Biodegradability of Novel Polylactide and Polycaprolactone Materials with Bacteriostatic Properties Due to Embedded Birch Tar in Different Environments
Abstract
:1. Introduction
2. Results
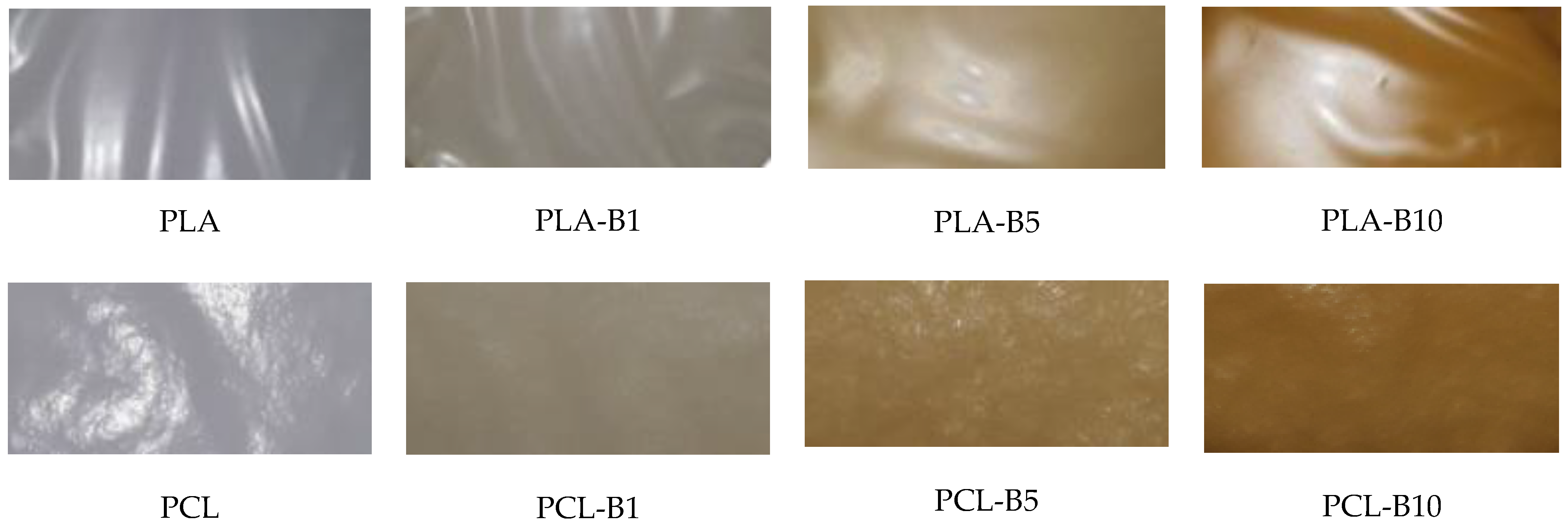
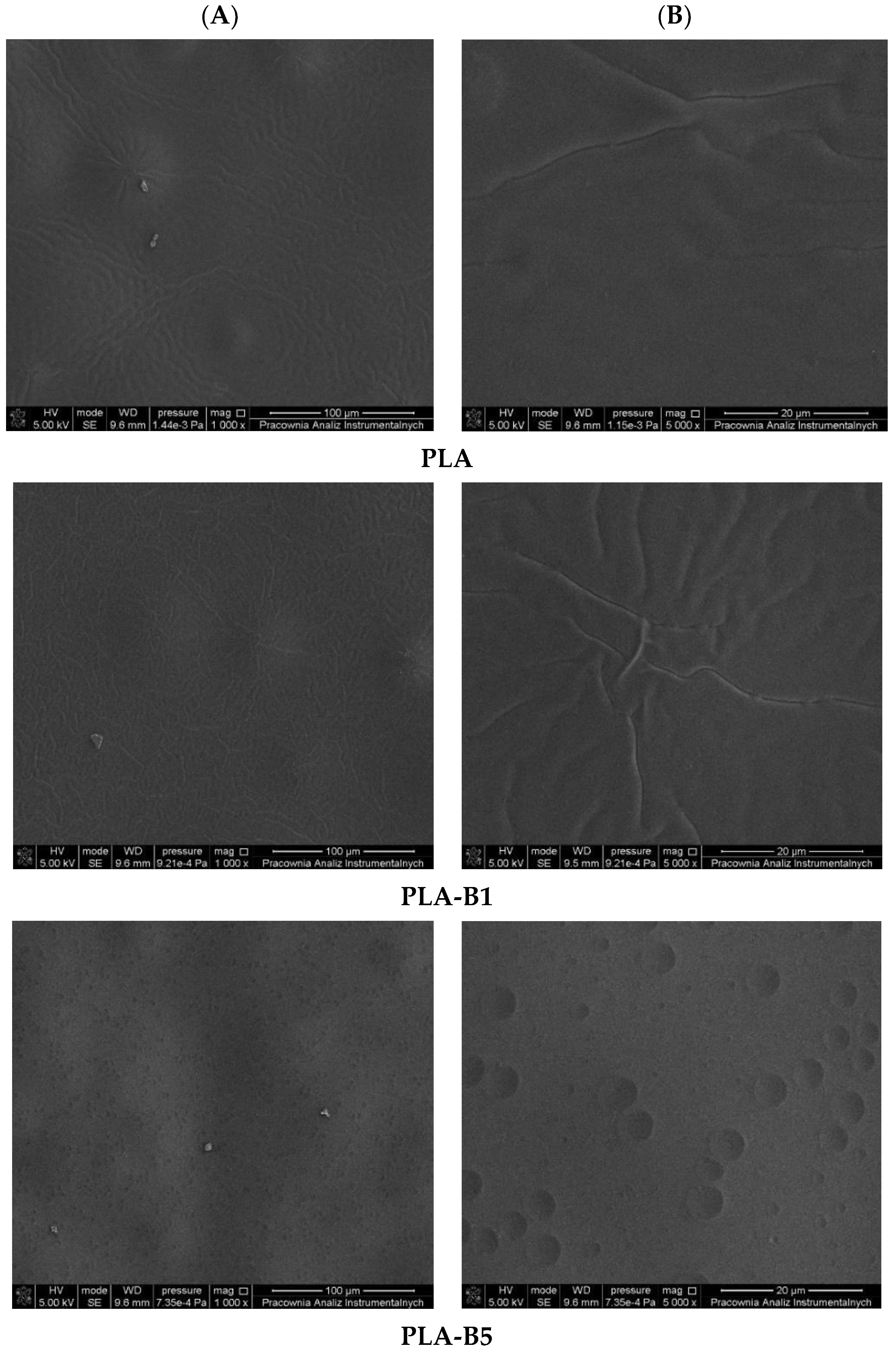
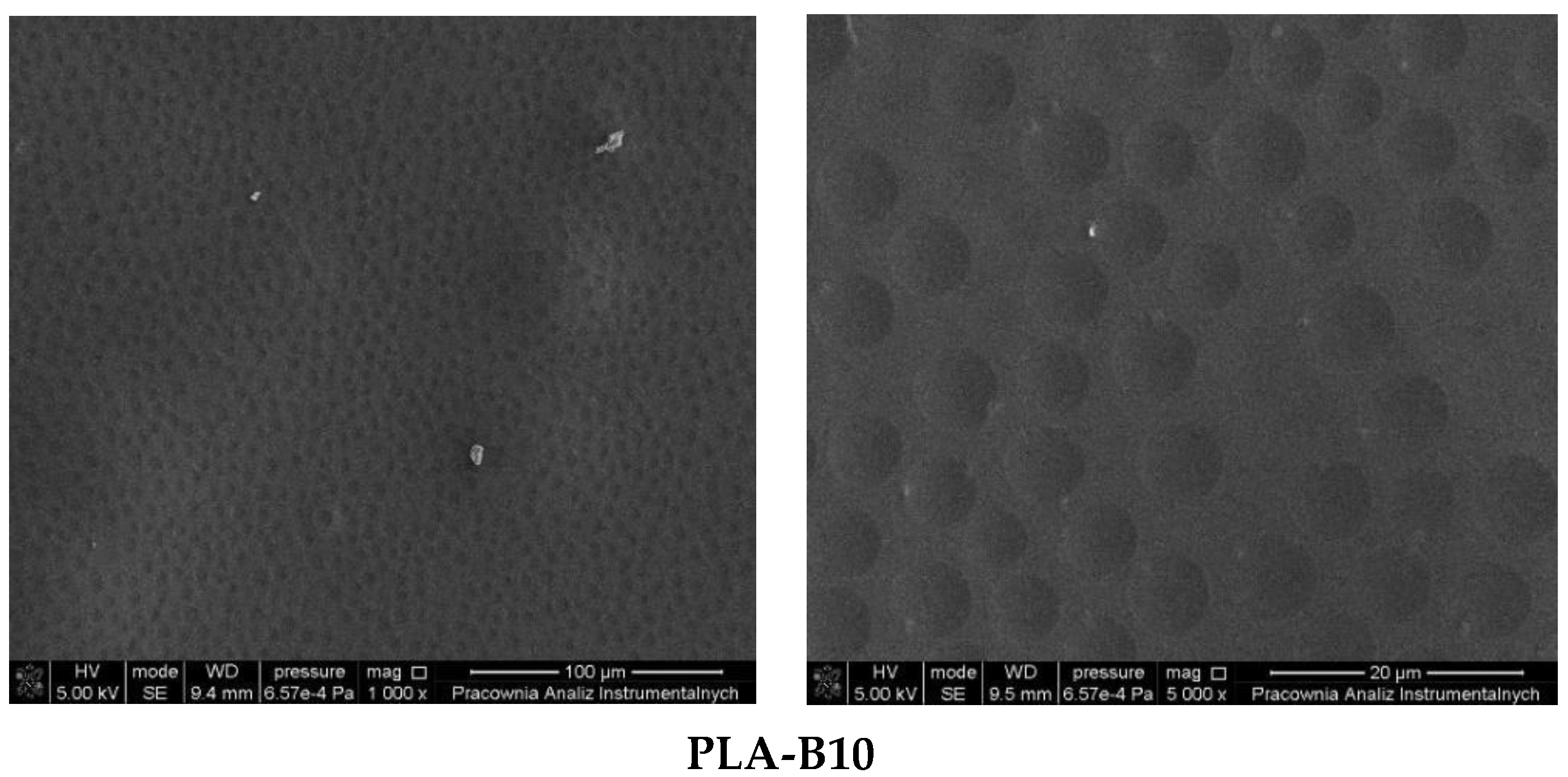
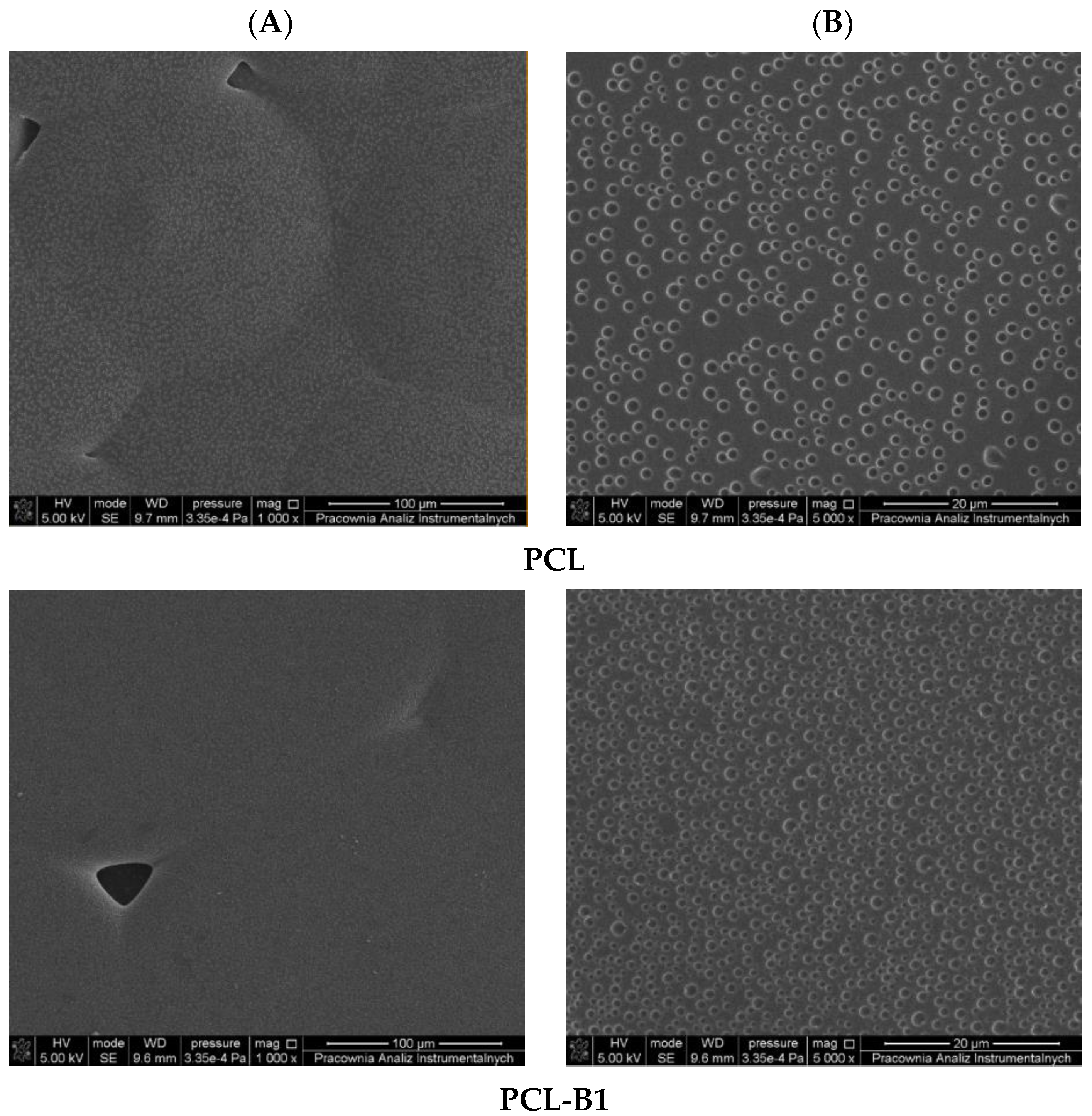
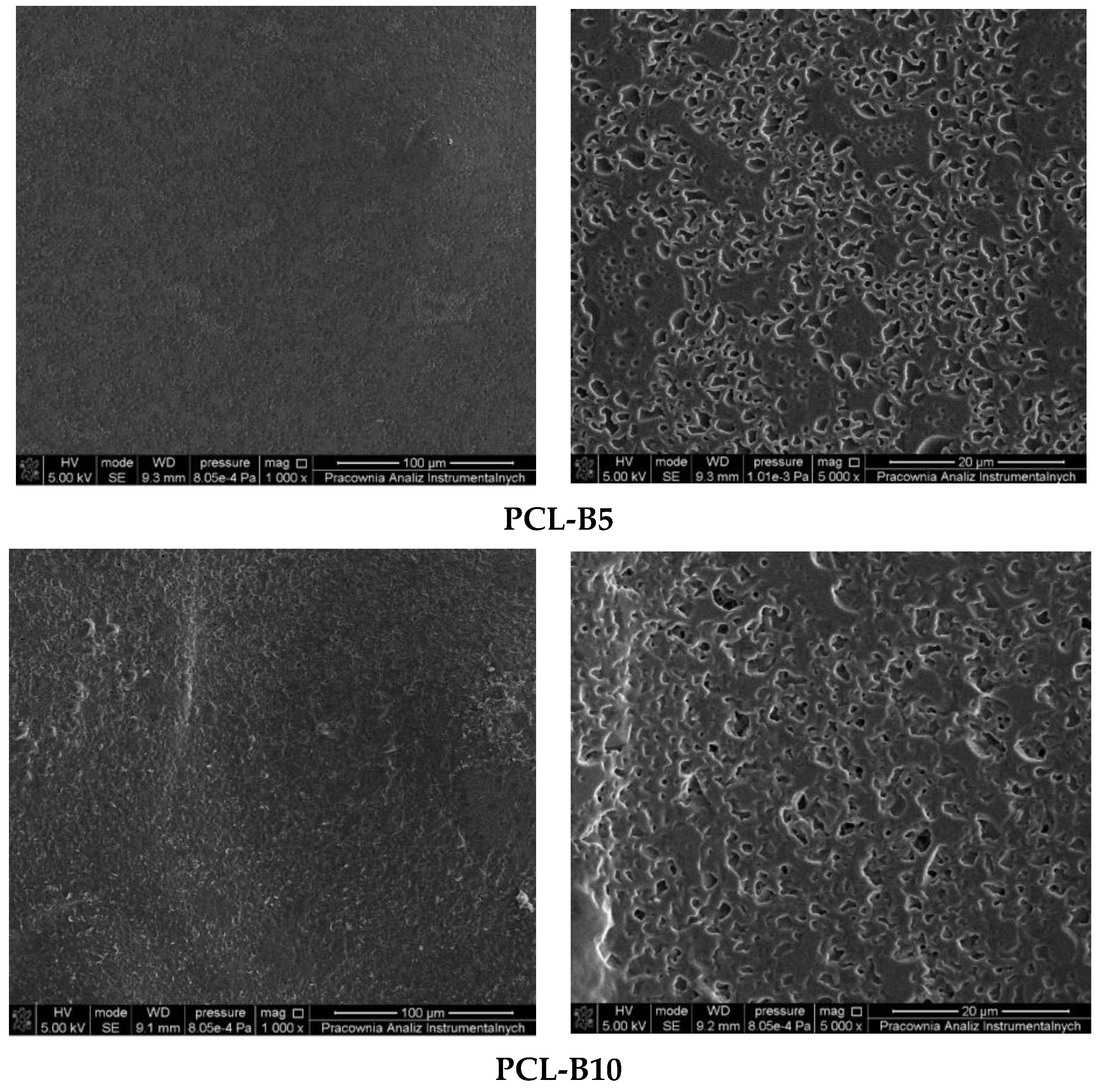
2.1. Description of the Produced Materials—Visual and Microscopic Analysis (SEM)
2.2. The Impact of Microorganisms from Various Environments on Materials Containing Birch Tar
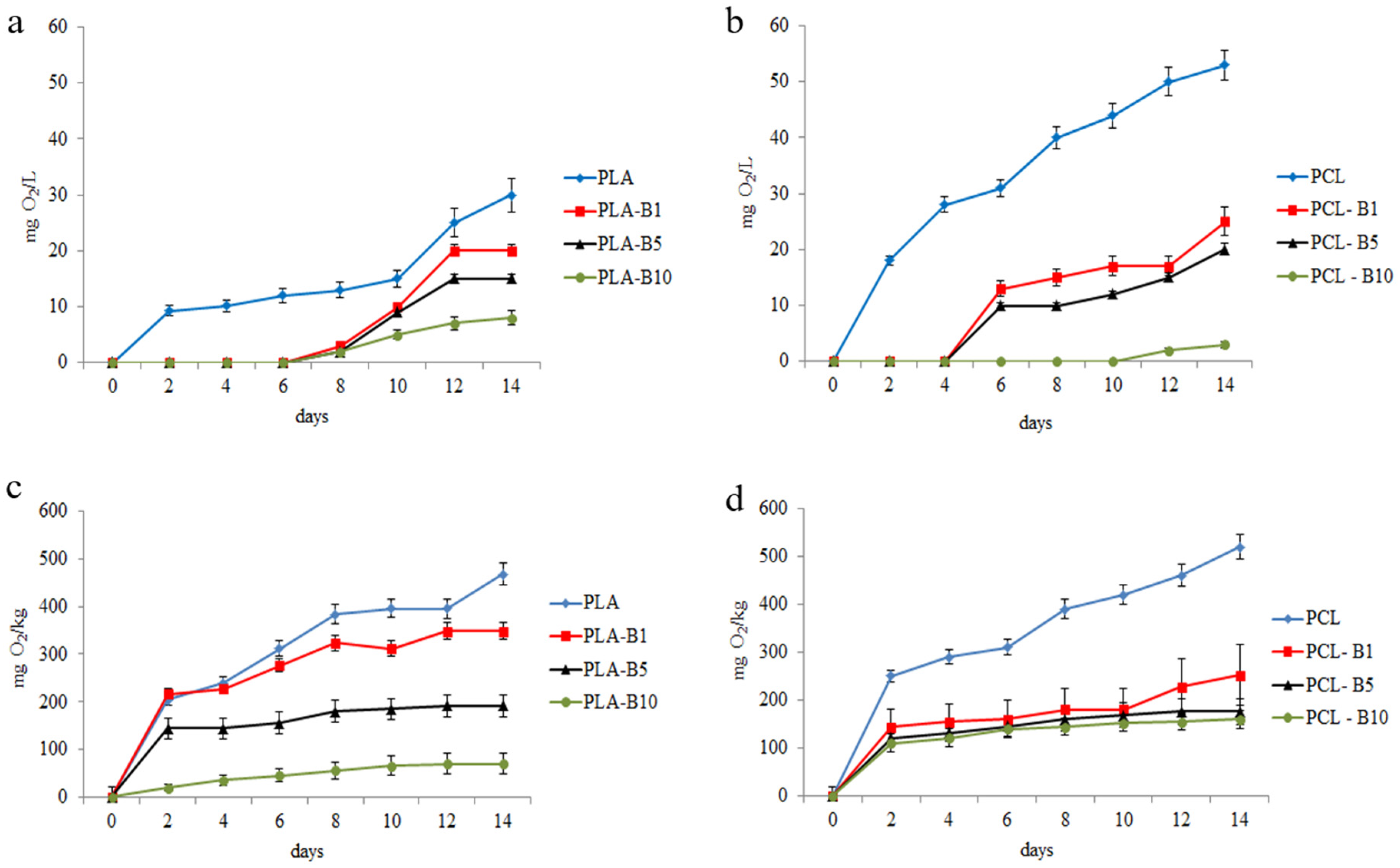
2.2.1. Biological Oxygen Demand
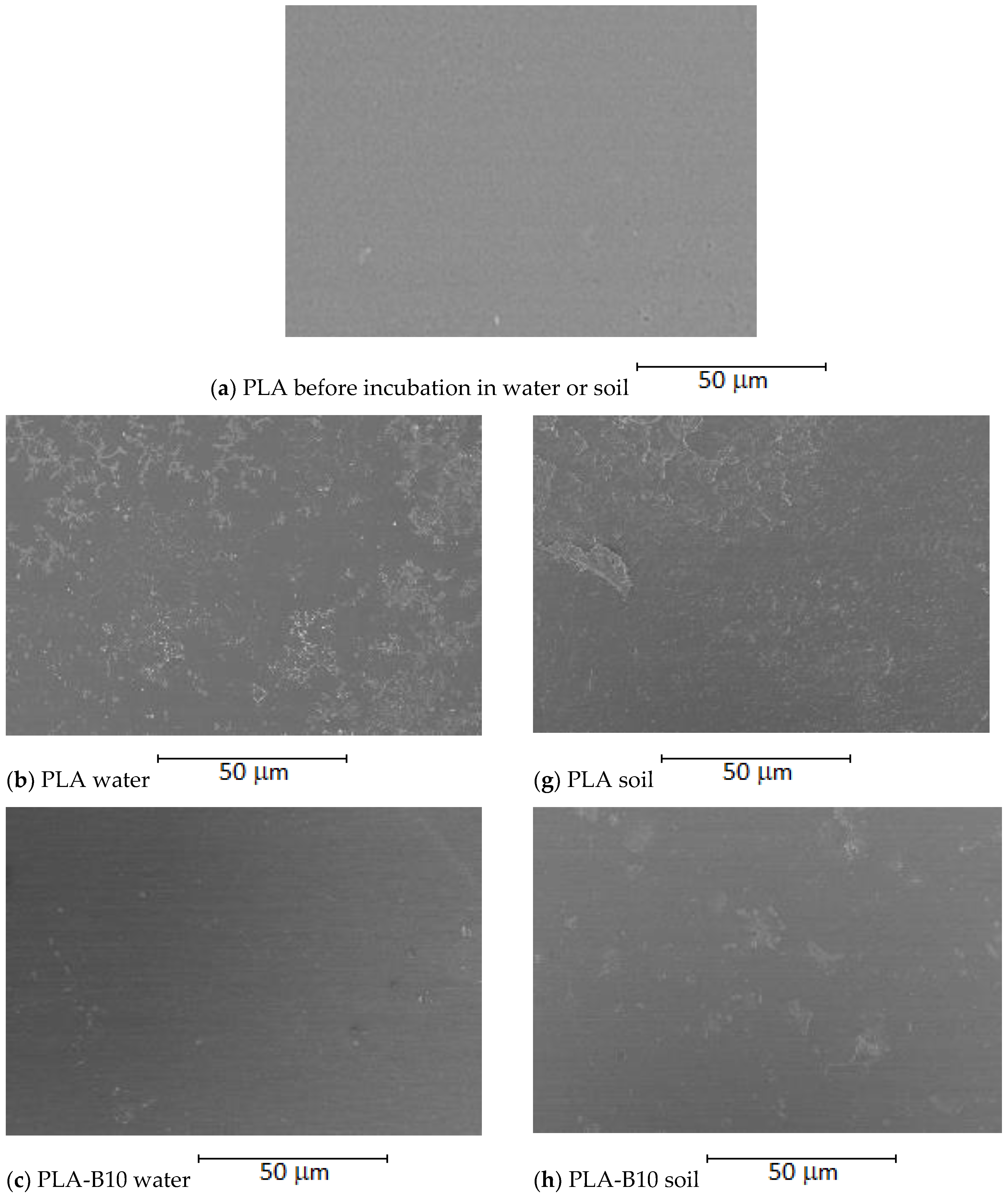
2.2.2. Biofilm Analysis Using SEM
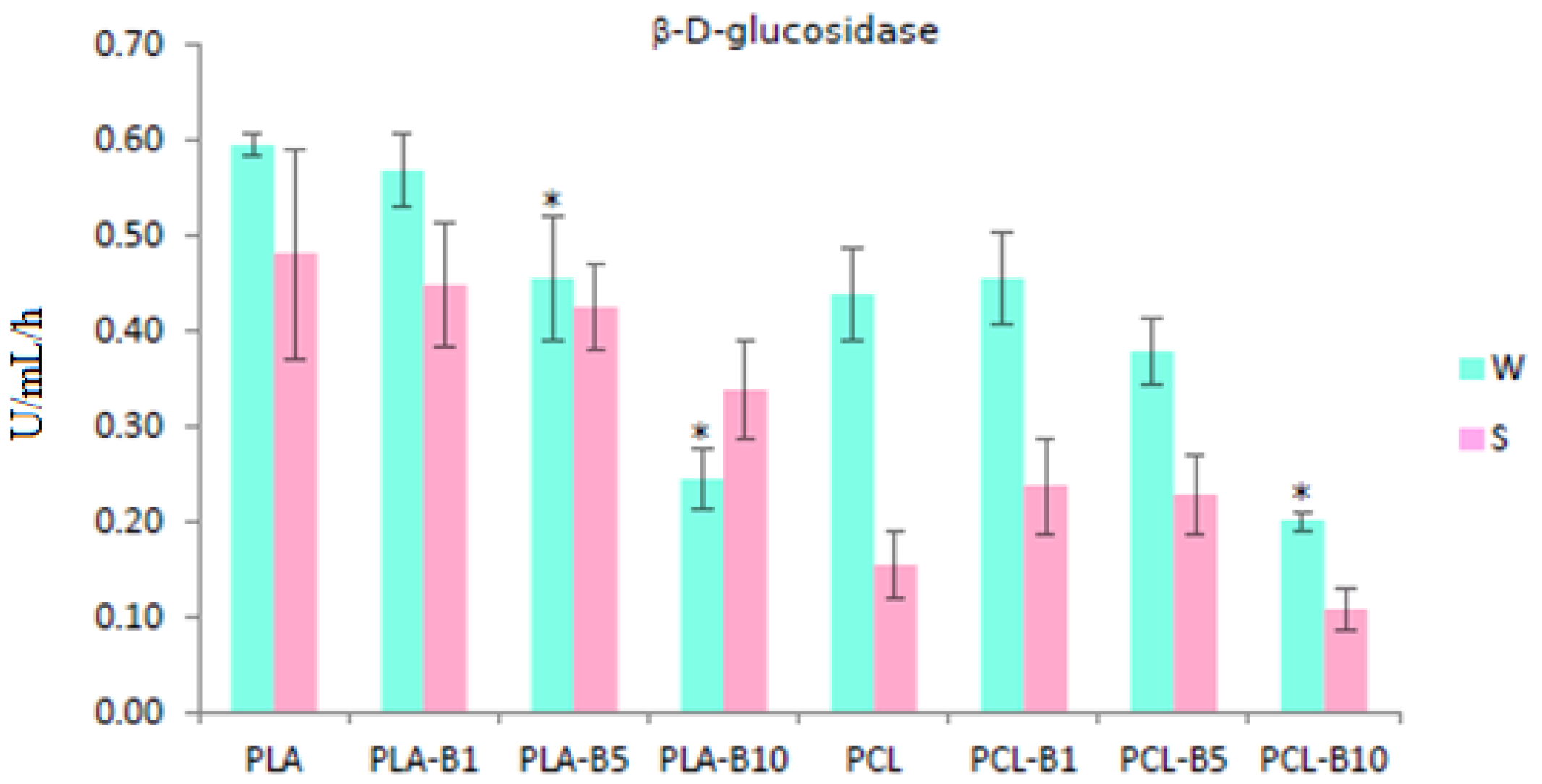
2.2.3. The Effect of Birch Tar Embedded into PLA and PCL on Bacterial Enzymatic Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Films
4.3. Biodegradation of Birch Tar-Containing PLA and PCL
