The Potential Roles of Dec1 and Dec2 in Periodontal Inflammation
Abstract
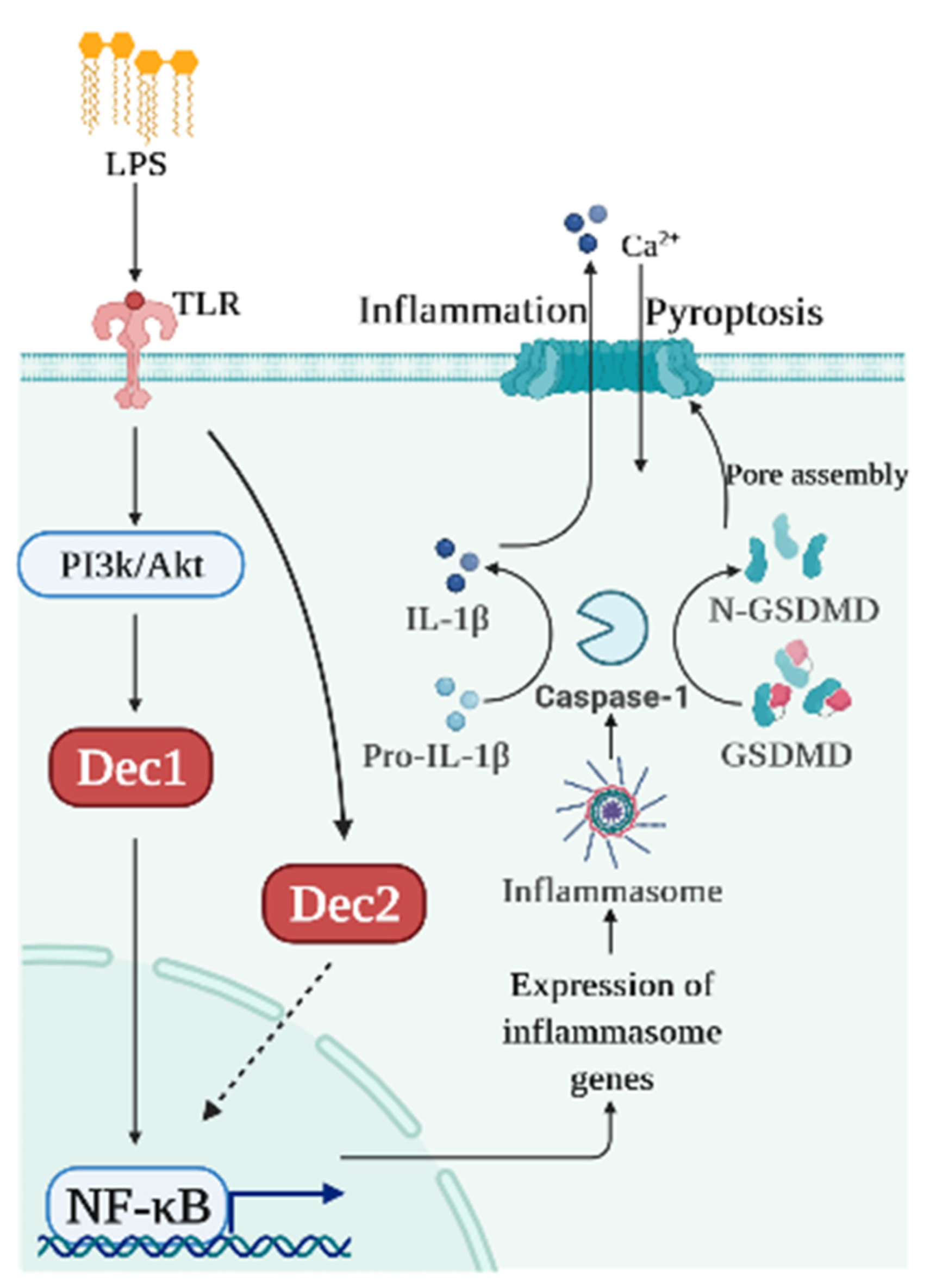
:1. Introduction
2. Autophagy
2.1. Dec1 and Autophagy
2.2. Dec2 and Autophagy
3. Pyroptosis
3.1. Dec1 and Pyroptosis
3.2. Dec2 and Pyroptosis
4. Clock Genes and Other Inflammatory Diseases
4.1. Brain and Muscle Arnt-like Protein-1 (BMAL1) an Alzheimer Disease (AD)
4.2. NR1D1 and Atherogenesis
4.3. Dec1 and Cardiac Hypertrophy
4.4. Dec 1 and Ischemia/Reperfusion-Induced Myocardial Inflammation
4.5. Dec2 and Rheumatoid Arthritis (RA)
5. Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kinane, D.F. Causation and Pathogenesis of Periodontal Disease. Periodontology 2000 2001, 25, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Hu, T.; Bhowmick, N.A. Be Resistant to Apoptosis: A Host Factor from Gingival Fibroblasts. Cell Death Dis. 2015, 6, e2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and Their Roles in Health and Disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, C.-M.; Zhang, P.; Wang, X.; Chen, J.; Yang, J.; Lu, W.; Zhou, W.; Yuan, W.; Feng, Y. Expression of Programmed Death 1 Ligand 1 on Periodontal Tissue Cells as a Possible Protective Feedback Mechanism against Periodontal Tissue Destruction. Mol. Med. Rep. 2016, 13, 2423–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Awady, A.R.; Messer, R.L.W.; Gamal, A.Y.; Sharawy, M.M.; Wenger, K.H.; Lapp, C.A. Periodontal Ligament Fibroblasts Sustain Destructive Immune Modulators of Chronic Periodontitis. J. Periodontol. 2010, 81, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rossi, F.M.V.; Putnins, E.E. Periodontal Regeneration Using Engineered Bone Marrow Mesenchymal Stromal Cells. Biomaterials 2010, 31, 8574–8582. [Google Scholar] [CrossRef]
- Onizuka, S.; Iwata, T. Application of Periodontal Ligament-Derived Multipotent Mesenchymal Stromal Cell Sheets for Periodontal Regeneration. Int. J. Mol. Sci. 2019, 20, 2796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haus, E.; Smolensky, M.H. Biologic Rhythms in the Immune System. Chronobiol. Int. 1999, 16, 581–622. [Google Scholar] [CrossRef]
- Castanon-Cervantes, O.; Wu, M.; Ehlen, J.C.; Paul, K.; Gamble, K.L.; Johnson, R.L.; Besing, R.C.; Menaker, M.; Gewirtz, A.T.; Davidson, A.J. Dysregulation of Inflammatory Responses by Chronic Circadian Disruption. J. Immunol. 2010, 185, 5796–5805. [Google Scholar] [CrossRef] [Green Version]
- Cutolo, M. Chronobiology and the Treatment of Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2012, 24, 312–318. [Google Scholar] [CrossRef]
- Gupta, A.; Shetty, H. Circadian Variation in Stroke—A Prospective Hospital-Based Study. Int. J. Clin. Pract. 2005, 59, 1272–1275. [Google Scholar] [CrossRef]
