The MiR-320 Family Is Strongly Downregulated in Patients with COVID-19 Induced Severe Respiratory Failure
Abstract
:1. Introduction
2. Results
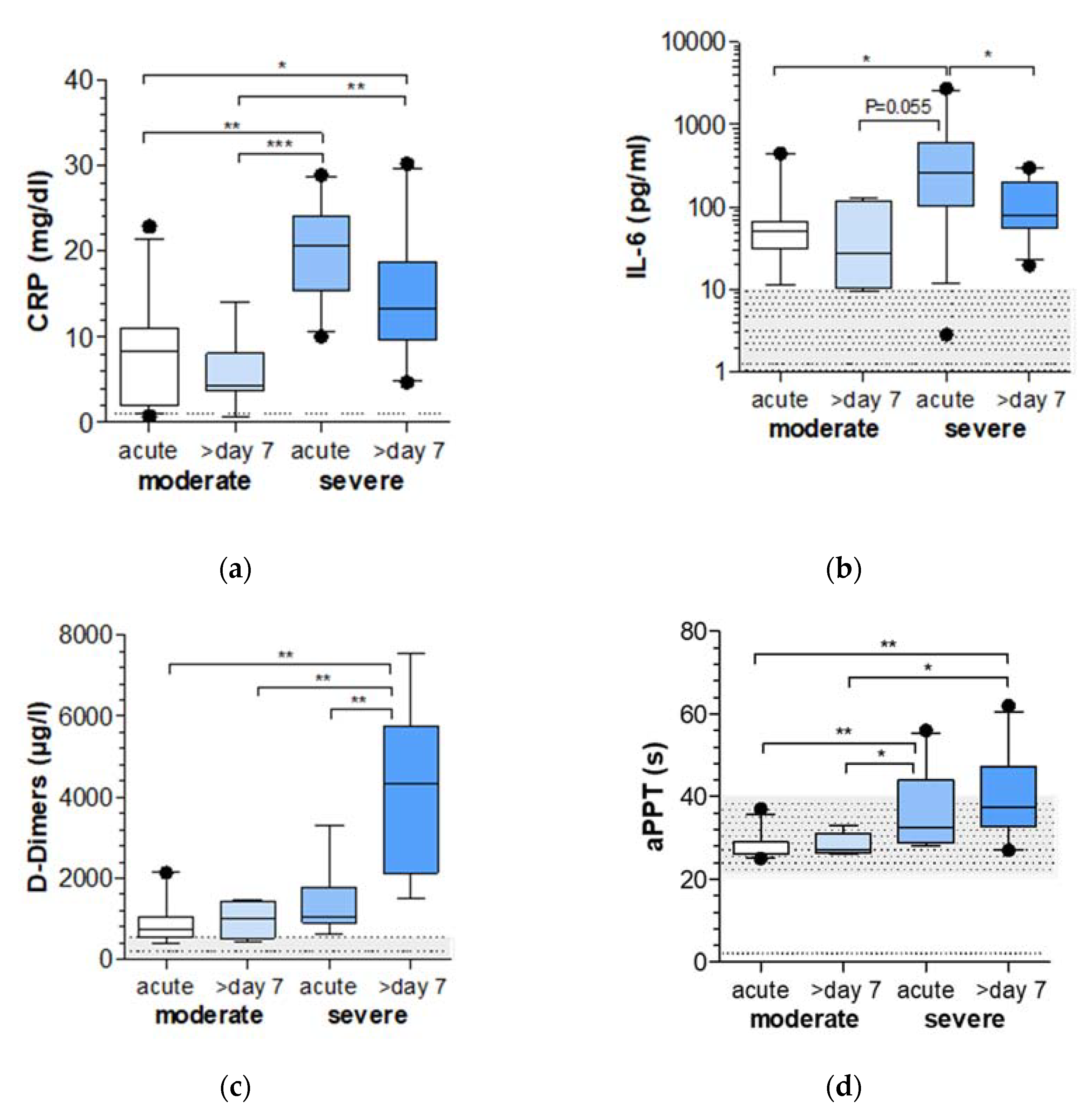
2.1. Markers of Inflammation and Coagulation Are Increased in COVID-19 Patients with Severe Respiratory Failure
2.2. MiR-320 Family Is Strongly Downregulated in COVID-19 Patients with Severe Respiratory Failure
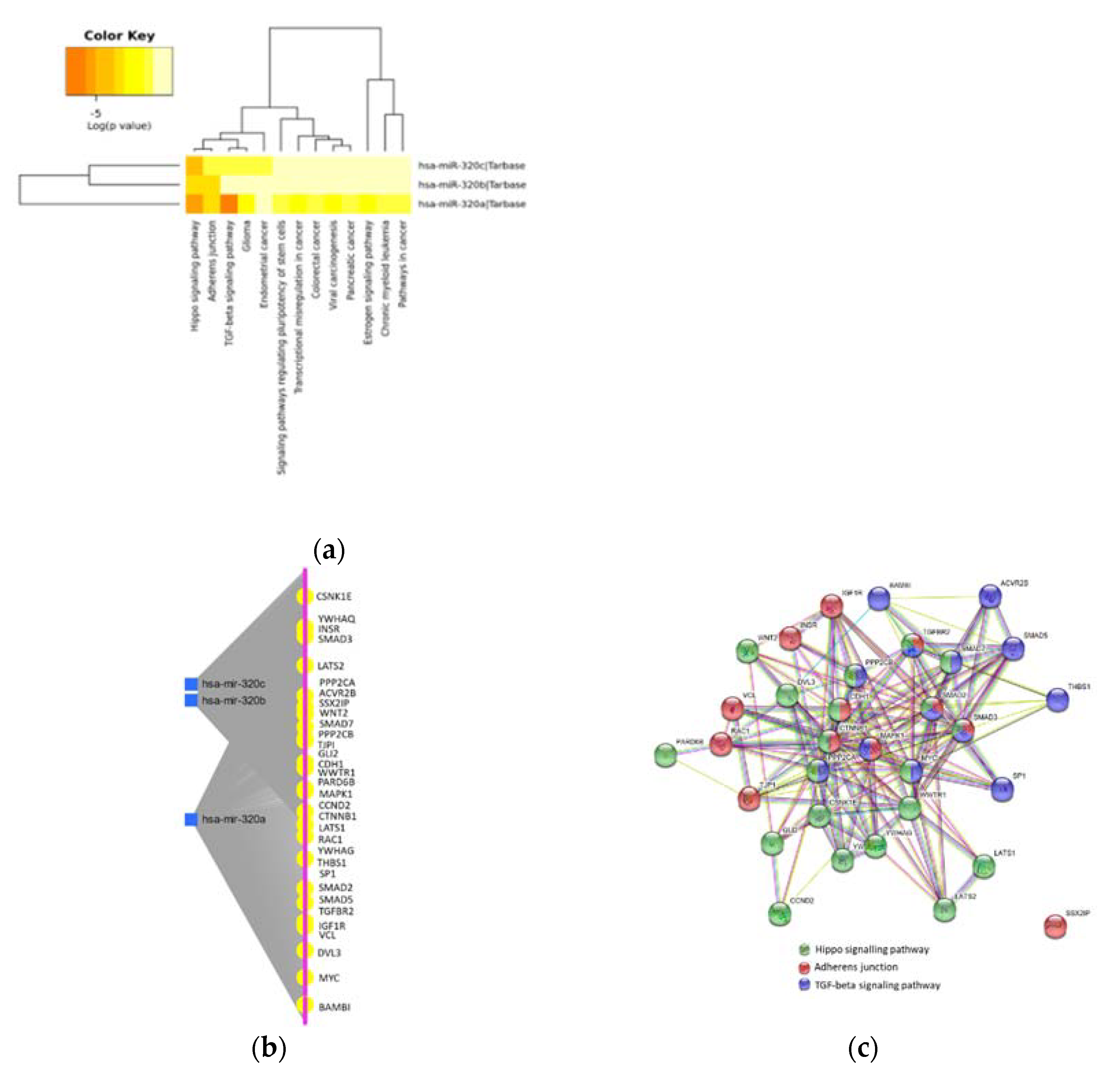
2.3. MiR-320 Family Is Involved in Inflammation and Endothelial Dysfunction
2.4. Protein–Protein Interaction in Inflammation and Endothelial Dysfunction
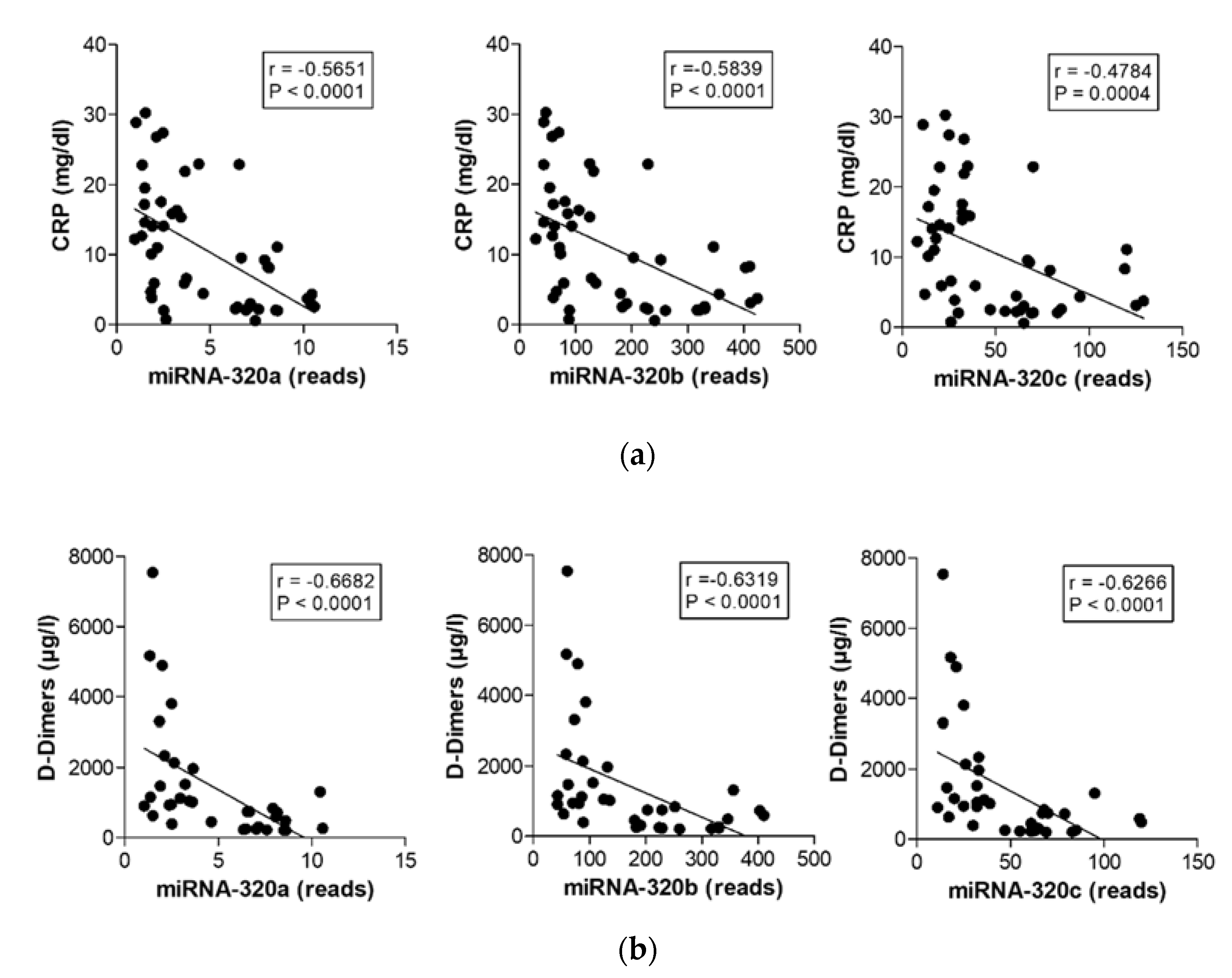
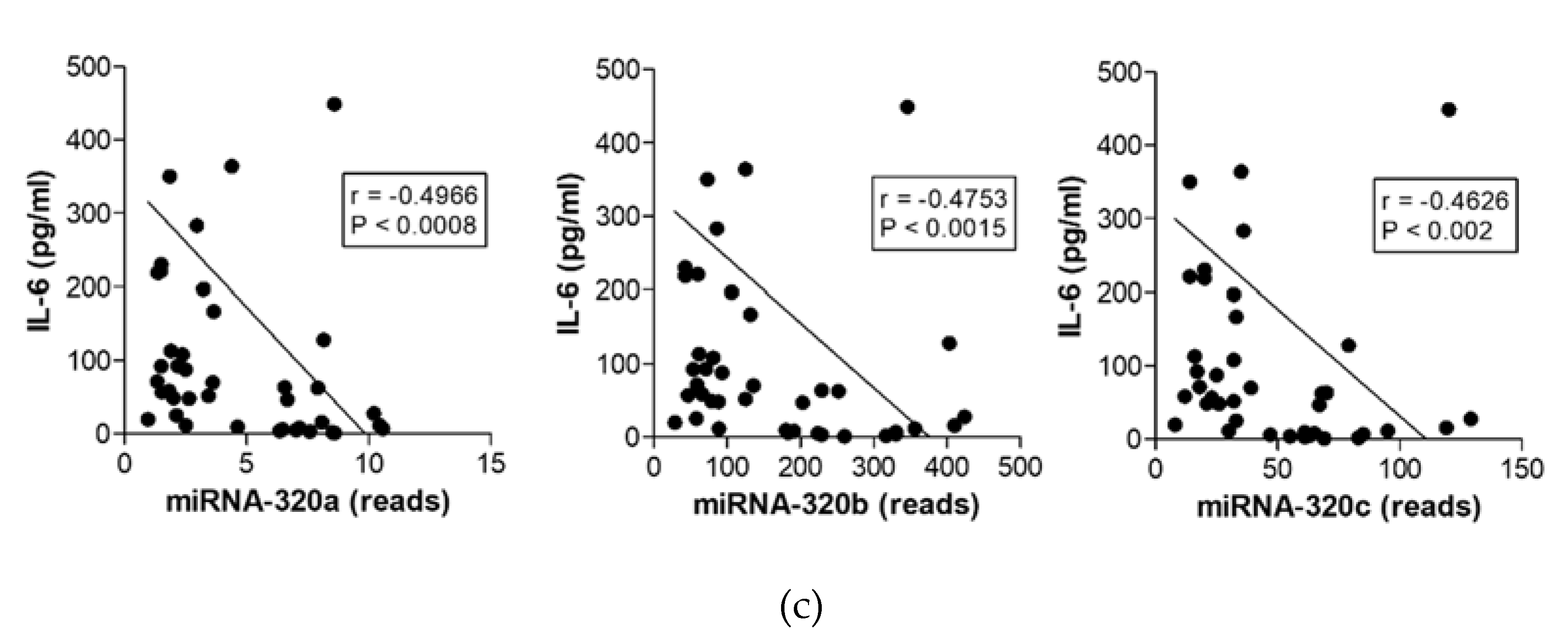
2.5. MiR-320 Family Members’ Expression Correlates with Inflammation and Coagulation
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Biomarker for Inflammation and Coagulation
4.3. MiRNA Sequencing and Analysis
4.4. Pathway Enrichment Analysis of Target Genes and Protein–Protein Interaction Network
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Wolf, M.S.; Serper, M.; Opsasnick, L.; O’Conor, R.M.; Curtis, L.M.; Benavente, J.Y.; Wismer, G.; Batio, S.; Eifler, M.; Zheng, P.; et al. Awareness, Attitudes, and Actions Related to COVID-19 Among Adults with Chronic Conditions at the Onset of the U.S. Outbreak. Ann. Intern. Med. 2020, 173, 100–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elezkurtaj, S.; Greuel, S.; Ihlow, J.; Michaelis, E.G.; Bischoff, P.; Kunze, C.A.; Sinn, B.V.; Gerhold, M.; Hauptmann, K.; Ingold-Heppner, B.; et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Yang, X.; Du, H.; Zhang, C.; Song, Y.; Ran, X.; Zhang, A.; Yang, M. Early Improvement of Acute Respiratory Distress Syndrome in Patients With COVID-19 in the Intensive Care Unit: Retrospective Analysis. JMIR Public Health Surveill. 2021, 7, e24843. [Google Scholar] [CrossRef]
