Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine
Abstract
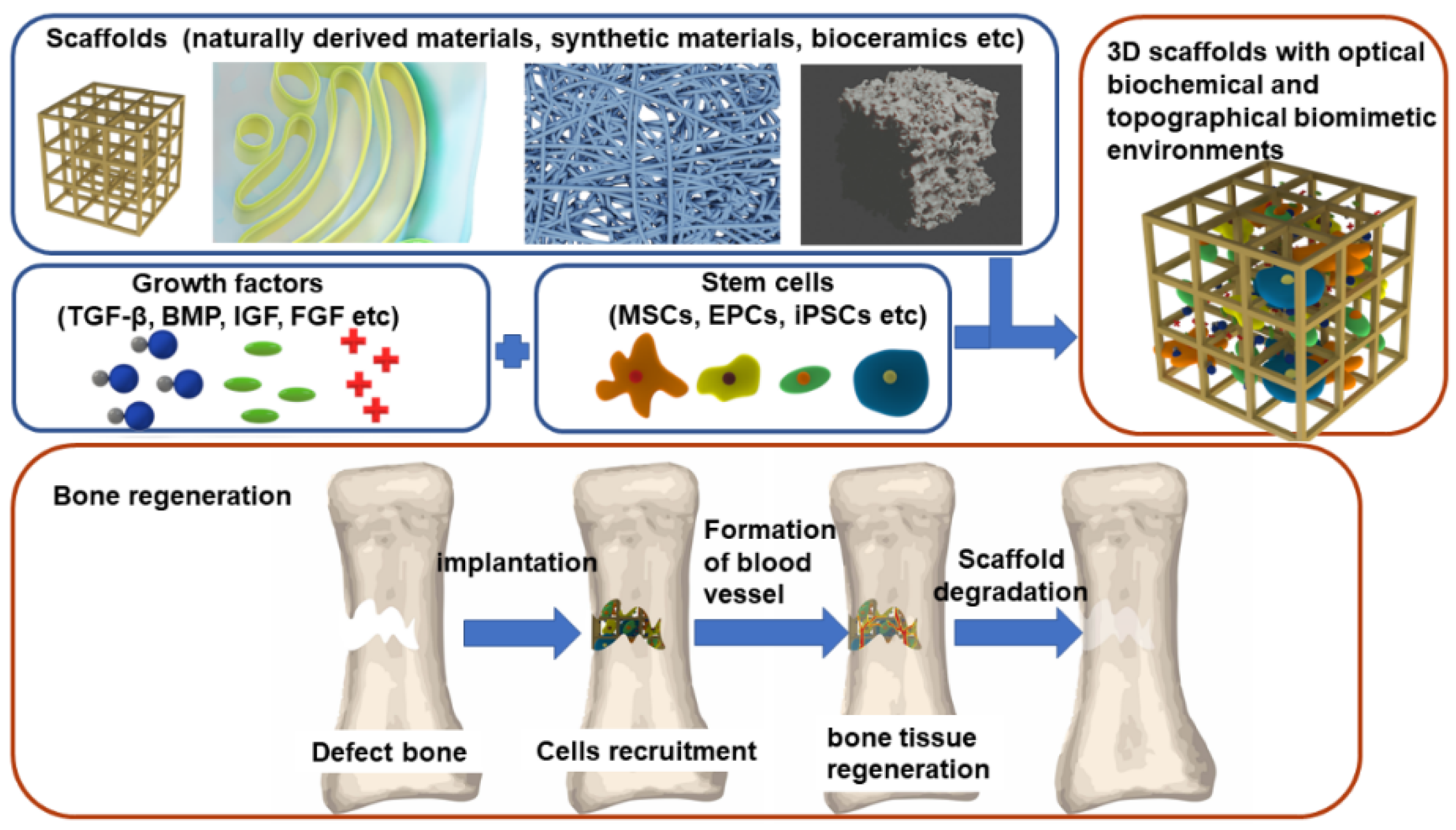
:1. Introduction
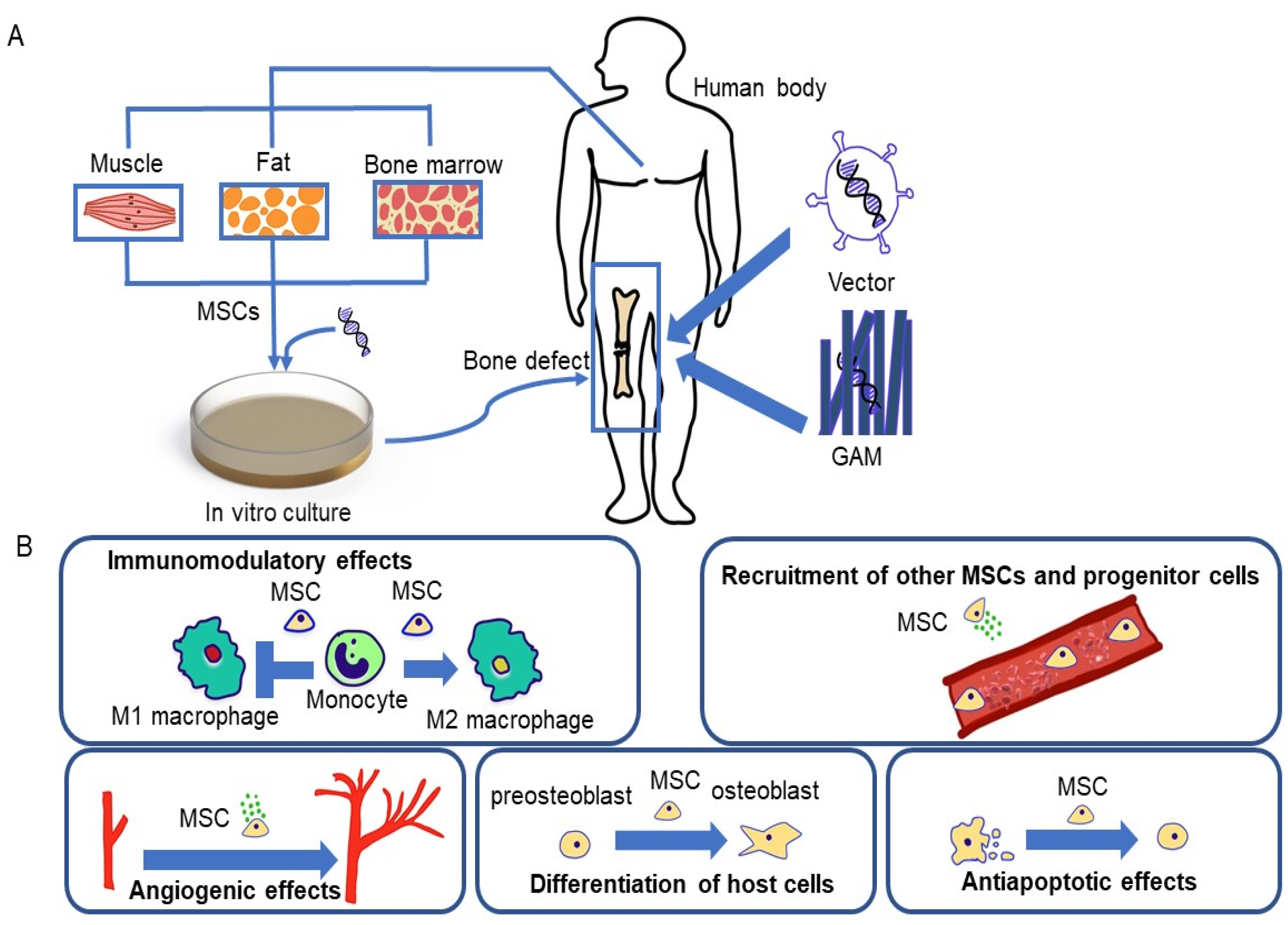
2. Stem Cells
2.1. Mesenchymal Stem Cells (MSCs)
2.2. Endothelial Progenitor Cells (EPCs)
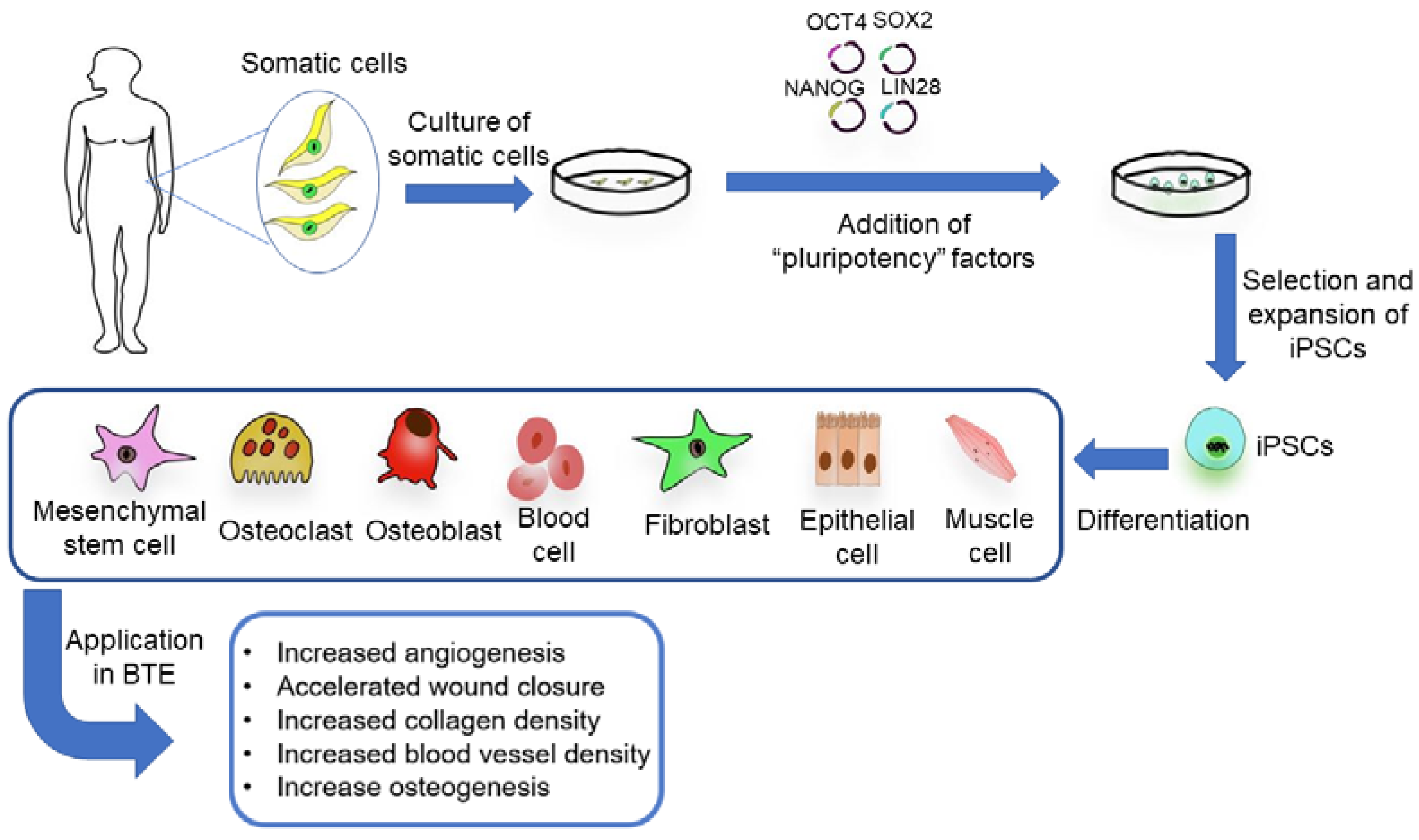
2.3. Induced Pluripotent Stem Cells (iPSCs)
3. Scaffolds
3.1. Naturally Derived Biomaterials
3.1.1. Fibrin
3.1.2. Collagen
3.1.3. Chitosan
3.1.4. Polyhydroxyalkanoates (PHA)
3.2. Ceramics
3.3. Metallic Materials
3.3.1. Tantalum
3.3.2. Titanium
3.4. Synthetic Biomaterials
3.4.1. Polymer Organic Synthetic Materials
3.4.2. Synthetic Inorganic Materials
3.4.3. Composite Materials
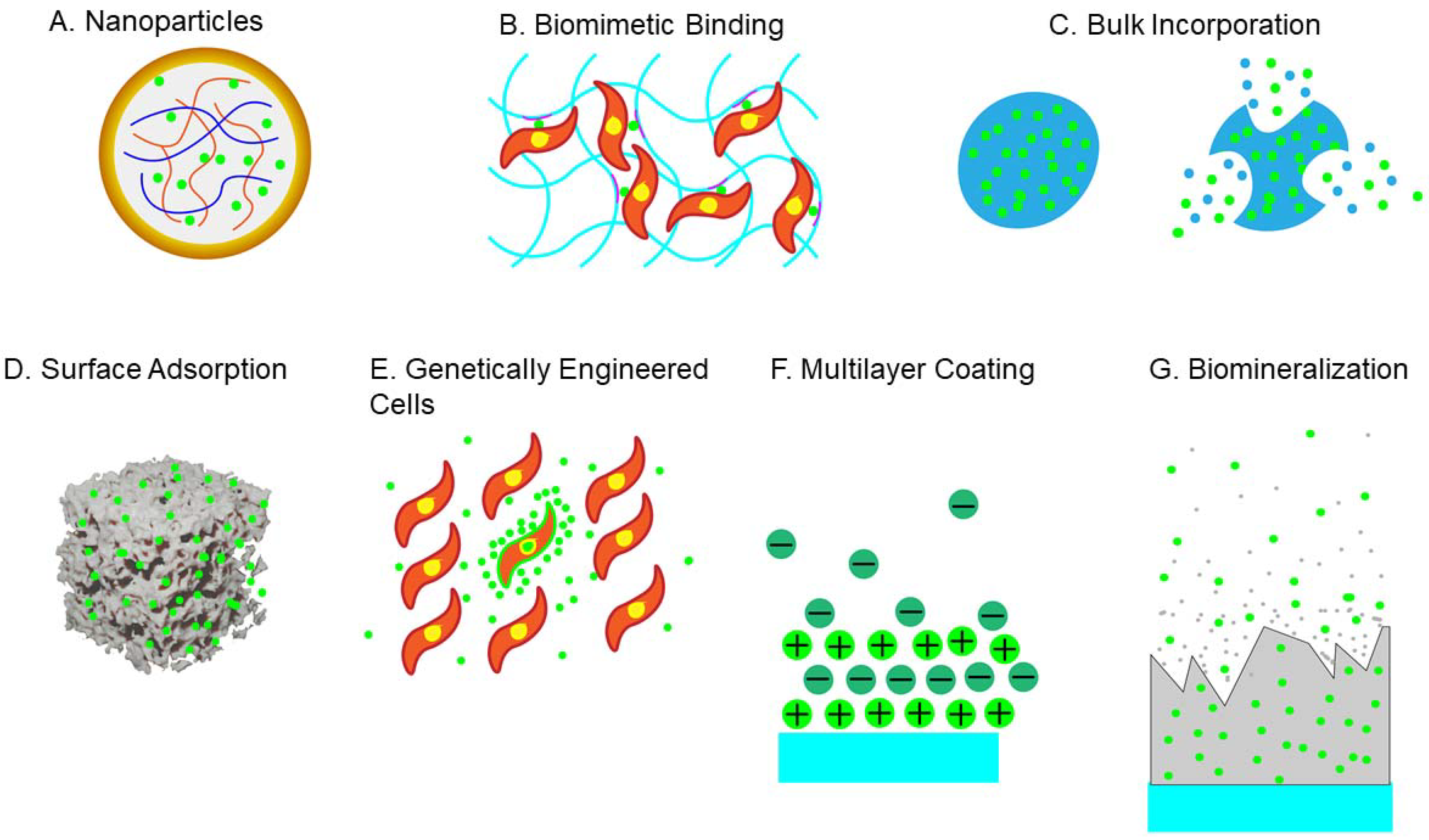
4. Growth Factors
4.1. Transforming Growth Factor β (TGF-β)
4.2. Bone Morphogenetic Protein (BMP)
4.3. Insulin-like Growth Factor (IGF)
4.4. Fibroblast Growth Factor (FGF)
5. BTE Clinical Application and Challenges
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Schuknecht, H.F. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Ann. Otol. Rhinol. Laryngol. 1975, 84, 704–718. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of Bone Grafting Materials for Bone Repair and Regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Fu, S.; Zhu, Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: An overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Liu, X.; Zhao, L.; Weir, M.D.; Sun, J.; Chen, W. Bone Tissue Engineering Via Human Induced Pluripotent, Umbilical Cord and Bone Marrow Mesenchymal Stem Cells in Rat Cranium. Acta Biomater 2015, 18, 236–248. [Google Scholar] [CrossRef] [Green Version]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef] [Green Version]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- Vats, A.; Tolley, N.S.; Polak, J.M.; Buttery, L.D.K. Stem Cells: Sources and Applications. Clin. Otolaryngol. Allied Sci. 2002, 27, 227–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An In Vitro Comparative Study of Multisource Derived Human Mesenchymal Stem Cells for Bone Tissue Engineering. Stem Cells Dev. 2018, 27, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Tevlin, R.; Walmsley, G.G.; Marecic, O.; Hu, M.S.; Wan, D.C.; Longaker, M.T. Stem and progenitor cells: Advancing bone tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 159–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeynayake, N.; Arthur, A.; Gronthos, S. Crosstalk between skeletal and neural tissues is critical for skeletal health. Bone 2021, 142, 115645. [Google Scholar] [CrossRef]
- Mizuno, H. Adipose-Derived Stem Cells for Tissue Repair and Regeneration: Ten Years of Research and a Literature Review. J. Nippon. Med. Sch. 2009, 76, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aust, L.; Devlin, B.; Foster, S.J.; Halvorsen, Y.D.C.; Hicok, K.; du Laney, T.; Sen, A.; Willingmyre, G.; Gimble, J. Yield of Human Adipose-Derived Adult Stem Cells From Liposuction Aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef]