- Fujimoto, K.; Shen, M.; Noshiro, M.; Matsubara, K.; Shingu, S.; Honda, K.; Yoshida, E.; Suardita, K.; Matsuda, Y.; Kato, Y. Molecular Cloning and Characterization of DEC2, a New Member of Basic Helix-Loop-Helix Proteins. Biochem. Biophys. Res. Commun. 2001, 280, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Bhawal, U.K.; Yoshimura, T.; Muragaki, Y. DEC1 and DEC2 Crosstalk between Circadian Rhythm and Tumor Progression. J. Cancer 2016, 7, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudjelal, M.; Taneja, R.; Matsubara, S.; Bouillet, P.; Dolle, P.; Chambon, P. Overexpression of Stra13, a Novel Retinoic Acid-Inducible Gene of the Basic Helix-Loop-Helix Family, Inhibits Mesodermal and Promotes Neuronal Differentiation of P19 Cells. Genes Dev. 1997, 11, 2052–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.; Kawamoto, T.; Yan, W.; Nakamasu, K.; Tamagami, M.; Koyano, Y.; Noshiro, M.; Kato, Y. Molecular Characterization of the Novel Basic Helix-Loop-Helix Protein DEC1 Expressed in Differentiated Human Embryo Chondrocytes. Biochem. Biophys. Res. Commun. 1997, 236, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Noshiro, M.; Sato, F.; Maemura, K.; Takeda, N.; Nagai, R.; Iwata, T.; Fujimoto, K.; Furukawa, M.; Miyazaki, K.; et al. A Novel Autofeedback Loop of Dec1 Transcription Involved in Circadian Rhythm Regulation. Biochem. Biophys. Res. Commun. 2004, 313, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, H.; Fujimoto, K.; Kawamoto, T.; Noshiro, M.; Maemura, K.; Takeda, N.; Nagai, R.; Furukawa, M.; Honma, S.; Honma, K.; et al. Expression of the Gene for Dec2, a Basic Helix-Loop-Helix Transcription Factor, Is Regulated by a Molecular Clock System. Biochem. J. 2004, 382, 43–50. [Google Scholar] [CrossRef]
- Miyazaki, K.; Kawamoto, T.; Tanimoto, K.; Nishiyama, M.; Honda, H.; Kato, Y. Identification of Functional Hypoxia Response Elements in the Promoter Region of the DEC1 and DEC2 Genes. J. Biol. Chem. 2002, 277, 47014–47021. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Llordella, M.; Esensten, J.H.; Bailey-Bucktrout, S.L.; Lipsky, R.H.; Marini, A.; Chen, J.; Mughal, M.; Mattson, M.P.; Taub, D.D.; Bluestone, J.A. CD28-Inducible Transcription Factor DEC1 Is Required for Efficient Autoreactive CD4+ T Cell Response. J. Exp. Med. 2013, 210, 1603–1619. [Google Scholar] [CrossRef]
- Camponeschi, A.; Todi, L.; Cristofoletti, C.; Lazzeri, C.; Carbonari, M.; Mitrevski, M.; Marrapodi, R.; Del Padre, M.; Fiorilli, M.; Casato, M.; et al. DEC1/STRA13 Is a Key Negative Regulator of Activation-Induced Proliferation of Human B Cells Highly Expressed in Anergic Cells. Immunol. Lett. 2018, 198, 7–11. [Google Scholar] [CrossRef]
- Yang, X.O.; Angkasekwinai, P.; Zhu, J.; Peng, J.; Liu, Z.; Nurieva, R.; Liu, X.; Chung, Y.; Chang, S.H.; Sun, B.; et al. Requirement for the Basic Helix-Loop-Helix Transcription Factor Dec2 in Initial TH2 Lineage Commitment. Nat. Immunol. 2009, 10, 1260–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhawal, U.K.; Ito, Y.; Tanimoto, K.; Sato, F.; Fujimoto, K.; Kawamoto, T.; Sasahira, T.; Hamada, N.; Kuniyasu, H.; Arakawa, H.; et al. IL-1β-Mediated up-Regulation of DEC1 in Human Gingiva Cells via the Akt Pathway. J. Cell. Biochem. 2012, 113, 3246–3253. [Google Scholar] [CrossRef]
- Zhang, F.; Suzuki, M.; Kim, I.S.; Kobayashi, R.; Hamada, N.; Sato, F.; Bhawal, U.K. Transcription Factor DEC1 Is Required for Maximal Experimentally Induced Periodontal Inflammation. J. Periodontal Res. 2018, 53, 883–893. [Google Scholar] [CrossRef]