- Kruse, J.M.; Zickler, D.; Lüdemann, W.M.; Piper, S.K.; Gotthardt, I.; Ihlow, J.; Greuel, S.; Horst, D.; Kahl, A.; Eckardt, K.-U.; et al. Evidence for a thromboembolic pathogenesis of lung cavitations in severely ill COVID-19 patients. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef]
- Tan, B.K.; Mainbourg, S.; Friggeri, A.; Bertoletti, L.; Douplat, M.; Dargaud, Y.; Grange, C.; Lobbes, H.; Provencher, S.; Lega, J.-C. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax 2021, 76, 970–979. [Google Scholar] [CrossRef]
- Jalde, F.C.; Beckman, M.O.; Svensson, A.M.; Bell, M.; Sköld, M.; Strand, F.; Nyren, S.; Kistner, A. Widespread Parenchymal Abnormalities and Pulmonary Embolism on Contrast-Enhanced CT Predict Disease Severity and Mortality in Hospitalized COVID-19 Patients. Front. Med. 2021, 8, 666723. [Google Scholar] [CrossRef]
- Sharma, P.; Ng, J.H.; Bijol, V.; Jhaveri, K.D.; Wanchoo, R. Pathology of COVID-19-associated acute kidney injury. Clin. Kidney J. 2021, 14, i30–i39. [Google Scholar] [CrossRef] [PubMed]
- Cañas, J.A.; Rodrigo-Muñoz, J.M.; Sastre, B.; Gil-Martinez, M.; Redondo, N.; Del Pozo, V. MicroRNAs as Potential Regulators of Immune Response Networks in Asthma and Chronic Obstructive Pulmonary Disease. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Manzoor, S.; Siddiqui, S.; Mariappan, N.; Zafar, I.; Ahmad, A.; Ahmad, A. Epigenetic underpinnings of inflammation: Connecting the dots between pulmonary diseases, lung cancer and COVID-19. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef]
- Bayarsaihan, D. Epigenetic Mechanisms in Inflammation. J. Dent. Res. 2010, 90, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A Brief Review on the Mechanisms of miRNA Regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Farr, R.J.; Rootes, C.L.; Rowntree, L.C.; Nguyen, T.H.O.; Hensen, L.; Kedzierski, L.; Cheng, A.C.; Kedzierska, K.; Au, G.G.; Marsh, G.A.; et al. Altered microRNA expression in COVID-19 patients enables identification of SARS-CoV-2 infection. PLOS Pathog. 2021, 17, e1009759. [Google Scholar] [CrossRef]