- Gu, X.; Li, C.; Yin, F.; Yang, G. Adipose-Derived Stem Cells in Articular Cartilage Regeneration: Current Concepts and Optimization Strategies. Histol. Histopathol. 2018, 33, 639–653. [Google Scholar]
- Lendeckel, S.; Jödicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.-P. Autologous Stem Cells (Adipose) and Fibrin Glue Used to Treat Widespread Traumatic Calvarial Defects: Case Report. J. Cranio-Maxillofac. Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef]
- Sándor, G.K.; Numminen, J.; Wolff, J.; Thesleff, T.; Miettinen, A.; Tuovinen, V.J.; Mannerström, B.; Patrikoski, M.; Seppänen, R.; Miettinen, S.; et al. Adipose Stem Cells Used to Reconstruct 13 Cases with Cranio-Maxillofacial Hard-Tissue Defects. Stem Cells Transl. Med. 2014, 3, 530–540. [Google Scholar] [CrossRef]
- Vériter, S.; André, W.; Aouassar, N.; Poirel, H.A.; Lafosse, A.; Docquier, P.-L.; Dufrane, D. Human adipose-derived mesenchymal stem cells in cell therapy: Safety and feasibility in different “hospital exemption” clinical applications. PLoS ONE 2015, 10, e0139566. [Google Scholar] [CrossRef]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oryan, A.; Kamali, A.; Moshirib, A.; Eslaminejad, M.B. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs. 2017, 204, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Umebayashi, M.; Sumita, Y.; Kawai, Y.; Watanabe, S.; Asahina, I. Gene-Activated Matrix Comprised of Atelocollagen and Plasmid DNA Encoding BMP4 or Runx2 Promotes Rat Cranial Bone Augmentation. Biores Open Access 2015, 4, 164–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vural, A.C.; Odabas, S.; Korkusuz, P.; Yar Sağlam, A.S.; Bilgiç, E.; Çavuşoğlu, T.; Piskin, E.; Vargel, I. Cranial Bone Regeneration via BMP-2 Encoding Mesenchymal Stem Cells. Artif. Cells Nanomed. Biotechnol. 2017, 45, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Gamradt, S.C.; Abe, N.; Bahamonde, M.E.; Lee, Y.P.; Nelson, S.D.; Lyons, K.M.; Lieberman, J.R. Tracking Expression of Virally Mediated BMP-2 in Gene Therapy for Bone Repair. Clin. Orthop. Relat. Res. 2006, 450, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Wright, V.; Usas, A.; Gearhart, B.; Shen, H.C.; Cummins, J.; Huard, J. Synergistic Enhancement of Bone Formation and Healing by Stem Cell-Expressed VEGF and Bone Morphogenetic Protein-4. J. Clin. Investig. 2002, 110, 751–759. [Google Scholar] [CrossRef]
- Dickson, K.F.; Katzman, S.; Paiement, G. The Importance of the Blood Supply in the Healing of Tibial Fractures. Contemp. Orthop. 1995, 30, 489–493. [Google Scholar]
- Atesok, K.; Matsumoto, T.; Karlsson, J.; Asahara, T.; Atala, A.; Doral, M.N.; Verdonk, R.; Li, R. An Emerging Cell-Based Strategy in Orthopaedics: Endothelial Progenitor Cells. Knee Surg. Sport Traumatol. Arthrosc. 2012, 20, 1366–1377. [Google Scholar] [CrossRef]
- Kawamoto, A.; Gwon, H.-C.; Iwaguro, H.; Yamaguchi, J.-I.; Uchida, S.; Masuda, H.; Silver, M.; Ma, H.; Kearney, M.; Isner, J.M.; et al. Therapeutic Potential of Ex Vivo Expanded Endothelial Progenitor Cells for Myocardial Ischemia. Circulation 2001, 103, 634–637. [Google Scholar] [CrossRef]
