Looking for In Vitro Models for Retinal Diseases
Abstract
:1. Introduction
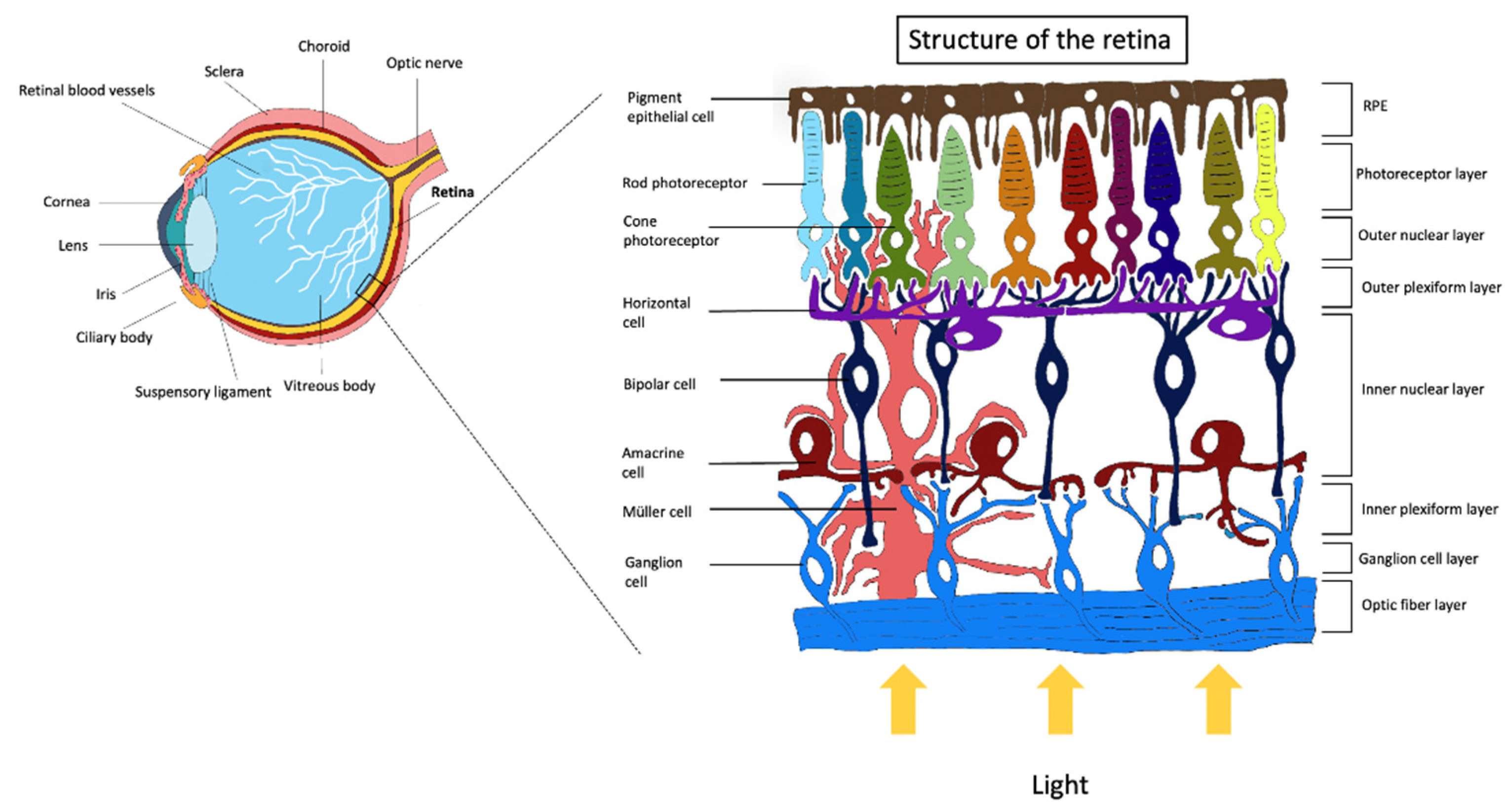
Retinal Cells and Function
2. Retinal Pathologies and In Vivo Models
2.1. Age-Related Macular Degeneration and Diabetic Retinopathy
2.2. Glaucoma
2.3. Retinitis Pigmentosa
2.4. Retinoblastoma
3. From Animal Studies to In Vitro Models
4. Alternative 2D In Vitro Models
4.1. Neoangiogenesis In Vitro Models
4.2. Blood–Retinal Barrier Models
4.3. Retinal Pigmented Epithelium Cultures
4.4. In Vitro Models of AMD
4.5. In Vitro Models of Glaucoma
4.6. 2D Models of Retinoblastoma
5. Alternative 3D In Vitro Models
5.1. 3D Models for the Posterior Segment of the Eye and RP
5.2. 3D Models for Retinoblastoma
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pearring, J.N.; Salinas, R.Y.; Baker, S.A.; Arshavsky, V.Y. Protein Sorting, Targeting and Trafficking in Photoreceptor Cells. Prog. Retin. Eye Res. 2013, 36, 24–51. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.P.; Herzlich, A.A.; Sauer, T.; Chan, C.-C. Retinal Anatomy and Pathology. Dev. Ophthalmol. 2016, 55, 7–17. [Google Scholar] [CrossRef]
- Shah, M.; Cabrera-Ghayouri, S.; Christie, L.-A.; Held, K.S.; Viswanath, V. Translational Preclinical Pharmacologic Disease Models for Ophthalmic Drug Development. Pharm. Res. 2019, 36, 58. [Google Scholar] [CrossRef] [Green Version]
- Schnichels, S.; Paquet-Durand, F.; Löscher, M.; Tsai, T.; Hurst, J.; Joachim, S.C.; Klettner, A. Retina in a Dish: Cell Cultures, Retinal Explants and Animal Models for Common Diseases of the Retina. Prog. Retin. Eye Res. 2021, 81, 100880. [Google Scholar] [CrossRef]
- Masland, R.H. The Fundamental Plan of the Retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef]
- Nickla, D.L.; Wallman, J. The Multifunctional Choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.M.F.; Bergen, A.A.B. The Dynamic Nature of Bruch’s Membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef]
- Fuhrmann, S.; Zou, C.; Levine, E.M. Retinal Pigment Epithelium Development, Plasticity, and Tissue Homeostasis. Exp. Eye Res. 2014, 123, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The Retinal Pigment Epithelium in Health and Disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Tarau, I.-S.; Berlin, A.; Curcio, C.A.; Ach, T. The Cytoskeleton of the Retinal Pigment Epithelium: From Normal Aging to Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 3578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilha, V.L.; Rayborn, M.E.; Bhattacharya, S.K.; Gu, X.; Crabb, J.S.; Crabb, J.W.; Hollyfield, J.G. The Retinal Pigment Epithelium Apical Microvilli and Retinal Function. Adv. Exp. Med. Biol. 2006, 572, 519–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C.; Charniga, C.; Temple, S.; Finnemann, S.C. Quantified F-Actin Morphology Is Predictive of Phagocytic Capacity of Stem Cell-Derived Retinal Pigment Epithelium. Stem Cell Rep. 2018, 10, 1075–1087. [Google Scholar] [CrossRef] [Green Version]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-Retinal Barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.; Hopkins, A.; Hudson, N.; Campbell, M. Tight Junctions of the Outer Blood Retina Barrier. Int. J. Mol. Sci. 2019, 21, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istrate, M.; Vlaicu, B.; Poenaru, M.; Hasbei-Popa, M.; Salavat, M.C.; Iliescu, D.A. Photoprotection Role of Melanin in the Human Retinal Pigment Epithelium. Imaging Techniques for Retinal Melanin. Rom. J. Ophthalmol. 2020, 64, 100–104. [Google Scholar] [CrossRef]
- Salesse, C. Physiology of the visual retinal signal: From phototransduction to the visual cycle. J. Fr. Ophtalmol. 2017, 40, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Euler, T.; Haverkamp, S.; Schubert, T.; Baden, T. Retinal Bipolar Cells: Elementary Building Blocks of Vision. Nat. Rev. Neurosci. 2014, 15, 507–519. [Google Scholar] [CrossRef]
- Chapot, C.A.; Euler, T.; Schubert, T. How Do Horizontal Cells “talk” to Cone Photoreceptors? Different Levels of Complexity at the Cone-Horizontal Cell Synapse. J. Physiol. 2017, 595, 5495–5506. [Google Scholar] [CrossRef]
- Detwiler, P.B. Phototransduction in Retinal Ganglion Cells. Yale J. Biol. Med. 2018, 91, 49–52. [Google Scholar]
- Ingram, N.T.; Sampath, A.P.; Fain, G.L. Why Are Rods More Sensitive than Cones? J. Physiol. 2016, 594, 5415–5426. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.P.; Boehm, A.E.; Tuten, W.S.; Roorda, A. Spatial Summation of Individual Cones in Human Color Vision. PLoS ONE 2019, 14, e0211397. [Google Scholar] [CrossRef] [Green Version]