- Mirzaei, R.; Mahdavi, F.; Badrzadeh, F.; Hosseini-Fard, S.R.; Heidary, M.; Jeda, A.S.; Mohammadi, T.; Roshani, M.; Yousefimashouf, R.; Keyvani, H.; et al. The emerging role of microRNAs in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Int. Immunopharmacol. 2021, 90, 107204. [Google Scholar] [CrossRef]
- Zhang, S.; Amahong, K.; Sun, X.; Lian, X.; Liu, J.; Sun, H.; Lou, Y.; Zhu, F.; Qiu, Y. The miRNA: A small but powerful RNA for COVID-19. Brief. Bioinform. 2021, 22, 1137–1149. [Google Scholar] [CrossRef]
- Stolzenburg, L.; Harris, A. The role of microRNAs in chronic respiratory disease: Recent insights. Biol. Chem. 2018, 399, 219–234. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020, 69, 1181–1189. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Long, H.; Nie, L.; Xiang, X.; Li, H.; Zhang, X.; Fu, X.; Ren, H.; Liu, W.; Wang, Q.; Wu, Q. D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis. BioMed Res. Int. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Ma, J.; Wang, Q.; Wu, F.; Ping, J.; Ming, L. Combination of Circulating miRNA-320a/b and D-Dimer Improves Diagnostic Accuracy in Deep Vein Thrombosis Patients. Med. Sci. Monit. 2018, 24, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Starikova, I.; Jamaly, S.; Sorrentino, A.; Blondal, T.; Latysheva, N.; Sovershaev, M.; Hansen, J.-B. Differential expression of plasma miRNAs in patients with unprovoked venous thromboembolism and healthy control individuals. Thromb. Res. 2015, 136, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Guo, Y.; You, Y.; Hu, K.; Cai, F.; Xie, M.; Yang, L.; Ling, K.; Ye, D.; Misra, S.; et al. Deep Venous Thrombosis in COVID-19 Patients: A Cohort Analysis. Clin. Appl. Thromb. 2020, 26. [Google Scholar] [CrossRef]
- Zhu, X.-A.; Gao, L.-F.; Zhang, Z.-G.; Xiang, D.-K. Down-regulation of miR-320 exerts protective effects on myocardial I-R injury via facilitating Nrf2 expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1730–1741. [Google Scholar]