- Liu, Y.; Teoh, S.H.; Chong, M.S.K.; Lee, E.S.M.; Mattar, C.N.Z.; Randhawa, N.K.; Zhang, Z.-Y.; Medina, R.J.; Kamm, R.D.; Fisk, N.M.; et al. Vasculogenic and Osteogenesis-Enhancing Potential of Human Umbilical Cord Blood Endothelial Colony-Forming Cells. Stem Cells 2012, 30, 1911–1924. [Google Scholar] [CrossRef]
- Rozen, N.; Bick, T.; Bajayo, A.; Shamian, B.; Schrift-Tzadok, M.; Gabet, Y.; Yayon, A.; Bab, I.; Soudry, M.; Lewinson, D. Transplanted Blood-Derived Endothelial Progenitor Cells (EPC) Enhance Bridging of Sheep Tibia Critical Size Defects. Bone 2009, 45, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Atesok, K.; Li, R.; Stewart, D.J.; Schemitsch, E.H. Endothelial Progenitor Cells Promote Fracture Healing in a Segmental Bone Defect Model. J. Orthop. Res. 2010, 28, 1007–1014. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Kähling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial Progenitor Cells and Mesenchymal Stem Cells Seeded onto β-tcp Granules Enhance Early Vascularization and Bone Healing in a Critical-Sized Bone Defect in Rats. Tissue Eng. Part A 2010, 16, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. Methods of Induced Pluripotent Stem Cells for Clinical Application. World J. Stem Cells 2015, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived From Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Lian, Q.; Zhang, Y.; Zhang, J.; Zhang, H.K.; Wu, X.; Zhang, Y.; Lam, F.F.-Y.; Kang, S.; Xia, J.C.; Lai, W.-H.; et al. Functional Mesenchymal Stem Cells Derived From Human Induced Pluripotent Stem Cells Attenuate Limb Ischemia in Mice. Circulation 2010, 121, 1113–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyranoski, D. Stem Cells Cruise to Clinic. Nature 2013, 494, 413. [Google Scholar] [CrossRef] [Green Version]
- Hollinger, J.O.; Einhorn, T.A.; Doll, B.A.; Sfeir, C. Bone tissue engineering. Bone Tissue Eng. 2004, 11, 277–302. [Google Scholar]
- Wang, P.; Song, Y.; Weir, M.D.; Sun, J.; Zhao, L.; Simon, C.G.; Xu, H.H. A Self-Setting iPSMSC-Alginate-Calcium Phosphate Paste for Bone Tissue Engineering. Dent. Mater. 2016, 32, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef] [Green Version]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. Biomed. Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.O.; Vorwald, C.E.; Dreher, M.L.; Mott, E.J.; Cheng, M.-H.; Cinar, A.; Mehdizadeh, H.; Somo, S.; Dean, D.; Brey, E.M.; et al. Evaluating 3D-printed biomaterials as scaffolds for vascularized bone tissue engineering. Adv. Mater. 2014, 27, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Nanditha, S.; Chandrasekaran, B.; Muthusamy, S.; Muthu, K. Apprising the Diverse Facets of Platelet Rich Fibrin in Surgery through a Systematic Review. Int. J. Surg. 2017, 46, 186–194. [Google Scholar] [CrossRef]
