Nogo-A Induced Polymerization of Microtubule Is Involved in the Inflammatory Heat Hyperalgesia in Rat Dorsal Root Ganglion Neurons
Abstract
:1. Introduction
2. Results
2.1. Polymerization of Microtubule in DRG Neurons Was Increased in CFA-Induced Inflammatory Pain
2.2. Nogo-A Protein in DRG Promotes Inflammatory Heat Hyperalgesia by Increasing Microtubule Polymerization
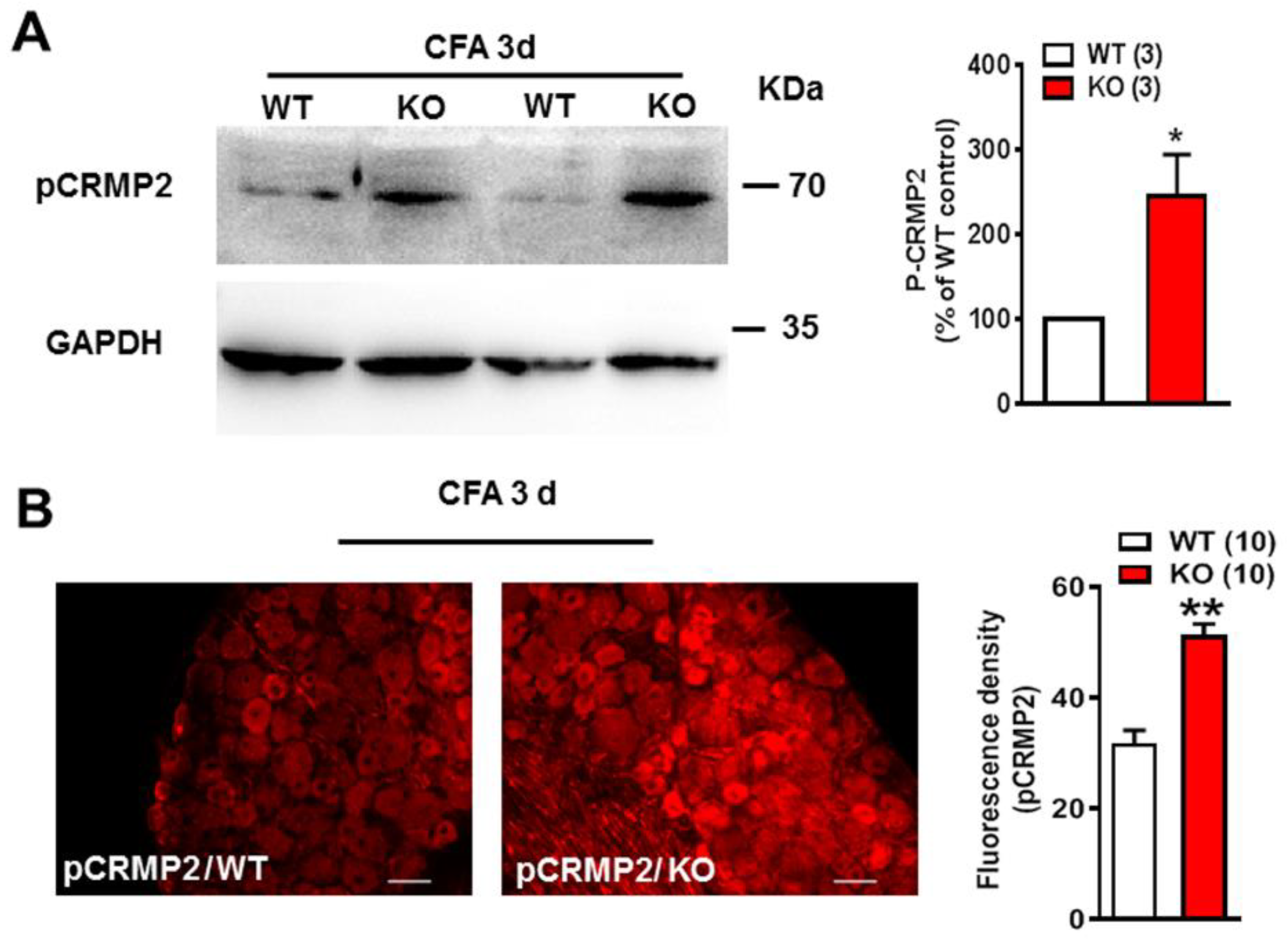
2.3. pCRMP2 Was Increased in DRG in Nogo-A KO Rats after CFA Administration
2.4. Nogo-A Was Necessary to Maintain Colocalization of Acetylated Microtubules and TRPV1
2.5. Inhibition of Microtubule Polymerization Attenuated Both CFA Induced Inflammatory Heat Hypersensitivity and Mechanical Pain in Spared Nerve Injury
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Inflammatory Pain Model
4.3. Spared Nerve Injury (SNI) Model
4.4. Behavior Test
4.4.1. Assessment of Heat Hypersensitivity
4.4.2. Assessment of Mechanical Allodynia
4.5. Intrathecal Injection of Nocodazole or Paclitaxel
4.6. Western Blot Analysis
4.7. Immunofluorescence Staining
4.8. Transmission Electron Microscopy
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, R.; Chen, Y.; Liedtke, W.B.; Morales-Lazaro, S.L. TRP Channels and Pain. In Neurobiology of TRP Channels, 2nd ed.; Emir, T.L.R., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 125–147. [Google Scholar]
- Dina, O.A.; McCarter, G.C.; de Coupade, C.; Levine, J.D. Role of the Sensory Neuron Cytoskeleton in Second Messenger Signaling for Inflammatory Pain. Neuron 2003, 39, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Liu, H.-C.; Su, D.-Q.; Chen, H.-J.; Chan, S.-O.; Wang, Y.; Wang, J. Nogo-A promotes inflammatory heat hyperalgesia by maintaining TRPV-1 function in the rat dorsal root ganglion neuron. FASEB J. 2018, 33, 668–682. [Google Scholar] [CrossRef]