- Fu, S.; Zheng, Y.; Sun, Y.; Lai, M.; Qiu, J.; Gui, F.; Zeng, Q.; Liu, F. Suppressing long noncoding RNA OGRU ameliorates diabetic retinopathy by inhibition of oxidative stress and inflammation via miR-320/USP14 axis. Free Radic. Biol. Med. 2021, 169, 361–381. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, M.; Li, X.; Pan, N.; Bian, X.; Wu, W. Protective Effect of miR-193a-5p and miR-320-5p on Caerulein-Induced Injury in AR42J Cells. Dig. Dis. Sci. 2021, 1–11. [Google Scholar] [CrossRef]
- Xiao, C.; Yu, Y.; Liu, Y.; Yang, J. Aerosol inhalation of edaravone can improve inflammation, oxidative stress and pulmonary function of rats with smoke inhalation injury by down-regulating miR-320. Am. J. Transl. Res. 2021, 13, 2563–2570. [Google Scholar]
- Liu, L.; Li, X. Downregulation of miR-320 Alleviates Endoplasmic Reticulum Stress and Inflammatory Response in 3T3-L1 Adipocytes. Exp. Clin. Endocrinol. Diabetes 2021, 129, 131–137. [Google Scholar] [CrossRef]
- Pierdomenico, M.; Cesi, V.; Cucchiara, S.; Vitali, R.; Prete, E.; Costanzo, M.; Aloi, M.; Oliva, S.; Stronati, L. NOD2 Is Regulated By Mir-320 in Physiological Conditions but this Control Is Altered in Inflamed Tissues of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 315–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Chen, M.; Tellgren-Roth, C.; Pettersson, U. Fluctuating expression of microRNAs in adenovirus infected cells. Virology 2015, 478, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Tatsumi, T.; Hosui, A.; Nawa, T.; Kodama, T.; Shimizu, S.; Hikita, H.; Hiramatsu, N.; Kanto, T.; Hayashi, N.; et al. Alterations in microRNA expression profile in HCV-infected hepatoma cells: Involvement of miR-491 in regulation of HCV replication via the PI3 kinase/Akt pathway. Biochem. Biophys. Res. Commun. 