- Nawy, S.; von Gersdorff, H. Bipolar Cells in the Vertebrate Retina: From Form to Function. Introduction. Vis. Neurosci. 2011, 28, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-Neuron Interactions in the Mammalian Retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazawa, M.; Hara, A.; Ishiguro, S.-I. Optical Coherence Tomography of Animal Models of Retinitis Pigmentosa: From Animal Studies to Clinical Applications. BioMed Res. Int. 2019, 2019, 8276140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.-H.; Wang, B.; Lu, Y.; Son, T.; Yao, X. Functional Optical Coherence Tomography Enables in Vivo Optoretinography of Photoreceptor Dysfunction Due to Retinal Degeneration. Biomed. Opt. Express 2020, 11, 5306–5320. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-Related Macular Degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Rita Machado, A.; Carvalho Pereira, A.; Ferreira, F.; Ferreira, S.; Quendera, B.; Silva, E.; Castelo-Branco, M. Structure-Function Correlations in Retinitis Pigmentosa Patients with Partially Preserved Vision: A Voxel-Based Morphometry Study. Sci. Rep. 2017, 7, 11411. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.-Y.; Cringle, S.J.; Yu, P.K.; Balaratnasingam, C.; Mehnert, A.; Sarunic, M.V.; An, D.; Su, E.-N. Retinal Capillary Perfusion: Spatial and Temporal Heterogeneity. Prog. Retin. Eye Res. 2019, 70, 23–54. [Google Scholar] [CrossRef]
- Joyal, J.-S.; Gantner, M.L.; Smith, L.E.H. Retinal Energy Demands Control Vascular Supply of the Retina in Development and Disease: The Role of Neuronal Lipid and Glucose Metabolism. Prog. Retin. Eye Res. 2018, 64, 131–156. [Google Scholar] [CrossRef]
- Mrugacz, M.; Bryl, A.; Zorena, K. Retinal Vascular Endothelial Cell Dysfunction and Neuroretinal Degeneration in Diabetic Patients. J. Clin. Med. 2021, 10, 458. [Google Scholar] [CrossRef]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 591. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Adeosun, A.M.; Akinloye, O.A. Alloxan-Induced Diabetes, a Common Model for Evaluating the Glycemic-Control Potential of Therapeutic Compounds and Plants Extracts in Experimental Studies. Medicina 2017, 53, 365–374. [Google Scholar] [CrossRef]
- Wu, K.K.; Huan, Y. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2008, Chapter 5. 1–14. [Google Scholar] [CrossRef]
- Olivares, A.M.; Althoff, K.; Chen, G.F.; Wu, S.; Morrisson, M.A.; DeAngelis, M.M.; Haider, N. Animal Models of Diabetic Retinopathy. Curr. Diab. Rep. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal Pigment Epithelium and Age-Related Macular Degeneration: A Review of Major Disease Mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal Models of Age Related Macular Degeneration. Mol. Aspects Med. 2012, 33, 487–509. [Google Scholar] [CrossRef] [Green Version]
- Rastoin, O.; Pagès, G.; Dufies, M. Experimental Models in Neovascular Age Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4627. [Google Scholar] [CrossRef]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.-L.A.; Struman, I.; Sounni, N.E.; Rozet, E.; de Tullio, P.; Foidart, J.M.; et al. Laser-Induced Choroidal Neovascularization Model to Study Age-Related Macular Degeneration in Mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef]
- Zipfel, P.F.; Lauer, N.; Skerka, C. The Role of Complement in AMD. Adv. Exp. Med. Biol. 2010, 703, 9–24. [Google Scholar] [CrossRef]
- Quigley, H.A.; McKinnon, S.J.; Zack, D.J.; Pease, M.E.; Kerrigan-Baumrind, L.A.; Kerrigan, D.F.; Mitchell, R.S. Retrograde Axonal Transport of BDNF in Retinal Ganglion Cells Is Blocked by Acute IOP Elevation in Rats. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3460–3466. [Google Scholar]
- Biswas, S.; Wan, K.H. Review of Rodent Hypertensive Glaucoma Models. Acta Ophthalmol. 2019, 97, e331–e340. [Google Scholar] [CrossRef]