- Du, X.; Wei, D.; Huang, L.; Zhu, M.; Zhang, Y.; Zhu, Y. 3D Printing of Mesoporous Bioactive Glass/Silk Fibroin Composite Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 103, 109731. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Roux, B.M.; Posukonis, M.; Bodamer, E.; Brey, E.M.; Fisher, J.P.; Dean, D. Effect of Prevascularization on In Vivo Vascularization of Poly(Propylene Fumarate)/Fibrin Scaffolds. Biomaterials 2016, 77, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Borciani, G.; Pontremoli, C.; Ciapetti, G.; Mattioli-Belmonte, M.; Fiorilli, S.; Vitale-Brovarone, C. Development and Biocompatibility of Collagen-Based Composites Enriched with Nanoparticles of Strontium Containing Mesoporous Glass. Materials 2019, 12, 3719. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Ye, X.; Fan, Y.; Ma, L.; Tan, Y.; Qing, F.; Zhang, X. Biomimetic Fabrication of a Three-Level Hierarchical Calcium Phosphate/Collagen/Hydroxyapatite Scaffold for Bone Tissue Engineering. Biofabrication 2014, 6, 035013. [Google Scholar] [CrossRef] [PubMed]
- Yunus Basha, R.; Sampath, S.K.; Doble, M. Design of Biocomposite Materials for Bone Tissue Regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The Development of Collagen Based Composite Scaffolds for Bone Regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Ong Abdullah, J.; Ho, C.L. Biosynthesis of Agar in Red Seaweeds: A Review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nady, N.; Kandil, S.H. Novel Blend for Producing Porous Chitosan-Based Films Suitable for Biomedical Applications. Membranes 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhumathi, K.; Shalumon, K.T.; Rani, V.V.D.; Tamura, H.; Furuike, T.; Selvamurugan, N.; Nair, S.; Jayakumar, R. Wet Chemical Synthesis of Chitosan Hydrogel-Hydroxyapatite Composite Membranes for Tissue Engineering Applications. Int. J. Biol. Macromol. 2009, 45, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cheng, X.; Zhu, W.; Holmes, B.; Keidar, M.; Zhang, L.G. Design of Biomimetic and Bioactive Cold Plasma-Modified Nanostructured Scaffolds for Enhanced Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Tissue Eng.-Part A 2014, 20, 1060–1071. [Google Scholar] [CrossRef]
- Ansari, S.; Sami, N.; Yasin, D.; Ahmad, N.; Fatma, T. Biomedical applications of environmental friendly poly-hydroxyalkanoates. Int. J. Biol. Macromol. 2021, 183, 549–563. [Google Scholar] [CrossRef]
- Lim, J.; You, M.; Li, J.; Li, Z. Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater. Sci. Eng. C 2017, 79, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Galego, N.; Rozsa, C.; Sánchez, R.; Fung, J.; Analía Vázquez Santo Tomás, J. Characterization and application of poly(β-hydroxyalkanoates) family as composite biomaterials. Polym. Test. 2000, 19, 485–492. [Google Scholar] [CrossRef]
- Dwivedi, R.; Pandey, R.; Kumar, S.; Mehrotra, D. Poly Hydroxyalkanoates (PHA): Role in Bone Scaffolds. J. Oral. Biol. Craniofacial. Res. 2020, 10, 389. [Google Scholar] [CrossRef]
- Martínez, V.; Herencias, C.; Jurkevitch, E.; Prieto, M.A. Engineering a Predatory Bacterium as a Proficient Killer Agent for Intracellular Bio-Products Recovery: The Case of the Polyhydroxyalkanoates. Nat. Publ. Gr. 2016. Available online: www.nature.com/scientificreports (accessed on 13 September 2021).