- Liu, C.; Mo, L.; Niu, Y.; Li, X.; Zhou, X.; Xu, X. The Role of Reactive Oxygen Species and Autophagy in Periodontitis and Their Potential Linkage. Front. Physiol. 2017, 8, 439. [Google Scholar] [CrossRef]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-Consumption: The Interplay of Autophagy and Apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Sanchez-Lopez, E.; Karin, M. Autophagy, Inflammation, and Immunity: A Troika Governing Cancer and Its Treatment. Cell 2016, 166, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; An, Y.; An, Y.; Fei, D.; Wang, Q. Activation of Autophagy in Periodontal Ligament Mesenchymal Stem Cells Promotes Angiogenesis in Periodontitis. J. Periodontol. 2018, 89, 718–727. [Google Scholar] [CrossRef]
- Memmert, S.; Damanaki, A.; Nogueira, A.V.B.; Eick, S.; Nokhbehsaim, M.; Papadopoulou, A.K.; Till, A.; Rath, B.; Jepsen, S.; Götz, W.; et al. Role of Cathepsin S in Periodontal Inflammation and Infection. Mediat. Inflamm. 2017, 2017, 4786170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, A.; Kim, S.; Goldshteyn, V.; Kim, T.; Park, N.-H.; Wang, C.-Y.; Kim, R.H. Beclin1 Modulates Bone Homeostasis by Regulating Osteoclast and Chondrocyte Differentiation. J. Bone Miner. Res. 2019, 34, 1753–1766. [Google Scholar] [CrossRef]
- An, Y.; Liu, W.; Xue, P.; Zhang, Y.; Wang, Q.; Jin, Y. Increased Autophagy Is Required to Protect Periodontal Ligament Stem Cells from Apoptosis in Inflammatory Microenvironment. J. Clin. Periodontol. 2016, 43, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Zimmermann, A.; Maiuri, M.C.; Kroemer, G. Essential Role for Autophagy in Life Span Extension. J. Clin. Investig. 2015, 125, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.C.; Juhász, G.; Neufeld, T.P. Direct Induction of Autophagy by Atg1 Inhibits Cell Growth and Induces Apoptotic Cell Death. Curr. Biol. 2007, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeifer, U.; Strauss, P. Autophagic Vacuoles in Heart Muscle and Liver. A Comparative Morphometric Study Including Circadian Variations in Meal-Fed Rats. J. Mol. Cell. Cardiol. 1981, 13, 37–49. [Google Scholar] [CrossRef]
- Ma, D.; Panda, S.; Lin, J.D. Temporal Orchestration of Circadian Autophagy Rhythm by C/EBPβ. EMBO J. 2011, 30, 4642–4651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubrovsky, Y.V.; Samsa, W.E.; Kondratov, R.V. Deficiency of Circadian Protein CLOCK Reduces Lifespan and Increases Age-Related Cataract Development in Mice. Aging 2010, 2, 936–944. [Google Scholar] [CrossRef] [Green Version]
- Tonetti, M.S.; Van Dyke, T.E. Working group 1 of the joint EFP/AAP workshop Periodontitis and Atherosclerotic Cardiovascular Disease: Consensus Report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Li, X.; Zhang, F.; Tewari, N.; Kim, I.