- Harada, C.; Kimura, A.; Guo, X.; Namekata, K.; Harada, T. Recent Advances in Genetically Modified Animal Models of Glaucoma and Their Roles in Drug Repositioning. Br. J. Ophthalmol. 2019, 103, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, K.A.; Harder, J.M.; Williams, P.A.; Rausch, R.L.; Kiernan, A.E.; Nair, K.S.; Anderson, M.G.; John, S.W.M.; Howell, G.R.; Libby, R.T. Using Genetic Mouse Models to Gain Insight into Glaucoma: Past Results and Future Possibilities. Exp. Eye Res. 2015, 141, 42–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Grinchuk, O.; Tomarev, S.I. Transgenic Mice Expressing the Tyr437His Mutant of Human Myocilin Protein Develop Glaucoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1932–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal Models of Glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struebing, F.L.; Geisert, E.E. What Animal Models Can Tell Us About Glaucoma. Prog. Mol. Biol. Transl. Sci. 2015, 134, 365–380. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis Pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Mir, T.A. The Mechanism of Cone Cell Death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 62, 24–37. [Google Scholar] [CrossRef]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-Syndromic Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Tsang, S.H.; Sharma, T. Autosomal Dominant Retinitis Pigmentosa. Adv. Exp. Med. Biol. 2018, 1085, 69–77. [Google Scholar] [CrossRef]
- Tsang, S.H.; Sharma, T. X-Linked Retinitis Pigmentosa. Adv. Exp. Med. Biol. 2018, 1085, 31–35. [Google Scholar] [CrossRef]
- Kalloniatis, M.; Nivison-Smith, L.; Chua, J.; Acosta, M.L.; Fletcher, E.L. Using the Rd1 Mouse to Understand Functional and Anatomical Retinal Remodelling and Treatment Implications in Retinitis Pigmentosa: A Review. Exp. Eye Res. 2016, 150, 106–121. [Google Scholar] [CrossRef]
- Caruso, R.C.; Aleman, T.S.; Cideciyan, A.V.; Roman, A.J.; Sumaroka, A.; Mullins, C.L.; Boye, S.L.; Hauswirth, W.W.; Jacobson, S.G. Retinal Disease in Rpe65-Deficient Mice: Comparison to Human Leber Congenital Amaurosis Due to RPE65 Mutations. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5304. [Google Scholar] [CrossRef] [Green Version]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal Degeneration Mutants in the Mouse. Vis. Res. 2002, 42, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Fahim, A. Retinitis Pigmentosa: Recent Advances and Future Directions in Diagnosis and Management. Curr. Opin. Pediatr. 2018, 30, 725–733. [Google Scholar] [CrossRef]
- Dimaras, H.; Corson, T.W. Retinoblastoma, the Visible CNS Tumor: A Review. J. Neurosci. Res. 2019, 97, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Vélez-Cruz, R.; Johnson, D.G. The Retinoblastoma (RB) Tumor Suppressor: Pushing Back against Genome Instability on Multiple Fronts. Int. J. Mol. Sci. 2017, 18, 1776. [Google Scholar] [CrossRef]
- Macpherson, D. Insights from Mouse Models into Human Retinoblastoma. Cell Div. 2008, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, R.M.; Kaliki, S.; Vemuganti, G.K. Animal Models in Retinoblastoma Research. Saudi J. Ophthalmol. 2013, 27, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Donovan, S.L.; Schweers, B.; Martins, R.; Johnson, D.; Dyer, M.A. Compensation by Tumor Suppressor Genes during Retinal Development in Mice and Humans. BMC Biol. 2006, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschulakow, A.V.; Schraermeyer, U.; Rodemann, H.P.; Julien-Schraermeyer, S. Establishment of a Novel Retinoblastoma (Rb) Nude Mouse Model by Intravitreal Injection of Human Rb Y79 Cells—Comparison of in Vivo Analysis versus Histological Follow Up. Biol. Open 2016, 5, 1625–1630. [Google Scholar] [CrossRef] [Green Version]
- Cassoux, N.; Thuleau, A.; Assayag, F.; Aerts, I.; Decaudin, D. Establishment of an Orthotopic Xenograft Mice Model of Retinoblastoma Suitable for Preclinical Testing. Ocul. Oncol. Pathol. 2015, 1, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benavente, C.A.; Dyer, M.A. Genetically Engineered Mouse and Orthotopic Human Tumor Xenograft Models of Retinoblastoma. Methods Mol. Biol. 2015, 1267, 307–317. [Google Scholar] [CrossRef]
- Krebs, M.P.; Collin, G.B.; Hicks, W.L.; Yu, M.; Charette, J.R.; Shi, L.Y.; Wang, J.; Naggert, J.K.; Peachey, N.S.; Nishina, P.M. Mouse Models of Human Ocular Disease for Translational Research. PLoS ONE 2017, 12, e0183837. [Google Scholar] [CrossRef] [Green Version]
- Alex, A.F.; Alnawaiseh, M.; Heiduschka, P.; Eter, N. Retinal Fundus Imaging in Mouse Models of Retinal Diseases. Methods Mol. Biol. 2019, 1834, 253–283. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.L.; Jobling, A.I.; Vessey, K.A.; Luu, C.; Guymer, R.H.; Baird, P.N. Animal Models of Retinal Disease. Prog. Mol. Biol. Transl. Sci. 2011, 100, 211–286. [Google Scholar] [CrossRef]
