J.; Thomas, C.; Borkowetz, A. Vascular-targeted Photodynamic Therapy in Unilateral Low-risk Prostate Cancer in Germany: 2-yr Single-centre Experience in a Real-world Setting Compared with Radical Prostatectomy. Eur. Urol. Focus 2021. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutz, J.; Benchellal, A.; Kassabra, W.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Juengel, E.; Blaheta, R.A. Growth, Proliferation and Metastasis of Prostate Cancer Cells Is Blocked by Low-Dose Curcumin in Combination with Light Irradiation. Int. J. Mol. Sci. 2021, 22, 9966. https://doi.org/10.3390/ijms22189966
Rutz J, Benchellal A, Kassabra W, Maxeiner S, Bernd A, Kippenberger S, Zöller N, Chun FK-H, Juengel E, Blaheta RA. Growth, Proliferation and Metastasis of Prostate Cancer Cells Is Blocked by Low-Dose Curcumin in Combination with Light Irradiation. International Journal of Molecular Sciences. 2021; 22(18):9966. https://doi.org/10.3390/ijms22189966
Chicago/Turabian StyleRutz, Jochen, Aicha Benchellal, Wajdi Kassabra, Sebastian Maxeiner, August Bernd, Stefan Kippenberger, Nadja Zöller, Felix K.-H. Chun, Eva Juengel, and Roman A. Blaheta. 2021. "Growth, Proliferation and Metastasis of Prostate Cancer Cells Is Blocked by Low-Dose Curcumin in Combination with Light Irradiation" International Journal of Molecular Sciences 22, no. 18: 9966. https://doi.org/10.3390/ijms22189966