-S.; Chen, C.; Zhong, L.; Hamada, N.; Oi, Y.; Makishima, M.; et al. Loss of Dec1 Prevents Autophagy in Inflamed Periodontal Ligament Fibroblast. Mol. Biol. Rep. 2021, 48, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Karp, C.; Strohecker, A.M.; Guo, Y.; Mathew, R. Role of Autophagy in Suppression of Inflammation and Cancer. Curr. Opin. Cell Biol. 2010, 22, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Veskovic, M.; Mladenovic, D.; Milenkovic, M.; Tosic, J.; Borozan, S.; Gopcevic, K.; Labudovic-Borovic, M.; Dragutinovic, V.; Vucevic, D.; Jorgacevic, B.; et al. Betaine Modulates Oxidative Stress, Inflammation, Apoptosis, Autophagy, and Akt/MTOR Signaling in Methionine-Choline Deficiency-Induced Fatty Liver Disease. Eur. J. Pharmacol. 2019, 848, 39–48. [Google Scholar] [CrossRef]
- Li, P.; Shi, J.; He, Q.; Hu, Q.; Wang, Y.Y.; Zhang, L.J.; Chan, W.T.; Chen, W.-X. Streptococcus Pneumoniae Induces Autophagy through the Inhibition of the PI3K-I/Akt/MTOR Pathway and ROS Hypergeneration in A549 Cells. PLoS ONE 2015, 10, e0122753. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhang, S.; Cao, X.; Zhang, Y. Lipopolysaccharide of Porphyromonas gingivalis promotes the autophagy of human gingival fibroblasts. Chin. J. Cell. Mol. Immunol. 2017, 33, 315–319. [Google Scholar]
- Kim, J.-H.; Hong, S.-K.; Wu, P.-K.; Richards, A.L.; Jackson, W.T.; Park, J.-I. Raf/MEK/ERK Can Regulate Cellular Levels of LC3B and SQSTM1/P62 at Expression Levels. Exp. Cell Res. 2014, 327, 340–352. [Google Scholar] [CrossRef] [Green Version]
- Oka, S.; Li, X.; Sato, F.; Zhang, F.; Tewari, N.; Chen, C.; Zhong, L.; Makishima, M.; Liu, Y.; Bhawal, U.K. Dec2 Attenuates Autophagy in Inflamed Periodontal Tissues. Immun. Inflamm. Dis. 2021, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Feng, Y.; Zhang, R.; Liu, W.; Lei, L.; Hu, T. The Extent of Pyroptosis Varies in Different Stages of Apical Periodontitis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory Caspases Are Innate Immune Receptors for Intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and Functions of Inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-KB in Development and Progression of Human Cancer. Virchows Arch. Int. J. Pathol. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Yuan, S.; Wen, S.; Liu, X.; Wang, C.; Qu, Z.; Li, J.; Liu, H.; Sun, L.; et al. TLR4/NF-ΚB Signaling Induces GSDMD-Related Pyroptosis in Tubular Cells in Diabetic Kidney Disease. Front. Endocrinol. 2019, 10, 603. [Google Scholar] [CrossRef] [Green Version]
- Pourcet, B.; Zecchin, M.; Ferri, L.; Beauchamp, J.; Sitaula, S.; Billon, C.; Delhaye, S.; Vanhoutte, J.; Mayeuf-Louchart, A.; Thorel, Q.; et al. Nuclear Receptor Subfamily 1 Group D Member 1 Regulates Circadian Activity of NLRP3 Inflammasome to Reduce the Severity of Fulminant Hepatitis in Mice. Gastroenterology 2018, 154, 1449–1464.e20. [Google Scholar] [CrossRef]
- Yu, D.; Fang, X.; Xu, Y.; Xiao, H.; Huang, T.; Zhang, Y.; Ge, Y.; Li, Y.; Zong, L.; Gao, J. Rev-Erbα Can Regulate the NF-ΚB/NALP3 Pathway to Modulate Lipopolysaccharide-Induced Acute Lung Injury and Inflammation. Int. Immunopharmacol. 2019, 73, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Li, X.; Zhang, F.; Tewari, N.; Wang, C.; Kim, I.-S.; Zhong, L.; Hamada, N.; Oi, Y.; Makishima, M.; et al. Inhibition of Dec1 Provides Biological Insights into Periodontal Pyroptosis. Life 2021, 14, 300–307. [Google Scholar] [CrossRef]