- Denayer, T.; Stöhr, T.; Roy, M.V. Animal Models in Translational Medicine: Validation and Prediction. Eur. J. Mol. Clin. Med. 2014, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Marmorstein, A.D.; Marmorstein, L.Y. The Challenge of Modeling Macular Degeneration in Mice. Trends Genet. 2007, 23, 225–231. [Google Scholar] [CrossRef]
- Volland, S.; Esteve-Rudd, J.; Hoo, J.; Yee, C.; Williams, D.S. A Comparison of Some Organizational Characteristics of the Mouse Central Retina and the Human Macula. PLoS ONE 2015, 10, e0125631. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in Vitro Experiments to in Vivo and Clinical Studies; Pros and Cons. Curr. Drug Discov. Technol. 2015, 12, 218–224. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Witmer, A.N.; Vrensen, G.F.J.M.; van Noorden, C.J.F.; Schlingemann, R.O. Vascular Endothelial Growth Factors and Angiogenesis in Eye Disease. Prog. Retin. Eye Res. 2003, 22, 1–29. [Google Scholar] [CrossRef]
- Abcouwer, S.F. Angiogenic Factors and Cytokines in Diabetic Retinopathy. J. Clin. Cell Immunol. 2013, 7 (Suppl. 1), 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bressler, S.B. Introduction: Understanding the Role of Angiogenesis and Antiangiogenic Agents in Age-Related Macular Degeneration. Ophthalmology 2009, 116, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, J.; Wertheimer, C.; Wolf, A.; Liegl, R.; Priglinger, C.; Priglinger, S.; Eibl-Lindner, K. Combined VEGF and PDGF Inhibition for Neovascular AMD: Anti-Angiogenic Properties of Axitinib on Human Endothelial Cells and Pericytes in Vitro. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Van der Giet, M.; Henkel, C.; Schuchardt, M.; Tolle, M. Anti-VEGF Drugs in Eye Diseases: Local Therapy with Potential Systemic Effects. Curr. Pharm. Des. 2015, 21, 3548–3556. [Google Scholar] [CrossRef] [PubMed]
- Eyre, J.J.; Williams, R.L.; Levis, H.J. A Human Retinal Microvascular Endothelial-Pericyte Co-Culture Model to Study Diabetic Retinopathy in Vitro. Exp. Eye Res. 2020, 201, 108293. [Google Scholar] [CrossRef] [PubMed]
- Stryker, Z.I.; Rajabi, M.; Davis, P.J.; Mousa, S.A. Evaluation of Angiogenesis Assays. Biomedicines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, C.J.; Nguyen, M. Human Microvascular Endothelial Cells Differ from Macrovascular Endothelial Cells in Their Expression of Matrix Metalloproteinases. Int. J. Biochem. Cell Biol. 1997, 29, 1167–1177. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and Neutralization of Vascular Endothelial Growth Factor (VEGF) and Related Ligands by VEGF Trap, Ranibizumab and Bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef] [Green Version]
- Djigo, A.D.; Bérubé, J.; Landreville, S.; Proulx, S. Characterization of a Tissue-Engineered Choroid. Acta Biomaterialia 2019, 84, 305–316. [Google Scholar] [CrossRef]
- Shokoohmand, A.; Jeon, J.E.; Theodoropoulos, C.; Baldwin, J.G.; Hutmacher, D.W.; Feigl, B. A Novel 3D Cultured Model for Studying Early Changes in Age-Related Macular Degeneration. Macromol. Biosci. 2017, 17, 1700221. [Google Scholar] [CrossRef]
- Eshaq, R.S.; Aldalati, A.M.Z.; Alexander, J.S.; Harris, N.R. Diabetic Retinopathy: Breaking the Barrier. Pathophysiology 2017, 24, 229–241. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Caruso, G.; Caraci, F.; Giblin, F.J.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. A New Human Blood-Retinal Barrier Model Based on Endothelial Cells, Pericytes, and Astrocytes. Int. J. Mol. Sci. 2020, 21, 1636. [Google Scholar] [CrossRef] [Green Version]
- Castelli, V.; Paladini, A.; d’Angelo, M.; Allegretti, M.; Mantelli, F.; Brandolini, L.; Cocchiaro, P.; Cimini, A.; Varrassi, G. Taurine and Oxidative Stress in Retinal Health and Disease. CNS Neurosci. Ther. 2021, 27, 403–412. [Google Scholar] [CrossRef]
- Hamilton, R.D.; Leach, L. Isolation and Properties of an in Vitro Human Outer Blood-Retinal Barrier Model. Methods Mol. Biol. 2011, 686, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Bhutto, I.; Lutty, G. Understanding Age-Related Macular Degeneration (AMD): Relationships between the Photoreceptor/Retinal Pigment Epithelium/Bruch’s Membrane/Choriocapillaris Complex. Mol. Aspects Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.R.; Truong, Y.B.; O’Brien, C.M.; Glattauer, V. Bio-Inspired Human in Vitro Outer Retinal Models: Bruch’s Membrane and Its Cellular Interactions. Acta Biomater. 