- Salinas, A.J.; Vallet-Regí, M. Bioactive ceramics: From bone grafts to tissue engineering. RSC Adv. 2013, 3, 11116–11131. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [Green Version]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Myoui, A. Bone tissue engineering with porous hydroxyapatite ceramics. J. Artif. Organs. 2005, 8, 131–136. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale Hydroxyapatite Particles for Bone Tissue Engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass Scaffolds for Bone Tissue Engineering: State of the Art and Future Perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1245–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass-Derived Glass-Ceramic Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, W.; Wang, R. Retraction: The Role of Tantalum Nanoparticles in Bone Regeneration Involves the bmp2/smad4/runx2 Signaling Pathway. Int. J. Nanomed. 2020, 15, 2419–2435. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhu, Z.; Xiao, H.; Luo, C.; Luo, X.; Lv, F.; Liao, J.; Huang, W. Three-Dimensional, Multiscale, and Interconnected Trabecular Bone Mimic Porous Tantalum Scaffold for Bone Tissue Engineering. ACS Omega. 2020, 5, 22520–22528. [Google Scholar] [CrossRef]
- Martel-Frachet, V.; Ivanova, E.P.; Le Clainche, T.; Linklater, D.; Wong, S.; Le, P.; Juodkazis, S.; Le Guével, X.; Coll, J.-L.; Ivanova, E.P. Mechano-Bactericidal Titanium Surfaces for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 48272–48283. [Google Scholar]
- Thi Hiep, N.; Chan Khon, H.; Dai Hai, N.; Byong-Taek, L.; Van Toi, V.; Thanh Hung, L. Biocompatibility of PCL/PLGA-BCP Porous Scaffold for Bone Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2017, 28, 864–878. [Google Scholar] [CrossRef]
- Lin, Y.J.; Huang, C.C.; Wan, W.L.; Chiang, C.H.; Chang, Y.; Sung, H.W. Recent Advances in CO2 Bubble-Generating Carrier Systems for Localized Controlled Release. Biomaterials 2017, 133, 154–164. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Knoke, I.Y.; Han, J.; Klug, C.A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Fluorescent PLLA-Nanodiamond Composites for Bone Tissue Engineering. Biomaterials 2011, 32, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shen, H.; Yang, F.; Bei, J.; Wang, S. Preparation and Cell Affinity of Microtubular Orientation-Structured PLGA(70/30) Blood Vessel Scaffold. Biomaterials 2008, 29, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-K.; Cai, S.-X.; Liu, B.; Xu, Z.-L.; Dai, X.-Z.; Ma, K.-W.; Li, S.-Q.; Yang, L.; Sung, K.P.; Fu, X.-B. Characteristics of PLGA-Gelatin Complex as Potential Artificial Nerve Scaffold. Colloids Surf. B Biointerfaces 2007, 57, 198–203. [Google Scholar] [CrossRef]
- Frezzo, J.A.; Montclare, J.K. Natural Composite Systems for Bioinspired Materials. Adv. Exp. Med. Biol. 2016, 940, 143–166. [Google Scholar]
- Huang, X.; Bai, S.; Lu, Q.; Liu, X.; Liu, S.; Zhu, H. Osteoinductive-Nanoscaled Silk/HA Composite Scaffolds for Bone Tissue Engineering Application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Isikli, C.; Hasirci, V.; Hasirci, N. Development of Porous Chitosan-Gelatin/Hydroxyapatite Composite Scaffolds for Hard Tissue-Engineering Applications. J. Tissue Eng. Regen. Med. 2012, 6, 135–143. [Google Scholar] [CrossRef]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of Hydroxyapatite for Biomedical Applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Türe, H. Characterization of Hydroxyapatite-Containing Alginate–Gelatin Composite Films as a Potential Wound Dressing. Int. J. Biol. Macromol. 2019, 123, 878–888. [Google Scholar] [CrossRef]
- Smith, B.T.; Shum, J.; Wong, M.; Mikos, A.G.; Young, S. Bone Tissue Engineering Challenges in Oral & Maxillofacial Surgery. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2015; pp. 57–78. [Google Scholar]
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic Calcium Phosphates Bioceramics (HA/TCP): Concept, Physicochemical Properties and the Impact of Standardization of Study Protocols in Biomaterials Research. Mater. Sci. Eng. C 2017, 71, 1293–1312. [Google Scholar] [CrossRef]
- Bosetti, M.; Cannas, M. The Effect of Bioactive Glasses on Bone Marrow Stromal Cells Differentiation. Biomaterials 2005, 26, 3873–3879. [Google Scholar] [CrossRef]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of Nanoscale and Microscale Bioactive Glass on the Properties of P(3HB)/Bioglass® Composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Ayarza, J.; Steeves, T.; Hu, Z.; Manna, S.; Esser-Kahn, A.P. Bio-Inspired Mechanically Adaptive Materials through Vibration-Induced Crosslinking. Nat. Mater. 2021, 20, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Devescovi, V.; Leonardi, E.; Ciapetti, G.; Cenni, E. Growth factors in bone repair. Chir. Organi. Mov. 2008, 92, 161–168. [Google Scholar] [CrossRef]
- Dole, N.S.; Mazur, C.M.; Acevedo, C.; Lopez, J.P.; Monteiro, D.A.; Fowler, T.W.; Gludovatz, B.; Walsh, F.; Regan, J.N.; Messina, S.; et al. Osteocyte-Intrinsic TGF-β Signaling Regulates Bone Quality through Perilacunar/Canalicular Remodeling. Cell Rep. 2017, 21, 2585–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramoshebi, L.N.; Matsaba, T.N.; Teare, J.; Renton, L.; Patton, J.; Ripamonti, U. Tissue Engineering: TGF-β Superfamily Members and Delivery Systems in Bone Regeneration. Expert Rev. Mol. Med. 2002, 4, 1–11. Available online: http://www.expertreviews.org/ (accessed on 5 July 2021). [CrossRef]