- Oka, S.; Li, X.; Sato, F.; Zhang, F.; Tewari, N.; Kim, I.-S.; Zhong, L.; Hamada, N.; Makishima, M.; Liu, Y.; et al. A Deficiency of Dec2 Triggers Periodontal Inflammation and Pyroptosis. J. Periodontal Res. 2021, 56, 492–500. [Google Scholar] [CrossRef]
- Musiek, E.S.; Xiong, D.D.; Holtzman, D.M. Sleep, Circadian Rhythms, and the Pathogenesis of Alzheimer Disease. Exp. Mol. Med. 2015, 47, e148. [Google Scholar] [CrossRef] [Green Version]
- Circadian Clock Proteins Regulate Neuronal Redox Homeostasis and Neurodegeneration—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/24270424/ (accessed on 13 August 2021).
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early Aging and Age-Related Pathologies in Mice Deficient in BMAL1, the Core Componentof the Circadian Clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [Green Version]
- Masri, S.; Patel, V.R.; Eckel-Mahan, K.L.; Peleg, S.; Forne, I.; Ladurner, A.G.; Baldi, P.; Imhof, A.; Sassone-Corsi, P. Circadian Acetylome Reveals Regulation of Mitochondrial Metabolic Pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 3339–3344. [Google Scholar] [CrossRef] [Green Version]
- Pourcet, B.; Duez, H. Circadian Control of Inflammasome Pathways: Implications for Circadian Medicine. Front. Immunol. 2020, 11, 1630. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I. Macrophage Death and Defective Inflammation Resolution in Atherosclerosis. Nat. Rev. Immunol. 2010, 10, 36–46. [Google Scholar] [CrossRef]
- Libby, P.; Okamoto, Y.; Rocha, V.Z.; Folco, E. Inflammation in Atherosclerosis: Transition from Theory to Practice. Circ. J. Off. J. Jpn. Circ. Soc. 2010, 74, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Zhong, W.; Jiang, Y.; Fontaine, C.; Li, S.; Fu, J.; Olkkonen, V.M.; Staels, B.; Yan, D. Increased Atherosclerotic Lesions in LDL Receptor Deficient Mice with Hematopoietic Nuclear Receptor Rev-Erbα Knock-Down. J. Am. Heart Assoc. 2013, 2, e000235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitaula, S.; Billon, C.; Kamenecka, T.M.; Solt, L.A.; Burris, T.P. Suppression of Atherosclerosis by Synthetic REV-ERB Agonist. Biochem. Biophys. Res. Commun. 2015, 460, 566–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisheit, C.; Zhang, Y.; Faron, A.; Köpke, O.; Weisheit, G.; Steinsträsser, A.; Frede, S.; Meyer, R.; Boehm, O.; Hoeft, A.; et al. Ly6C(Low) and Not Ly6C(High) Macrophages Accumulate First in the Heart in a Model of Murine Pressure-Overload. PLoS ONE 2014, 9, e112710. [Google Scholar] [CrossRef]
- Li, X.; Le, H.T.; Sato, F.; Kang, T.H.; Makishima, M.; Zhong, L.; Liu, Y.; Guo, L.; Bhawal, U.K. Dec1 Deficiency Protects the Heart from Fibrosis, Inflammation, and Myocardial Cell Apoptosis in a Mouse Model of Cardiac Hypertrophy. Biochem. Biophys. Res. Commun. 2020, 532, 513–519. [Google Scholar] [CrossRef]