2020, 104, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shadforth, A.M.A.; George, K.A.; Kwan, A.S.; Chirila, T.V.; Harkin, D.G. The Cultivation of Human Retinal Pigment Epithelial Cells on Bombyx Mori Silk Fibroin. Biomaterials 2012, 33, 4110–4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galloway, C.A.; Dalvi, S.; Shadforth, A.M.A.; Suzuki, S.; Wilson, M.; Kuai, D.; Hashim, A.; MacDonald, L.A.; Gamm, D.M.; Harkin, D.G.; et al. Characterization of Human IPSC-RPE on a Prosthetic Bruch’s Membrane Manufactured From Silk Fibroin. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2792–2800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nebel, C.; Aslanidis, A.; Rashid, K.; Langmann, T. Activated Microglia Trigger Inflammasome Activation and Lysosomal Destabilization in Human RPE Cells. Biochem. Biophys. Res. Commun. 2017, 484, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhao, L.; Fontainhas, A.M.; Fariss, R.N.; Wong, W.T. Microglia in the Mouse Retina Alter the Structure and Function of Retinal Pigmented Epithelial Cells: A Potential Cellular Interaction Relevant to AMD. PLoS ONE 2009, 4, e7945. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-Z.; Song, Z.; Fu, S.; Zhu, M.; Le, Y.-Z. RPE Barrier Breakdown in Diabetic Retinopathy: Seeing Is Believing. J. Ocul. Biol. Dis. Inform. 2011, 4, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fronk, A.H.; Vargis, E. Methods for Culturing Retinal Pigment Epithelial Cells: A Review of Current Protocols and Future Recommendations. J. Tissue Eng. 2016, 7, 2041731416650838. [Google Scholar] [CrossRef] [Green Version]
- Adijanto, J.; Philp, N.J. Cultured Primary Human Fetal Retinal Pigment Epithelium (HfRPE) as a Model for Evaluating RPE Metabolism. Exp. Eye Res. 2014, 126, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.-T.; Li, X.; Huang, J.; Guidry, C.; Wang, S.-Z. Photoreceptor-like Cells from Reprogramming Cultured Mammalian RPE Cells. Mol. Vis. 2013, 19, 1178–1187. [Google Scholar] [PubMed]
- Jiang, X.R.; Jimenez, G.; Chang, E.; Frolkis, M.; Kusler, B.; Sage, M.; Beeche, M.; Bodnar, A.G.; Wahl, G.M.; Tlsty, T.D.; et al. Telomerase Expression in Human Somatic Cells Does Not Induce Changes Associated with a Transformed Phenotype. Nat. Genet. 1999, 21, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Hellinen, L.; Pirskanen, L.; Tengvall-Unadike, U.; Urtti, A.; Reinisalo, M. Retinal Pigment Epithelial Cell Line with Fast Differentiation and Improved Barrier Properties. Pharmaceutics 2019, 11, 412. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, R.; Bayatti, N.; Scharff, R.; Szula, E.; Tilakaratna, V.; Udsen, M.S.; McHarg, S.; Askari, J.A.; Humphries, M.J.; Bishop, P.N.; et al. FHL-1 Interacts with Human RPE Cells through the A5β1 Integrin and Confers Protection against Oxidative Stress. Sci. Rep. 2021, 11, 14175. [Google Scholar] [CrossRef]
- Alge, C.S.; Hauck, S.M.; Priglinger, S.G.; Kampik, A.; Ueffing, M. Differential Protein Profiling of Primary versus Immortalized Human RPE Cells Identifies Expression Patterns Associated with Cytoskeletal Remodeling and Cell Survival. J. Proteome Res. 2006, 5, 862–878. [Google Scholar] [CrossRef]
- Kawasaki, H.; Suemori, H.; Mizuseki, K.; Watanabe, K.; Urano, F.; Ichinose, H.; Haruta, M.; Takahashi, M.; Yoshikawa, K.; Nishikawa, S.-I.; et al. Generation of Dopaminergic Neurons and Pigmented Epithelia from Primate ES Cells by Stromal Cell-Derived Inducing Activity. Proc. Natl. Acad. Sci. USA 2002, 99, 1580–1585. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M.; Sasai, Y.; Kawasaki, H.; Amemiya, K.; Ooto, S.; Kitada, M.; Suemori, H.; Nakatsuji, N.; Ide, C.; Honda, Y.; et al. In Vitro and in Vivo Characterization of Pigment Epithelial Cells Differentiated from Primate Embryonic Stem Cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1020–1025. [Google Scholar] [CrossRef] [Green Version]
- Mazzilli, J.L.; Domozhirov, A.Y.; Mueller-Ortiz, S.L.; Garcia, C.A.; Wetsel, R.A.; Zsigmond, E.M. Derivation and Characterization of the Human Embryonic Stem Cell Line CR-4: Differentiation to Human Retinal Pigment Epithelial Cells. Stem Cell Res. 2017, 18, 37–40. [Google Scholar] [CrossRef]
- Buchholz, D.E.; Hikita, S.T.; Rowland, T.J.; Friedrich, A.M.; Hinman, C.R.; Johnson, L.V.; Clegg, D.O. Derivation of Functional Retinal Pigmented Epithelium from Induced Pluripotent Stem Cells. Stem Cells 2009, 27, 2427–2434. [Google Scholar] [CrossRef]