- Wu, W.; Dan, Y.; Yang, S.-H.; Yang, C.; Shao, Z.-W.; Xu, W.-H.; Li, J.; Liu, X.-Z.; Zheng, D. Promotion of Chondrogenesis of Marrow Stromal Stem Cells by TGF-β3 Fusion Protein In Vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 692–699. [Google Scholar] [CrossRef]
- Kim, Y.I.; Ryu, J.-S.; Yeo, J.E.; Choi, Y.J.; Ko, K.; Koh, Y.-G. Overexpression of TGF-β1 Enhances Chondrogenic Differentiation and Proliferation of Human Synovium-Derived Stem Cells. Biochem. Biophys. Res. Commun. 2014, 450, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Hong, L.; Yamamoto, M.; Shigeno, K.; Inoue, M.; Toba, T.; Yoshitani, M.; Nakamura, T.; Tabata, Y.; Shimizu, Y. Use of collagen sponge incorporating transforming growth factor-β1 to promote bone repair in skull defects in rabbits. Biomaterials. 2002, 23, 1003–1010. [Google Scholar] [CrossRef] [Green Version]
- Herford, A.S.; Boyne, P.J.; Rawson, R.; Williams, R.P. Bone Morphogenetic Protein-Induced Repair of the Premaxillary Cleft. J. Oral. Maxillofac. Surg. 2007, 65, 2136–2141. [Google Scholar] [CrossRef]
- Herford, A.S.; Cicciù, M. Recombinant Human Bone Morphogenetic Protein Type 2 Jaw Reconstruction in Patients Affected by Giant Cell Tumor. J. Craniofacial Surg. 2010, 1970–1975. [Google Scholar] [CrossRef]
- Cahill, K.S.; Chi, J.H.; Day, A.; Claus, E.B. Prevalence, Complications, and Hospital Charges Associated with Use of Bone-Morphogenetic Proteins in Spinal Fusion Procedures. JAMA-J. Am. Med. Assoc. 2009, 302, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A Critical Review of Recombinant Human Bone Morphogenetic Protein-2 Trials in Spinal Surgery: Emerging Safety Concerns and Lessons Learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, T.; Sakata, R.; Reddi, A.H. Induction of Chondrogenesis and Expression of Superficial Zone Protein in Synovial Explants with TGF-β1 and BMP-7. Tissue Eng. Part A 2013, 19, 2638–2644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shintani, N.; Siebenrock, K.A.; Hunziker, E.B. TGF-ß1 Enhances the BMP-2-Induced Chondrogenesis of Bovine Synovial Explants and Arrests Downstream Differentiation at an Early Stage of Hypertrophy. PLoS ONE 2013, 8, e53086. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Bouxsein, M.L.; Faugere, M.-C.; Guldberg, R.E. The Hypoxia-Inducible Factor α Pathway Couples Angiogenesis to Osteogenesis during Skeletal Development. J. Clin. Investig. 2007, 117, 1616–1626. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.Z.; Sun, H.P.; Xu, J.Y.; Li, Y.M.; Xie, X. Sustained Dual Release of Placental Growth Factor-2 and Bone Morphogenic Protein-2 from Heparin-Based Nanocomplexes for Direct Osteogenesis. Int. J. Nanomed. 2016, 11, 1147–1158. [Google Scholar] [CrossRef] [Green Version]
- Nash, T.J.; Howlett, C.R.; Martin, C.; Steele, J.; Johnson, K.A.; Hicklin, D.J. Effect of Platelet-Derived Growth Factor on Tibial Osteotomies in Rabbits. Bone 1994, 15, 203–208. [Google Scholar] [CrossRef]
- Hock, J.M.; Centrella, M.; Canalis, E. Insulin-Like Growth Factor I Has Independent Effects on Bone Matrix Formation and Cell Replication. Endocrinology 1988, 122, 254–260. [Google Scholar] [CrossRef]
- Spencer, E.M.; Liu, C.C.; Si, E.C.C.; Howard, G.A. In Vivo Actions of Insulin-Like Growth Factor-I (IGF-I) on Bone Formation and Resorption in Rats. Bone 1991, 12, 21–26. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Schwabe, P.; Raschke, M.; Haas, N.P.; Wildemann, B. Collective Review: Bioactive Implants Coated with Poly(D,L-Lactide) and Growth Factors IGF-I, TGF-β1, or BMP-2 for Stimulation of Fracture Healing. J. Long Term Eff. Med. Implant. 2006, 16, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.E.; Colvin, R.B.; Antoniades, H.N. Growth Factors in Wound Healing. Single and Synergistic Effects on Partial Thickness Porcine Skin Wounds. J. Clin. Investig. 1989, 84, 640–646. [Google Scholar] [CrossRef]
- Charles, L.F.; Woodman, J.L.; Ueno, D.; Gronowicz, G.; Hurley, M.M.; Kuhn, L.T. Effects of Low Dose FGF-2 and BMP-2 on Healing of Calvarial Defects in Old Mice. Exp. Gerontol. 2015, 64, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Wamsley, H.L.; Iwaniec, U.T.; Wronski, T.J. Selected Extraskeletal Effects of Systemic Treatment with Basic Fibroblast Growth Factor in Ovariectomized Rats. Toxicol. Pathol. 2005, 33, 577–583. [Google Scholar] [CrossRef]
- Behr, B.; Leucht, P.; Longaker, M.T.; Quarto, N. Fgf-9 is Required for Angiogenesis and Osteogenesis in Long Bone Repair. Proc. Natl. Acad. Sci. USA 2010, 107, 11853–11858. [Google Scholar] [CrossRef] [Green Version]
- Hung, I.H.; Yu, K.; Lavine, K.J.; Ornitz, D.M. FGF9 Regulates Early Hypertrophic Chondrocyte Differentiation and Skeletal Vascularization in the Developing Stylopod. Dev. Biol. 2007, 307, 300–313. [Google Scholar] [CrossRef] [Green Version]
- Wallner, C.; Schira, J.; Wagner, J.M.; Schulte, M.; Fischer, S.; Hirsch, T.; Richter, W.; Abraham, S.; Kneser, U.; Lehnhardt, M.; et al. Application of VEGFA and FGF-9 Enhances Angiogenesis, Osteogenesis and Bone Remodeling in Type 2 Diabetic Long Bone Regeneration. PLoS ONE 2015, 10, e0118823. [Google Scholar]