- Godoy, L.C.; Lawler, P.R.; Farkouh, M.E.; Hersen, B.; Nicolau, J.C.; Rao, V. Urgent Revascularization Strategies in Patients With Diabetes Mellitus and Acute Coronary Syndrome. Can. J. Cardiol. 2019, 35, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, X.; Shi, J.; Wu, X. Involvement of Nrf2 in Myocardial Ischemia and Reperfusion Injury. Int. J. Biol. Macromol. 2019, 125, 496–502. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Hernandez-Resendiz, S.; Chinda, K.; Ong, S.-B.; Cabrera-Fuentes, H.; Zazueta, C.; Hausenloy, D.J. The Role of Redox Dysregulation in the Inflammatory Response to Acute Myocardial Ischaemia-Reperfusion Injury—Adding Fuel to the Fire. Curr. Med. Chem. 2018, 25, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, K.; Zhang, Y.; Ma, S.; Jin, D. Downregulation of DEC1 by RNA Interference Attenuates Ischemia/Reperfusion-Induced Myocardial Inflammation by Inhibiting the TLR4/NF-ΚB Signaling Pathway. Exp. Ther. Med. 2020, 20, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Cutolo, M. Circadian Rhythms in Rheumatoid Arthritis: Implications for Pathophysiology and Therapeutic Management. Arthritis Rheum. 2007, 56, 399–408. [Google Scholar] [CrossRef]
- Honma, S.; Kawamoto, T.; Takagi, Y.; Fujimoto, K.; Sato, F.; Noshiro, M.; Kato, Y.; Honma, K. Dec1 and Dec2 Are Regulators of the Mammalian Molecular Clock. Nature 2002, 419, 841–844. [Google Scholar] [CrossRef]
- Olkkonen, J.; Kouri, V.-P.; Hynninen, J.; Konttinen, Y.T.; Mandelin, J. Differentially Expressed in Chondrocytes 2 (DEC2) Increases the Expression of IL-1β and Is Abundantly Present in Synovial Membrane in Rheumatoid Arthritis. PLoS ONE 2015, 10, e0145279. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, M.; Kawamoto, T.; Noshiro, M.; Honda, K.K.; Sakai, M.; Fujimoto, K.; Honma, S.; Honma, K.; Hamada, T.; Kato, Y. Clock Gene Expression in the Submandibular Glands. J. Dent. Res. 2005, 84, 1193–1197. [Google Scholar] [CrossRef]
- Singh, A.; Gill, G.; Kaur, H.; Amhmed, M.; Jakhu, H. Role of Osteopontin in Bone Remodeling and Orthodontic Tooth Movement: A Review. Prog. Orthod. 2018, 19, 18. [Google Scholar] [CrossRef]
- Qin, X.; Li, Q.; Chen, W.; Bai, Y.; Baban, B.; Mao, J. The Circadian Expression of Osteogenic Factors in Periodontal Tissue Loading Mechanical Force: New Concepts of the Personalized Orthodontic Care. EPMA J. 2019, 10, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Verrusio, C.; Iorio-Siciliano, V.; Blasi, A.; Leuci, S.; Adamo, D.; Nicolò, M. The Effect of Orthodontic Treatment on Periodontal Tissue Inflammation: A Systematic Review. Quintessence Int. 2018, 49, 69–77. [Google Scholar] [CrossRef]
- Okanobu, A.; Matsuda, S.; Kajiya, M.; Fujita, T.; Kittaka, M.; Shiba, H.; Kurihara, H. A Novel Gingival Overgrowth Mouse Model Induced by the Combination of CsA and Ligature-Induced Inflammation. J. Immunol. Methods 2017, 445, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.T.; Hile, K.L.; Zhang, H.; Asanuma, H.; Vanderbrink, B.A.; Rink, R.R.; Meldrum, K.K. Toll-like Receptor 4: A Novel Signaling Pathway during Renal Fibrogenesis. J. Surg. Res. 2011, 168, e61–e69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegde, R.; Awan, K.H. Effects of Periodontal Disease on Systemic Health. Dis. Month 2019, 65, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease-Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef] [Green Version]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between Periodontal Pathogens and Systemic Disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Kim, I.