4.4. Scanning Electron Microscopy
4.5. Determination of the Effect of Birch Tar on Enzymatic Activity of Biofilm
4.6. Statistical Analyses
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swiontek, B.M.; Walczak, M.; Kalwasińska, A.; Richert, A.; Świątczak, J.; Deja-Sikora, E.; Burkowska-But, A. Biofilm formation during biodegradation of polylactide, poly(3,4 hydroxybutyrate) and poly(ε-caprolactone) in activated sludge. Int. J. Biol. Macromol. 2020, 159, 539–546. [Google Scholar] [CrossRef]
- Richert, A.; Dąbrowska, G.B. Enzymatic degradation and biofilm formation during biodegradation of polylactide and polycaprolactone polymers in various environments. Int. J. Biol. Macromol. 2021, 15, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Sip, A.; Jusik, P. Introducing antimicrobial substances into packaging. Package 2009, 1, 42–47. (In Polish) [Google Scholar]
- Richert, A. Structural and barrier properties of polylactide films with bacteriocins after biodegradation in a compost extract. Przem. Chem. 2017, 96, 1313–1316. (In Polish) [Google Scholar]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated Type B gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Gibas, E.; Richert, A. Impact of bacteria on oxy-degradable films containing essential oils and silver, and copper nanoparticles. Przem. Chem. 2018, 97, 291–293. (In Polish) [Google Scholar]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Ye, H.; Shen, S.; Xu, J.; Lin, S.; Yuan, Y.; Jones, G.S. Synergistic interactions of cinnamaldehyde in combination with carvacrol against food-borne bacteria. Food Control 2013, 34, 619–623. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active chicken meat packaging based on polylactide films and bimetallic Ag–Cu nanoparticles and essential oil. J. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef]