- Lu, J.; Dai, J.; Wang, X.; Zhang, M.; Zhang, P.; Sun, H.; Zhang, X.; Yu, H.; Zhang, W.; Zhang, L.; et al. Effect of Fibroblast Growth Factor 9 on the Osteogenic Differentiation of Bone Marrow Stromal Stem Cells and Dental Pulp Stem Cells. Mol. Med. Rep. 2015, 11, 1661–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohbayashi, N.; Shibayama, M.; Kurotaki, Y.; Imanishi, M.; Fujimori, T.; Itoh, N.; Takada, S. FGF18 is Required for Normal Cell Proliferation and Differentiation during Osteogenesis and Chondrogenesis. Genes Dev. 2002, 16, 870–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoaka, T.; Ogasawara, T.; Yonamine, A.; Chikazu, D.; Kawano, H.; Nakamura, K.; Itoh, N.; Kawaguchi, H. Regulation of Osteoblast, Chondrocyte, and Osteoclast Functions by Fibroblast Growth Factor (FGF)-18 in Comparison with FGF-2 and FGF-10. J. Biol. Chem. 2002, 277, 7493–7500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.S.; Kim, J.H.; Singh, R.K.; Jang, J.H.; Kim, H.W. Therapeutic-Designed Electrospun Bone Scaffolds: Mesoporous Bioactive Nanocarriers in Hollow Fiber Composites to Sequentially Deliver Dual Growth Factors. Acta Biomater. 2015, 16, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Does Implantation Site Influence Bone Ingrowth Into 3D-Printed Porous Implants? Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S152994301930837X?token=0E70949A956DA670E765B87E6B8D48D0F4AAE0DD1EA0EAAF10A0526540D96CC26478BB9201C5BC440462F060A7A136AA&originRegion=us-east-1&originCreation=20210912115038 (accessed on 12 September 2021).
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Jacome-Galarza, C.E.; Percin, G.I.; Muller, J.T.; Mass, E.; Lazarov, T.; Eitler, J.; Rauner, M.; Yadav, V.K.; Crozet, L.; Bohm, M.; et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature 2019, 568, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Diba, M.; Camargo, W.A.; Brindisi, M.; Farbod, K.; Klymov, A.; Schmidt, S.; Harrington, M.J.; Draghi, L.; Boccaccini, A.R.; Jansen, J.A.; et al. Composite Colloidal Gels Made of Bisphosphonate-Functionalized Gelatin and Bioactive Glass Particles for Regeneration of Osteoporotic Bone Defects. Adv. Funct. Mater. 2017, 27. Available online: www.advancedsciencenews.com (accessed on 12 September 2021). [CrossRef]
- Diesendruck, C.E.; Peterson, G.I.; Kulik, H.J.; Kaitz, J.A.; Mar, B.D.; May, P.A.; White, S.; Martínez, T.J.; Boydston, A.J.; Moore, J.S. Mechanically Triggered Heterolytic Unzipping of a Low-Ceiling-Temperature Polymer. Nat. Chem. 2014, 6. Available online: www.nature.com/naturechemistry (accessed on 12 September 2021). [CrossRef] [PubMed]
- Nasajpour, A.; Ansari, S.; Rinoldi, C.; Rad, S.; Aghaloo, T.; Shin, S.R.; Mishra, Y.; Adelung, R.; Swieszkowski, W.; Annabi, N.; et al. A Multifunctional Polymeric Periodontal Membrane with Osteogenic and Antibacterial Characteristics. Adv. Funct. Mater. 2017, 28, 1703437. [Google Scholar] [CrossRef]
- Holloway, J.L. One step solution for ghting bacteria and growing bone. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Johnson, C.T.; Wroe, J.A.; Agarwal, R.; Martin, K.E.; Guldberg, R.E.; Donlan, R.M.; Westblade, L.F.; García, A.J. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc. Natl. Acad. Sci. USA 2018, 115, E4960–E4969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell Therapy Induced Regeneration of Severely Atrophied Mandibular Bone in a Clinical Trial. Stem Cell Res. Ther. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Sándor, G.K.; Tuovinen, V.J.; Wolff, J.; Patrikoski, M.; Jokinen, J.; Nieminen, E.; Mannerström, B.; Lappalainen, O.-P.; Seppänen-Kaijansinkko, R.; Miettinen, S. Adipose Stem Cell Tissue-Engineered Construct Used to Treat Large Anterior Mandibular Defect: A Case Report and Review of the Clinical Application of Good Manufacturing Practice-Level Adipose Stem Cells for Bone Regeneration. J. Oral. Maxillofac. Surg. 2013, 71, 938–950. [Google Scholar] [CrossRef]
- Skogh, A.C.D.; Kihlström, L.; Neovius, E.; Persson, C.; Beckman, M.O.; Engstrand, T. Variation in Calvarial Bone Healing Capacity: A Clinical Study on the Effects of BMP-2-Hydrogel or Bone Autograft Treatments at Different Cranial Locations. J. Craniofac. Surg. 2013, 24, 339–343. [Google Scholar] [CrossRef]
- Dhote, R.; Charde, P.; Bhongade, M.; Rao, J. Stem Cells Cultured on Beta Tricalcium Phosphate (β-TCP) in Combination with Recombinant Human Platelet-Derived Growth FACTOR-BB (rh-PDGF-BB) for the Treatment of Human Infrabony Defects. J. Stem Cells 2015, 10, 243–254. [Google Scholar] [PubMed]
- Kim, K.T.; Kim, K.G.; Choi, U.Y.; Lim, S.H.; Kim, Y.J.; Sohn, S.; Sheen, S.H.; Heo, C.Y.; Han, I. Safety and Tolerability of Stromal Vascular Fraction Combined with β-Tricalcium Phosphate in Posterior Lumbar Interbody Fusion: Phase I Clinical Trial. Cells 2020, 9, 2250. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.P.; Becker, J.; Terheyden, H.; Capsius, B.; Wagner, W. A Prospective, Randomized Pilot Study on the Safety and Efficacy of Recombinant Human Growth and Differentiation Factor-5 Coated onto β-Tricalcium Phosphate for Sinus Lift Augmentation. Clin. Oral. Implant. Res. 2010, 21, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Redondo, L.M.; García, V.; Peral, B.; Verrier, A.; Becerra, J.; Sánchez, A.; García-Sancho, J. Repair of Maxillary Cystic Bone Defects with Mesenchymal Stem Cells Seeded on a Cross-Linked Serum Scaffold. J. Craniomaxillofac. Surg. 2018, 46, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, D.A.; Bakopoulou, A.A.; Kouzi-Koliakou, K.; Karagiannis, V.; Konstantinidis, A. A Tissue-Engineered Biocomplex for Periodontal Reconstruction. A Proof-of-Principle Randomized Clinical Study. J. Clin. Periodontol. 2021, 48, 1111–1125. [Google Scholar] [CrossRef]
| Bone Grafting Materials | Examples | Advantages | Disadvantages | |
|---|---|---|---|---|
| Polymers | Natural | Protein: collagen, fibrin, silk fibrin | Biodegradability | Low mechanical strength |
| Polysaccharides: hyaluronic acid, chitosan | Biocompatibility | High rates of degradation | ||
| Bacterially synthesized poly: polyhydroxyalkanoate | Bioactivity | High batch to batch variation | ||