2011, 412, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Q.; Li, B.; Qu, Y.; Li, Z.; Lu, L.; Li, R.; Cai, X. The Diagnosis Value of a Novel Model with 5 Circulating miRNAs for Liver Fibrosis in Patients with Chronic Hepatitis B. Mediat. Inflamm. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Perdaens, O.; Dang, H.A.; D’Auria, L.; van Pesch, V. CSF microRNAs discriminate MS activity and share similarity to other neuroinflammatory disorders. Neurol.-Neuroimmunol. Neuroinflamm. 2020, 7, e673. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wei, J.; Shang, F. Down-regulation of lncRNA SNHG5 relieves sepsis-induced acute kidney injury by regulating the miR-374a-3p/TLR4/NF-κB pathway. J. Biochem. 2021, 169, 575–583. [Google Scholar] [CrossRef]
- Dong, R.; Shen, Z.; Zheng, C.; Chen, G.; Zheng, S. Serum microRNA microarray analysis identifies miR-4429 and miR-4689 are potential diagnostic biomarkers for biliary atresia. Sci. Rep. 2016, 6, 21084. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Dai, W.-Z.; Zhu, X.-C.; Ma, T. Analysis of Serum miRNAs in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2021, 36. [Google Scholar] [CrossRef]
- Nikpay, M.; Beehler, K.; Valsesia, A.; Hager, J.; Harper, M.-E.; Dent, R.; McPherson, R. Genome-wide identification of circulating-miRNA expression quantitative trait loci reveals the role of several miRNAs in the regulation of cardiometabolic phenotypes. Cardiovasc. Res. 2019, 115, 1629–1645. [Google Scholar] [CrossRef]
- Donyavi, T.; Bokharaei-Salim, F.; Baghi, H.B.; Khanaliha, K.; Janat-Makan, M.A.; Karimi, B.; Nahand, J.S.; Mirzaei, H.; Khatami, A.; Garshasbi, S.; et al. Acute and post-acute phase of COVID-19: Analyzing expression patterns of miRNA-29a-3p, 146a-3p, 155-5p, and let-7b-3p in PBMC. Int. Immunopharmacol. 2021, 97, 107641. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; Benítez, I.D.; Pinilla, L.; Carratalá, A.; Moncusí-Moix, A.; Gort-Paniello, C.; Molinero, M.; González, J.; Torres, G.; Bernal, M.; et al. Circulating microRNA profiles predict the severity of COVID-19 in hospitalized patients. Transl. Res. 2021, 236, 147–159. [Google Scholar] [CrossRef]