- Wieczyńska, J.; Cavoski, I. Antimicrobial, antioxidant and sensory features of eugenol, carvacrol and trans-anethole in active packaging for organic ready-to-eat iceberg lettuce. Food Chem. 2018, 259, 251–260. [Google Scholar] [CrossRef]
- Richert, J.; Richert, A.; Żenkiewicz, M. Effect of silver nanoparticles on some physic-chemical and biological properties of polyethylene-matrix nanocomposites. Przem. Chem. 2012, 91, 1613–1616. (In Polish) [Google Scholar]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial efficacies of essential oils/nanoparticles incorporated polylactide films against L. monocytogenes and S. typhimurium on contaminated cheese. Int. J. Food Prop. 2017, 20, 53–67. [Google Scholar] [CrossRef]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef] [Green Version]
- Gijn, A.; Boon, J. Birch bark tar: A Neolithic settlement on the Dutch North Sea coast c. 3500 calbc. Archaeology 2006, 37, 261–266. [Google Scholar]
- Kozowyk, P.R.B.; van Gijn, A.L.; Langejans, G.H.J. Understanding preservation and identification biases of ancient adhesives through experimentation. Archaeol. Anthropol. Sci. 2020, 12, 209. [Google Scholar] [CrossRef]
- Schmidt, P.; Blessing, M.; Rageot, M.; Iovita, R.; Pfleging, J.; Nickel, G.; Righetti, L.; Tennie, C. Birch tar production does not prove Neanderthal behavioral complexity. Proc. Natl. Acad. Sci. USA 2019, 116, 17707–17711. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.Z.T.; Niemann, J.; Iversen, K.H. A 5700- year-old human genome and oral microbiome from chewed birch pitch. Nat. Commun. 2019, 10, 5520. [Google Scholar] [CrossRef] [Green Version]
- Kozowyk, P.R.B.; Soress, M.; Pomstra, D.; Langejans, G.H.J. Experimental methods for the Palaeolithic dry distillation of birch bark: Implications for the origin and development of Neandertal adhesive technology. Sci. Rep. 2017, 7, 8033. [Google Scholar] [CrossRef]
- Salonen, J.; Tiilikkala, K.; Ruttunen, P.; Lindqvist, I.; Lindqvist, B. Birch tar oil: A potential herbicide from the forests of Finland. In Proceedings of the Abstracts of the 5th International Weed Science Congress, Weeds Local Problems/Global Challenge, Vancouver, BC, Canada, 23–27 June 2008; pp. 286–287. [Google Scholar]
- Tiilikkala, K.; Fagernäs, L.; Tiilikkala, J. History and use of wood pyrolysis liquids as biocide and plant protection product. Open Agric. J. 2010, 4, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Tiilikkala, K.; Lindqvist, I.; Hagner, M.; Setälä, H.; Perdikis, D. Use of botanical pesticides in modern plant protection. In Pesticides in the Modern World—Pesticides Use and Management; Stoytcheva, M., Ed.; InTech: London, UK, 2011; pp. 259–272. [Google Scholar]
- Available online: https://pl.wikipedia.org/wiki/Dziegie%C4%87_brzozowy (accessed on 15 August 2021).