| Unlimited source (some of them) | ||||
| Synthetic | Poly-glycolic acid (PGA) | Biodegradability | Low mechanical strength | |
| Poly-lactic acid (PLA) | Biocompatibility | High local concentration of acidic degradation products | ||
| Poly-(lactide-co-glycolide) (PLGA) | Versatility | |||
| Poly-hydroxyethylmethacrylate (poly-HEMA) | ||||
| Poly-ε- caprolactone (PCL) | ||||
| Poly-etylene-glycol (PEG) | ||||
| Ceramics | Calcium-phosphate | Coralline or synthetic hydroxyapatite (HA) | Biocompatibility | Brittleness |
| Silicate-substituted HA | Biodegradability | Low fracture strength | ||
| β-Tricalcium phosphate (β-TCP) | Bioactivity | Degradation rates difficult to predict | ||
| Dicalcium phosphate dehydrate (DCPD) | Osteoconductivity | |||
| Bioglasses and glass-ceramics | Silicate bioactive glasses | Osteoinductivity (subject to structural and chemical properties) | ||
| Borate/borosilicate bioactive glasses | ||||
| Others | Alumina ceramic (Al2O3) | |||
| Metals | Titanium and its alloys | Excellent mechanical properties (high strength and wear resistance, ductility) | Lack of tissue adherence | |
| Tantalum | Biocompatibility | Corrosion | ||
| Stainless steel | Risk of toxicity due to release of metal ions | |||
| Magnesium and its alloys | ||||
| Composites | Calcium-phosphate coatings on metals | Combination of the above | Combination of the above | |
| HA/poly-(D,L-lactide) | ||||
| HA/chitosan-gelatin | ||||
| Indication | Stem Cell | Scaffold | Growth Factor | Outcome | Reference |
|---|---|---|---|---|---|
| Widespread traumatic calvarial defects | Adipose-derived stem cells | Fibrin | / | After 3 months, new bone formed with near complete calvarial continuity observed by axial and 3D-CT scans. | [18] |
| Severe mandibular ridge resorption | Bone marrow-derived mesenchymal stromal cells | Biphasic calcium phosphate | IGF-1, VEGF, and TGF-β | After 4 to 6 months, bone healed, as the mean volume of bone increased by 887.23 mm3, with little adverse events or side effects. | [121] |
| Large anterior mandibular defect | Adipose-derived stem cells | β-tricalcium phosphate | Recombinant human BMP-2 | After 10 months, dental implants were inserted into the grafted site to allow the harvest of bone cores, and prosthodontic rehabilitation was completed based on the visualization of panoramic radiographs. | [122] |
| Standardized critical-size cranial defects after neurosurgery | / | Hyaluronan | BMP-2 | After 3 to 6 weeks, bone was repaired with an increase in bone area of approximately 56 mm2, and no local or systemic side effects were observed. | [123] |
| Infrabony defects | Bone marrow-derived mesenchymal stromal cells | β-tricalcium phosphate | rh-PDGF-BB | 6 months after surgery, the treatment resulted in a significant added benefit in terms of clinical attachment level gain (3.91 mm compared to 2.08), probing pocket depth reduction (4.50 mm compared to 3.50 mm), greater radiographic defect fill (88.33% compared to 52.77%), and improvement in linear bone growth (3.58 mm compared to 1.83 mm) in comparison to open flap debridement alone. | [124] |
| Spinal stenosis | Stromal vascular fraction (SVF) | β-tricalcium phosphate | / | After 6 months, the SVF/β-TCP mixture possessed higher fusion grade (3.6 compared to 2.8) and fusion rate (54.5% compared to 18.1%) than the cages filled with β-TCP. Side effects were observed in 3 out of 10 patients. | [125] |
| Support bone formation after sinus lift augmentation | / | β-tricalcium phosphate | Recombinant human growth and differentiation factor-5 (rhGDF-5) | The amount of new bone was between 28–31.8%. Implants failed in 4 of 47 patients (8.5%) treated with RHGDF-5/β-TCP, in agreement with the general implant failure rate of 5–15%. | [126] |
| Maxillary cysts | Autologous bone-derived mesenchymal stem cells | BioMax cross-linked serum scaffold | / | After 7 months, the CT density of the cyst interior increased significantly, as the mean ratio of the CT values after/before treatment was 2.52, and importantly, the density of the contralateral control area of spongy alveolar bone without treatment did not change, as the average after/before ratio was 0.99. No inflammation or other adverse effects were observed. | [127] |
| Intrabony defects | Autologous clinical-grade alveolar bone marrow mesenchymal stem cells | Collagen enriched with autologous fibrin/platelet lysate | / | After 12 months, the bio-complex led to significant clinical improvements for all groups with an average 3.0 mm attachment gain, 3.7 mm probing pocket depth reduction, and 0.7 mm increase in recession, without adverse healing events. | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Yu, T.; Hu, B.; Wu, H.; Ouyang, H. Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine. Int. J. Mol. Sci. 2021, 22, 10233. https://doi.org/10.3390/ijms221910233
Qi J, Yu T, Hu B, Wu H, Ouyang H. Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine. International Journal of Molecular Sciences. 2021; 22(19):10233. https://doi.org/10.3390/ijms221910233
Chicago/Turabian StyleQi, Jingqi, Tianqi Yu, Bangyan Hu, Hongwei Wu, and Hongwei Ouyang. 2021. "Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine" International Journal of Molecular Sciences 22, no. 19: 10233. https://doi.org/10.3390/ijms221910233
APA StyleQi, J., Yu, T., Hu, B., Wu, H., & Ouyang, H. (2021). Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine. International Journal of Molecular Sciences, 22(19), 10233. https://doi.org/10.3390/ijms221910233