- Garg, A.; Seeliger, B.; Derda, A.A.; Xiao, K.; Gietz, A.; Scherf, K.; Sonnenschein, K.; Pink, I.; Hoeper, M.M.; Welte, T.; et al. Circulating cardiovascular microRNAs in critically ill COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Gao, Y.; Li, Z.; Miao, Y.; Huang, Z.; Liu, X.; Xie, L.; Li, H.; Wen, W.; Zheng, Y.; et al. The noncoding and coding transcriptional landscape of the peripheral immune response in patients with COVID-19. Clin. Transl. Med. 2020, 10. [Google Scholar] [CrossRef]
- Maes, T.; Cobos, F.A.; Schleich, F.; Sorbello, V.; Henket, M.; De Preter, K.; Bracke, K.; Conickx, G.; Mesnil, C.; Vandesompele, J.; et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum. J. Allergy Clin. Immunol. 2016, 137, 1433–1446. [Google Scholar] [CrossRef] [Green Version]
- Rout-Pitt, N.; Farrow, N.; Parsons, D.; Donnelley, M. Epithelial mesenchymal transition (EMT): A universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir. Res. 2018, 19, 1–10. [Google Scholar] [CrossRef]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine profile and disease severity in patients with COVID-19. Cytokine 2021, 137, 155323. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Gomes, M.; Kruglov, A.; Durek, P.; Heinrich, F.; Tizian, C.; Heinz, G.A.; Pascual-Reguant, A.; Du, W.; Mothes, R.; Fan, C.; et al. SARS-CoV-2 in severe COVID-19 induces a TGF-β-dominated chronic immune response that does not target itself. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Zhao, B.; Tumaneng, K.; Guan, K.-L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Taha, Z.; Van Rensburg, H.J.J.; Yang, X. The Hippo Pathway: Immunity and Cancer. Cancers 2018, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Shirvaliloo, M. The blood-gas barrier in COVID-19: An overview of the effects of SARS-CoV-2 infection on the alveolar epithelial and endothelial cells of the lung. Tissue Barriers 2021, 1937013. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Dreier, B.; Morgan, J.T.; Tuyen, B.C.; Rose, B.W.; Reilly, C.M.; Russell, P.; Murphy, C.J. Involvement of YAP, TAZ and HSP90 in Contact Guidance and Intercellular Junction Formation in Corneal Epithelial Cells. PLoS ONE 2014, 9, e109811. [Google Scholar] [CrossRef] [Green Version]
- Huertas, A.; Montani, D.; Savale, L.; Pichon, J.; Tu, L.; Parent, F.; Guignabert, C.; Humbert, M. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020, 56, 2001634. [Google Scholar] [CrossRef] [PubMed]
- Sugano, T.; Masuda, M.; Takeshita, F.; Motoi, N.; Hirozane, T.; Goto, N.; Kashimoto, S.; Uno, Y.; Moriyama, H.; Sawa, M.; et al. Pharmacological blockage of transforming growth factor-β signalling by a Traf2- and Nck-interacting kinase inhibitor, NCB-0846. Br. J. Cancer 2021, 124, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Fussbroich, D.; Kohnle, C.; Schwenger, T.; Driessler, C.; Dücker, R.; Eickmeier, O.; Gottwald, G.; Jerkic, S.; Zielen, S.; Kreyenberg, H.; et al. A combination of LCPUFAs regulates the expression of miRNA-146a-5p in a murine asthma model and human alveolar cells. Prostaglandins Other Lipid Mediat. 2020, 147, 106378. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Paraskevopoulou, M.D.; Karagkouni, D.; Georgakilas, G.; Vergoulis, T.; Kanellos, I.; Anastasopoulos, I.-L.; Maniou, S.; Karathanou, K.; Kalfakakou, D.; et al. DIANA-TarBase v7.0: Indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res. 2015, 43, D153–D159. [Google Scholar] [CrossRef]

| Control | COVID-19 with Respiratory Failure | ||
|---|---|---|---|
| Moderate | Severe | ||
| n = | 8 | 11 | 10 |
| Age (years) | 68 (49–89) | 76 (48–91) | 69 (52–79) |