- Hagner, M.; Pasanen, T.; Lindqvist, B.; Lindqvist, I.; Tiilikkala, K.; Penttinen, O.-P.; Setala, H. Effects of birch tar oils on soil organisms and plants. Agric. Food Sci. 2010, 19, 13–23. [Google Scholar] [CrossRef]
- Hagner, M.; Penttinen, O.; Pasanen, T. Acute toxicity of birch tar oil on aquatic organisms. Agric. Food Sci. 2010, 19, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Richert, A.; Dąbrowska, G.B. Bacterial biofilm on PLA film and methods of its identification. Ecol. Quest. 2020, 31, 1–13. [Google Scholar] [CrossRef]
- Lindqvist, I.; Lindqvist, B.; Tiilikkala, K.; Hagner, M.; Penttinen, O.-P.; Pasanen, T.; Setaälaä, H. Birch tar oil is an effective mollusc repellent: Field and laboratory experiments using Arianta arbustorum (Gastropoda: Helicidae) and Arion lusitanicus (Gastropoda: Aronidae). Agric. Food Sci. 2010, 19, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhuikov, V.A.; Akoulina, E.A.; Chesnokova, D.V.; Wenhao, Y.; Makhina, T.K.; Demyanova, I.V.; Zhuikova, Y.V.; Voinova, V.V.; Belishev, N.V.; Surmenev, R.A.; et al. The growth of 3T3 fibroblasts on PHB, PLA and PHB/PLA blend films at different stages of their biodegradation in vitro. Polymers 2021, 13, 108. [Google Scholar] [CrossRef]
- Sukkhum, S.; Tokuyama, S.; Tamura, T.; Kitpreechavanich, V. A novel poly (L-lactide) degrading actinomycetes isolated from Thai forest soil, phylogenic relationship and the enzyme characterization. J. Gen. Appl. Microbiol. 2009, 55, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E.; Ortega, Z. Enzymatic degradation study of PLA-based composite scaffolds. Rev. Adv. Mater. Sci. 2020, 59, 170–175. [Google Scholar] [CrossRef]
- Richert, A.; Dąbrowska, G.B.; Dąbrowski, H.P. Bactericidal Polylactide Film and the Method of Its Preparation. U.S. Patent Aplication 433,979, 16 September 2020. (In Polish). [Google Scholar]
- Richert, A.; Olewnik-Kruszkowska, E.; Dąbrowska, G.B.; Dąbrowski, H.P. The role of birch tar in changes of physicochemical and biocidal properties of polylactide-based films. Int. J. Biol. Macromol. 2021, 255, 117527. [Google Scholar]
- Shimizu, I.; Isshiki, Y.; Nomura, H.; Sakuda, K.; Sakuma, K.; Kondo, S. The antibacterial activity of fragrance ingredients against Legionella pneumophila. Biol. Pharm. Bull. 2009, 32, 1114–1117. [Google Scholar] [CrossRef] [Green Version]
- Swiontek, B.M.; Lalke-Porczyk, E.; Donderski, W.; Walczak, M. Degradation of chitin in natural environment: Role of actinomycetes. Pol. J. Ecol. 2009, 57, 229–238. [Google Scholar]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliver. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, W.; Zhuang, Z.; Gao, M. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 2015, 6, 5848. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, X.; Yan, H.; Meng, R.; Zhang, Y.; Li, W.; Liu, Z.; Guo, N. Effect of tea tree oil on Staphylococcus aureus growth and enterotoxin production. Food Control 2016, 62, 257–263. [Google Scholar] [CrossRef]
- Khan, S.J.; Abidi, S.N.F.; Skinner, A.; Tian, Y.; Smith-Bolton, R.K. The Drosophila Duox maturation factor is a key component of a positive feedback loop that sustains regeneration signaling. PLoS Genet. 2017, 13, e1006937. [Google Scholar] [CrossRef] [Green Version]
- Janczak, K.; Dąbrowska, G.B.; Raszkowska-Kaczor, A.; Hrynkiewicz, K.; Richert, A. Biodegradation of the plastics PLA and PET in cultivated soil with the participation of microorganisms and plants. Int. Biodeterior. Biodegrad. 2020, 155, 105087. [Google Scholar] [CrossRef]
- Janczak, K.; Hrynkiewicz, K.; Znajewska, Z.; Dąbrowska, G. Use of rhizosphere microorganisms in the biodegradation of PLA and PET polymers in compost soil. Int. Biodeterior. Biodegrad. 2018, 130, 65–75. [Google Scholar] [CrossRef]
- Siracusa, V. Microbial degradation of synthetic biopolymers waste. Polymers 2019, 11, 1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czernik, S. Environment, health and safety. In Fast Pyrolysis of Biomass, A Handbook; Bridgewater, A.V., Ed.; CPL Press: Newbury, UK, 2002; pp. 115–118. [Google Scholar]
- Rohn, S.; Rawel, H.M.; Kroll, J. Inhibitory effects of plant phenols on the activity of selected enzymes. J. Agric. Food Chem. 2002, 50, 3566–3571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Sun, Z.; Wang, T.; Yang, M.; Liu, M.; Zhang, J.; Li, Y. Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb. Pathog. 2020, 139, 103924. [Google Scholar] [CrossRef]