- Leach, L.L.; Croze, R.H.; Hu, Q.; Nadar, V.P.; Clevenger, T.N.; Pennington, B.O.; Gamm, D.M.; Clegg, D.O. Induced Pluripotent Stem Cell-Derived Retinal Pigmented Epithelium: A Comparative Study Between Cell Lines and Differentiation Methods. J. Ocul. Pharmacol. Ther. 2016, 32, 317–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeiss, C.J. Animals as Models of Age-Related Macular Degeneration: An Imperfect Measure of the Truth. Vet. Pathol. 2010, 47, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Melville, H.; Carpiniello, M.; Hollis, K.; Staffaroni, A.; Golestaneh, N. Stem Cells: A New Paradigm for Disease Modeling and Developing Therapies for Age-Related Macular Degeneration. J. Transl. Med. 2013, 11, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, W.; Jaworski, C.; Postnikova, O.A.; Kutty, R.K.; Duncan, T.; Tan, L.X.; Poliakov, E.; Lakkaraju, A.; Redmond, T.M. Appropriately Differentiated ARPE-19 Cells Regain Phenotype and Gene Expression Profiles Similar to Those of Native RPE Cells. Mol. Vis. 2017, 23, 60–89. [Google Scholar]
- McLenachan, S.; Hao, E.; Zhang, D.; Zhang, L.; Edel, M.; Chen, F. Bioengineered Bruch’s-like Extracellular Matrix Promotes Retinal Pigment Epithelial Differentiation. Biochem. Biophys. Rep. 2017, 10, 178–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowland, T.J.; Blaschke, A.J.; Buchholz, D.E.; Hikita, S.T.; Johnson, L.V.; Clegg, D.O. Differentiation of Human Pluripotent Stem Cells to Retinal Pigmented Epithelium in Defined Conditions Using Purified Extracellular Matrix Proteins. J. Tissue Eng. Regen. Med. 2013, 7, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Lim, K.-T. Biomimetic Polymer-Based Engineered Scaffolds for Improved Stem Cell Function. Materials 2019, 12, 2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoda, S.; Spee, C.; Barron, E.; Ryan, S.J.; Kannan, R.; Hinton, D.R. A Protocol for the Culture and Differentiation of Highly Polarized Human Retinal Pigment Epithelial Cells. Nat. Protoc. 2009, 4, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Sorkio, A.; Hongisto, H.; Kaarniranta, K.; Uusitalo, H.; Juuti-Uusitalo, K.; Skottman, H. Structure and Barrier Properties of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells Are Affected by Extracellular Matrix Protein Coating. Tissue Eng. Part A 2014, 20, 622–634. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.R.; de Paiva, M.R.B.; Ribeiro, M.C.S.; Andrade, G.F.; Carvalho, J.L.; Gomes, D.A.; Nehemy, M.; Fialho, S.L.; Silva-Cunha, A.; de Góes, A.M. Human Stem Cell-Derived Retinal Pigment Epithelial Cells as a Model for Drug Screening and Pre-Clinical Assays Compared to ARPE-19 Cell Line. Int. J. Stem Cells 2021, 14, 74–84. [Google Scholar] [CrossRef]
- Amin, S. Modulation of Sub-RPE Deposits In Vitro: A Potential Model for Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1281–1288. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.V.; Forest, D.L.; Banna, C.D.; Radeke, C.M.; Maloney, M.A.; Hu, J.; Spencer, C.N.; Walker, A.M.; Tsie, M.S.; Bok, D.; et al. Cell Culture Model That Mimics Drusen Formation and Triggers Complement Activation Associated with Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 18277–18282. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.V.; Li, Y.; Tsang, S.H. Patient-Specific IPSC-Derived RPE for Modeling of Retinal Diseases. J. Clin. Med. 2015, 4, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Di Foggia, V.; Makwana, P.; Ali, R.R.; Sowden, J.C. Induced Pluripotent Stem Cell Therapies for Degenerative Disease of the Outer Retina: Disease Modeling and Cell Replacement. J. Ocul. Pharmacol. Ther. 2016, 32, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, Y.; Chan, L.; Tsai, Y.-T.; Wu, W.-H.; Nguyen, H.V.; Hsu, C.-W.; Li, X.; Brown, L.M.; Egli, D.; et al. Validation of Genome-Wide Association Study (GWAS)-Identified Disease Risk Alleles with Patient-Specific Stem Cell Lines. Hum. Mol. Genet. 2014, 23, 3445–3455. [Google Scholar] [CrossRef] [Green Version]
- Ghareeb, A.E.; Lako, M.; Steel, D.H. Coculture Techniques for Modeling Retinal Development and Disease, and Enabling Regenerative Medicine. Stem Cells Transl. Med. 2020, 9, 1531–1548. [Google Scholar] [CrossRef]