| Length of stay in UKF 1 (days) | --- | 10 (8–43) | 26 (14–47) |
| Comorbidities (number of cases) | |||
| Diabetes mellitus | --- | 4 | 4 |
| Hypertension | --- | 9 | 4 |
| Coronary disease | --- | 7 | 6 |
| Chronic kidney disease | --- | 2 | 1 |
| Chronic lung disease | --- | 4 | 3 |
| Immune suppression | --- | 2 | 2 |
| Cancer | --- | 3 | 1 |
| Obesity BMI ≥ 30 (kg/m2) | --- | 2 | 6 |
| Treatment (number of cases) | |||
| Anticoagulation | --- | 11 | 10 |
| Antibiotic therapy | --- | 3 | 10 |
| Catecholamines | --- | 1 | 10 |
| Dexamethasone | --- | 5 | 1 |
| Remdesivir | --- | 2 | 1 |
| Intubation | --- | 0 | 10 |
| Non-invasive ventilation | --- | 3 | 9 |
| Oxygen without ventilation | --- | 11 | 9 |
| Extracorporeal membrane oxygenation | --- | 0 | 2 |
| Mortality | --- | 0 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duecker, R.P.; Adam, E.H.; Wirtz, S.; Gronau, L.; Khodamoradi, Y.; Eberhardt, F.J.; Donath, H.; Gutmann, D.; Vehreschild, M.J.G.T.; Zacharowski, K.; et al. The MiR-320 Family Is Strongly Downregulated in Patients with COVID-19 Induced Severe Respiratory Failure. Int. J. Mol. Sci. 2021, 22, 10351. https://doi.org/10.3390/ijms221910351
Duecker RP, Adam EH, Wirtz S, Gronau L, Khodamoradi Y, Eberhardt FJ, Donath H, Gutmann D, Vehreschild MJGT, Zacharowski K, et al. The MiR-320 Family Is Strongly Downregulated in Patients with COVID-19 Induced Severe Respiratory Failure. International Journal of Molecular Sciences. 2021; 22(19):10351. https://doi.org/10.3390/ijms221910351
Chicago/Turabian StyleDuecker, Ruth P., Elisabeth H. Adam, Sarah Wirtz, Lucia Gronau, Yascha Khodamoradi, Fabian J. Eberhardt, Helena Donath, Desiree Gutmann, Maria J. G. T. Vehreschild, Kai Zacharowski, and et al. 2021. "The MiR-320 Family Is Strongly Downregulated in Patients with COVID-19 Induced Severe Respiratory Failure" International Journal of Molecular Sciences 22, no. 19: 10351. https://doi.org/10.3390/ijms221910351