-S.; Zhang, F.; Bhawal, U.K. The Role of the Hypoxia Responsive Gene DEC1 in Periodontal Inflammation. J. Hard Tissue Biol. 2018, 27, 227–232. [Google Scholar] [CrossRef]
- Hu, S.; Shang, W.; Yue, H.; Chen, R.; Dong, Z.; Hu, J.; Mao, Z.; Yang, J. Differentiated Embryonic Chondrocytes 1 Expression of Periodontal Ligament Tissue and Gingival Tissue in the Patients with Chronic Periodontitis. Arch. Oral Biol. 2015, 60, 517–525. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Li, X.; Zhang, F.; Wang, C.; Liu, Y.; Bhawal, U.K.; Sun, J. Dec2 Inhibits Macrophage Pyroptosis to Promote Periodontal Homeostasis. J. Periodontal Implant Sci. 2021, 51, e35. [Google Scholar]

| Title | Authors |
|---|---|
| Loss of Dec1 prevents autophagy in inflamed periodontal ligament fibroblast | Oka et al. [40] |
| Inhibition of Dec1 provides biological insights into periodontal pyroptosis | Oka et al. [55] |
| Transcription factor DEC1 is required for maximal experimentally induced periodontal inflammation | Zhang et al. [23] |
| The role of the hypoxia responsive gene DEC1 in periodontal inflammation | Kim et al. [82] |
| Differentiated embryonic chondrocytes 1 expression of periodontal ligament tissue and gingival tissue in the patients with chronic periodontitis | Hu et al. [83] |
| IL-1β-mediated up-regulation of DEC1 in human gingiva cells via the Akt pathway | Bhawal et al. [22] |
| Dec2 inhibits macrophage pyroptosis to promote periodontal homeostasis | He et al. [84] |
| Dec2 attenuates autophagy in inflamed periodontal tissues | Oka et al. [46] |
| A deficiency of Dec2 triggers periodontal inflammation and pyroptosis | Oka et al. [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Sato, F.; Tanimoto, K.; Rajeshwaran, N.; Thangavelu, L.; Makishima, M.; Bhawal, U.K. The Potential Roles of Dec1 and Dec2 in Periodontal Inflammation. Int. J. Mol. Sci. 2021, 22, 10349. https://doi.org/10.3390/ijms221910349
Wang X, Sato F, Tanimoto K, Rajeshwaran N, Thangavelu L, Makishima M, Bhawal UK. The Potential Roles of Dec1 and Dec2 in Periodontal Inflammation. International Journal of Molecular Sciences. 2021; 22(19):10349. https://doi.org/10.3390/ijms221910349
Chicago/Turabian StyleWang, Xingzhi, Fuyuki Sato, Keiji Tanimoto, Niveda Rajeshwaran, Lakshmi Thangavelu, Makoto Makishima, and Ujjal K. Bhawal. 2021. "The Potential Roles of Dec1 and Dec2 in Periodontal Inflammation" International Journal of Molecular Sciences 22, no. 19: 10349. https://doi.org/10.3390/ijms221910349
APA StyleWang, X., Sato, F., Tanimoto, K., Rajeshwaran, N., Thangavelu, L., Makishima, M., & Bhawal, U. K. (2021). The Potential Roles of Dec1 and Dec2 in Periodontal Inflammation. International Journal of Molecular Sciences, 22(19), 10349. https://doi.org/10.3390/ijms221910349