- Aires, I.D.; Ambrósio, A.F.; Santiago, A.R. Modeling Human Glaucoma: Lessons from the in Vitro Models. Ophthalmic Res. 2017, 57, 77–86. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xu, L.; Wei, W.B.; Jonas, J.B. Intraocular Pressure and Its Normal Range Adjusted for Ocular and Systemic Parameters. The Beijing Eye Study 2011. PLoS ONE 2018, 13, e0196926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvador-Silva, M.; Ricard, C.S.; Agapova, O.A.; Yang, P.; Hernandez, M.R. Expression of Small Heat Shock Proteins and Intermediate Filaments in the Human Optic Nerve Head Astrocytes Exposed to Elevated Hydrostatic Pressure in Vitro. J. Neurosci. Res. 2001, 66, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ju, W.-K.; Crowston, J.G.; Xie, F.; Perry, G.; Smith, M.A.; Lindsey, J.D.; Weinreb, R.N. Oxidative Stress Is an Early Event in Hydrostatic Pressure Induced Retinal Ganglion Cell Damage. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4580–4589. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhong, Y.; Cheng, Y.; Shen, X.; Wang, J.; Wei, Y. Effect of High Hydrostatic Pressure on the Expression of Glutamine Synthetase in Rat Retinal Müller Cells Cultured in Vitro. Exp. Ther. Med. 2011, 2, 513–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tök, L.; Nazıroğlu, M.; Uğuz, A.C.; Tök, O. Elevated Hydrostatic Pressures Induce Apoptosis and Oxidative Stress through Mitochondrial Membrane Depolarization in PC12 Neuronal Cells: A Cell Culture Model of Glaucoma. J. Recept. Signal Transduct. Res. 2014, 34, 410–416. [Google Scholar] [CrossRef]
- Ju, W.-K.; Kim, K.-Y.; Lindsey, J.D.; Angert, M.; Duong-Polk, K.X.; Scott, R.T.; Kim, J.J.; Kukhmazov, I.; Ellisman, M.H.; Perkins, G.A.; et al. Intraocular Pressure Elevation Induces Mitochondrial Fission and Triggers OPA1 Release in Glaucomatous Optic Nerve. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4903–4911. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Pena, J.D.; Selvidge, J.A.; Salvador-Silva, M.; Yang, P. Hydrostatic Pressure Stimulates Synthesis of Elastin in Cultured Optic Nerve Head Astrocytes. Glia 2000, 32, 122–136. [Google Scholar] [CrossRef]
- Quigley, H.A.; Hohman, R.M.; Addicks, E.M.; Massof, R.W.; Green, W.R. Morphologic Changes in the Lamina Cribrosa Correlated with Neural Loss in Open-Angle Glaucoma. Am. J. Ophthalmol. 1983, 95, 673–691. [Google Scholar] [CrossRef]
- Kirwan, R.P.; Fenerty, C.H.; Crean, J.; Wordinger, R.J.; Clark, A.F.; O’Brien, C.J. Influence of Cyclical Mechanical Strain on Extracellular Matrix Gene Expression in Human Lamina Cribrosa Cells in Vitro. Mol. Vis. 2005, 11, 798–810. [Google Scholar] [PubMed]
- Quill, B.; Docherty, N.G.; Clark, A.F.; O’Brien, C.J. The Effect of Graded Cyclic Stretching on Extracellular Matrix-Related Gene Expression Profiles in Cultured Primary Human Lamina Cribrosa Cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1908–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, M.; Philippeit, C.; Weise, A.; Dünker, N. Re-Characterization of Established Human Retinoblastoma Cell Lines. Histochem. Cell Biol. 2015, 143, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Thakur, S.S.; Barnett, N.L.; Donaldson, M.J.; Parekh, H.S. Intravitreal Drug Delivery in Retinal Disease: Are We out of Our Depth? Expert Opin. Drug Deliv. 2014, 11, 1575–1590. [Google Scholar] [CrossRef]
- Cholkar, K.; Patel, S.P.; Vadlapudi, A.D.; Mitra, A.K. Novel Strategies for Anterior Segment Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 106–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barar, J.; Asadi, M.; Mortazavi-Tabatabaei, S.A.; Omidi, Y. Ocular Drug Delivery; Impact of in Vitro Cell Culture Models. J. Ophthalmic Vis. Res. 2009, 4, 238–252. [Google Scholar] [PubMed]
- Zhong, X.; Gutierrez, C.; Xue, T.; Hampton, C.; Vergara, M.N.; Cao, L.-H.; Peters, A.; Park, T.S.; Zambidis, E.T.; Meyer, J.S.; et al. Generation of Three-Dimensional Retinal Tissue with Functional Photoreceptors from Human IPSCs. Nat. Commun. 2014, 5, 4047. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wang, P.; Ma, J.H.; Cui, Z.; Yu, Q.; Liu, S.; Xue, Y.; Zhu, D.; Cao, J.; Li, Z.; et al. Modeling Retinitis Pigmentosa: Retinal Organoids Generated From the IPSCs of a Patient With the USH2A Mutation Show Early Developmental Abnormalities. Front. Cell Neurosci. 2019, 13, 361. [Google Scholar] [CrossRef] [Green Version]