- Ribes, S.; Ruiz-Rico, M.; Pérez-Esteve, É.; Fuentes, A.; Barat, J.M. Enhancing the antimicrobial activity of eugenol, carvacrol and vanillin immobilised on silica supports against Escherichia coli or Zygosaccharomyces rouxii in fruit juices by their binary combinations. LWT 2019, 113, 108326. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E. Influence of the type of buffer solution on thermal and structural properties of polylactide-based composites. Pol. Degrad. Stab. 2016, 129, 87–95. [Google Scholar] [CrossRef]
- Tarach, I.; Olewnik-Kruszkowska, E.; Richert, A.; Gierszewska, M.; Rudawska, A. Influence of tea tree essential oil and poly(ethylene glycol) on antibacterial and physicochemical properties of polylactide-based films. Materials 2020, 13, 4953. [Google Scholar] [CrossRef]
- Walczak, M.; Swiontek, B.M.; Richert, A.; Kalwasińska, A. The effect of polyhexamethylene guanidine hydrochlorideon biofilm formation on polylactide and polyhydroxybutyrate composites. Int. Biodeterior. Biodegrad. 2015, 98, 1–5. [Google Scholar] [CrossRef]
- Platen, H.; Wirtz, A. Measurement of the Respiration Activity of Soils Using the Oxitop Control Measuring System. Basic Principles and Process Characteristic Quantities; WTW (Wissenschaftlich-Technische Werkstätten): Weilheim, Germany, 1999. [Google Scholar]
- Niemi, T.T.; Kuitunen, A.H. Artificial colloids impair haemostasis. An in vitro study using thromboelastometry coagulation analysis. Acta Anaesthesiol. Scand. 2005, 49, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Adam, G.; Duncan, H. Development of sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soil. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.; Azam, F. Periplasmatic aminopeptidase and alkaline phosphatase activities in a marine bacterium: Implications for substrate processing in the sea. Mar. Ecol. Prog. Ser. 1993, 92, 89–97. [Google Scholar] [CrossRef]


| Film and Environment | Parameter | Lipase | Amino-Peptidase | Esterase | α-D-Gucosidase | β-D-Glucosidase |
|---|---|---|---|---|---|---|
| PLA water | Slope a: | −0.46306 | −0.44032 | −0.21339 | −0.03393 | −0.03484 |
| Intercept b: | 10.365 | 8.8021 | 3.2944 | 0.83987 | 0.60352 | |
| r2: | 0.89 | 0.879 | 0.96 | 0.78 | 0.93 | |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| PCL water | Slope a: | −0.45022 | −0.22081 | −0.28022 | −0.06753 | −0.02468 |
| Intercept b: | 11.467 | 8.5607 | 3.9009 | 0.99011 | 0.46538 | |
| r2: | 0.91 | 0.90 | 0.95 | 0.91 | 0.85 | |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| PLA soil | Slope a: | −0.52075 | −0.20855 | −0.14661 | −0.02737 | −0.01317 |
| Intercept b: | 11.191 | 5.345 | 2.1848 | 0.57113 | 0.47435 | |
| r2: | 0.97 | 0.83 | 0.96 | 0.65 | 0.43 | |
| p | <0.0001 | <0.0001 | <0.0001 | <0.01 | <0.05 | |
| Slope a: | −0.3543 | −0.23425 | −0.16446 | −0.0178 | −0.00737 | |
| PCL soil | Intercept b: | 11.784 | 7.8487 | 3.6212 | 0.34535 | 0.2103 |
| r2: | 0.89 | 0.80 | 0.72 | 0.88 | 0.22 | |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.125 |
| Enzyme | Mann-Whitney U | p | |
|---|---|---|---|
| PLA vs. PCL | Lipase | 178.5 | <0.05 |
| Amino | 122.0 | <0.01 | |
| Esterase | 135.5 | <0.01 | |
| α-D-Glucosidase | 236.0 | ns | |
| β-D-Glucosidase | 105.0 | <0.001 | |
| Water vs. Soil | Lipase | 237.0 | ns |
| Amino | 139.0 | <0.01 | |
| Esterase | 231.5 | ns | |
| α-D-Glucosidase | 78.0 | <0.0001 | |
| β-D-Glucosidase | 158.5 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richert, A.; Kalwasińska, A.; Brzezinska, M.S.; Dąbrowska, G.B. Biodegradability of Novel Polylactide and Polycaprolactone Materials with Bacteriostatic Properties Due to Embedded Birch Tar in Different Environments. Int. J. Mol. Sci. 2021, 22, 10228. https://doi.org/10.3390/ijms221910228
Richert A, Kalwasińska A, Brzezinska MS, Dąbrowska GB. Biodegradability of Novel Polylactide and Polycaprolactone Materials with Bacteriostatic Properties Due to Embedded Birch Tar in Different Environments. International Journal of Molecular Sciences. 2021; 22(19):10228. https://doi.org/10.3390/ijms221910228
Chicago/Turabian StyleRichert, Agnieszka, Agnieszka Kalwasińska, Maria Swiontek Brzezinska, and Grażyna B. Dąbrowska. 2021. "Biodegradability of Novel Polylactide and Polycaprolactone Materials with Bacteriostatic Properties Due to Embedded Birch Tar in Different Environments" International Journal of Molecular Sciences 22, no. 19: 10228. https://doi.org/10.3390/ijms221910228