- Lane, A.; Jovanovic, K.; Shortall, C.; Ottaviani, D.; Panes, A.B.; Schwarz, N.; Guarascio, R.; Hayes, M.J.; Palfi, A.; Chadderton, N.; et al. Modeling and Rescue of RP2 Retinitis Pigmentosa Using IPSC-Derived Retinal Organoids. Stem Cell Rep. 2020, 15, 67–79. [Google Scholar] [CrossRef]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem Cell 2012, 10, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Edgar, T.Y.S.; Yeong, W.Y.; Laude, A. Hybrid Three-Dimensional (3D) Bioprinting of Retina Equivalent for Ocular Research. Int. J. Bioprint. 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaeli, E.; Forster, V.; Picaud, S.; Karamali, F.; Nasr-Esfahani, M.H.; Marquette, C. Tissue Engineering of Retina through High Resolution 3-Dimensional Inkjet Bioprinting. Biofabrication 2020, 12, 025006. [Google Scholar] [CrossRef]
- Ortiz, M.V.; Dunkel, I.J. Retinoblastoma. J. Child Neurol. 2016, 31, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Kaewkhaw, R.; Rojanaporn, D. Retinoblastoma: Etiology, Modeling, and Treatment. Cancers 2020, 12, 2304. [Google Scholar] [CrossRef]
- Eagle, R.C. Ocular Tumors: Triumphs, Challenges and Controversies. Saudi J. Ophthalmol. 2013, 27, 129–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, S.; Rizvi, F.; Zia, N.; Ali, A.; Hamid, A.; Kumari, B. Outcomes of Group D Retinoblastoma With Resistant Vitreous Seeds After Integration of Intravitreal Chemotherapy to the Treatment Protocol. Cureus 2020, 12, e11757. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.; Mohanty, C.; Harilal, A.; Maheswari, U.K.; Sahoo, S.K.; Krishnakumar, S. A Novel in Vitro Three-Dimensional Retinoblastoma Model for Evaluating Chemotherapeutic Drugs. Mol. Vis. 2012, 18, 1361–1378. [Google Scholar] [PubMed]
- Kaushik, G.; Ponnusamy, M.P.; Batra, S.K. Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models. Stem Cells 2018, 36, 1329–1340. [Google Scholar] [CrossRef] [Green Version]
- Winter, U.; Aschero, R.; Fuentes, F.; Buontempo, F.; Zugbi, S.; Sgroi, M.; Sampor, C.; Abramson, D.H.; Carcaboso, A.M.; Schaiquevich, P. Tridimensional Retinoblastoma Cultures as Vitreous Seeds Models for Live-Cell Imaging of Chemotherapy Penetration. Int. J. Mol. Sci. 2019, 20, 1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saengwimol, D.; Rojanaporn, D.; Chaitankar, V.; Chittavanich, P.; Aroonroch, R.; Boontawon, T.; Thammachote, W.; Jinawath, N.; Hongeng, S.; Kaewkhaw, R. A Three-Dimensional Organoid Model Recapitulates Tumorigenic Aspects and Drug Responses of Advanced Human Retinoblastoma. Sci. Rep. 2018, 8, 15664. [Google Scholar] [CrossRef]
- Xi, X.; Yang, Y.; Ma, J.; Chen, Q.; Zeng, Y.; Li, J.; Chen, L.; Li, Y. MiR-130a Alleviated High-Glucose Induced Retinal Pigment Epithelium (RPE) Death by Modulating TNF-α/SOD1/ROS Cascade Mediated Pyroptosis. Biomed. Pharmacother. 2020, 125, 109924. [Google Scholar] [CrossRef]
- Fernandez-Godino, R.; Pierce, E.A. C3a Triggers Formation of Sub-Retinal Pigment Epithelium Deposits via the Ubiquitin Proteasome Pathway. Sci. Rep. 2018, 8, 9679. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.; Shahidullah, M.; Delamere, N.A. Hydrostatic Pressure-Induced Release of Stored Calcium in Cultured Rat Optic Nerve Head Astrocytes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3129–3138. [Google Scholar] [CrossRef] [Green Version]
- Doss, M.X.; Sachinidis, A. Current Challenges of IPSC-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara-Wright, M.; Gonzalez-Cordero, A. Retinal Organoids: A Window into Human Retinal Development. Development 2020, 147, dev189746. [Google Scholar] [CrossRef] [PubMed]
- Achberger, K.; Probst, C.; Haderspeck, J.; Bolz, S.; Rogal, J.; Chuchuy, J.; Nikolova, M.; Cora, V.; Antkowiak, L.; Haq, W.; et al. Merging Organoid and Organ-on-a-Chip Technology to Generate Complex Multi-Layer Tissue Models in a Human Retina-on-a-Chip Platform. eLife 2019, 8, e46188. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfonsetti, M.; Castelli, V.; d’Angelo, M.; Benedetti, E.; Allegretti, M.; Barboni, B.; Cimini, A. Looking for In Vitro Models for Retinal Diseases. Int. J. Mol. Sci. 2021, 22, 10334. https://doi.org/10.3390/ijms221910334
Alfonsetti M, Castelli V, d’Angelo M, Benedetti E, Allegretti M, Barboni B, Cimini A. Looking for In Vitro Models for Retinal Diseases. International Journal of Molecular Sciences. 2021; 22(19):10334. https://doi.org/10.3390/ijms221910334
Chicago/Turabian StyleAlfonsetti, Margherita, Vanessa Castelli, Michele d’Angelo, Elisabetta Benedetti, Marcello Allegretti, Barbara Barboni, and Annamaria Cimini. 2021. "Looking for In Vitro Models for Retinal Diseases" International Journal of Molecular Sciences 22, no. 19: 10334. https://doi.org/10.3390/ijms221910334