- Li, Y.; Hu, F.; Chen, H.-J.; Du, Y.-J.; Xie, Z.-Y.; Zhang, Y.; Wang, J.; Wang, Y. LIMK-Dependent Actin Polymerization in Primary Sensory Neurons Promotes the Development of Inflammatory Heat Hyperalgesia in Rats. Sci. Signal. 2014, 7, ra61. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Kapitein, L.C.; Hoogenraad, C.C. Building the Neuronal Microtubule Cytoskeleton. Neuron 2015, 87, 492–506. [Google Scholar] [CrossRef] [Green Version]
- Akhmanova, A.; Steinmetz, M.O. Tracking the ends: A dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 2008, 9, 309–322. [Google Scholar] [CrossRef]
- Muroyama, A.; Lechler, T. Microtubule organization, dynamics and functions in differentiated cells. Development 2017, 144, 3012–3021. [Google Scholar] [CrossRef] [Green Version]
- Cartelli, D.; Casagrande, F.; Busceti, C.L.; Bucci, D.; Molinaro, G.; Traficante, A.; Passarella, D.; Giavini, E.; Pezzoli, G.; Battaglia, G.; et al. Microtubule Alterations Occur Early in Experimental Parkinsonism and The Microtubule Stabilizer Epothilone D Is Neuroprotective. Sci. Rep. 2013, 3, 1837. [Google Scholar] [CrossRef] [Green Version]
- Brandt, R.; Bakota, L. Microtubule dynamics and the neurodegenerative triad of Alzheimer’s disease: The hidden connection. J. Neurochem. 2017, 143, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuckowree, J.A.; Zhu, Z.; Brizuela, M.; Lee, K.M.; Blizzard, C.A.; Dickson, T.C. The Microtubule-Modulating Drug Epothilone D Alters Dendritic Spine Morphology in a Mouse Model of Mild Traumatic Brain Injury. Front. Cell. Neurosci. 2018, 12, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, T.J.; Guo, J.L.; Hurtado, D.E.; Kwong, L.K.; Mills, I.P.; Trojanowski, J.Q.; Lee, V.M.Y. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, K.; Clatterbuck-Soper, S.F.; Jackrel, M.E.; Shorter, J.; Mili, S. FUS inclusions disrupt RNA localization by sequestering kinesin-1 and inhibiting microtubule detyrosination. J. Cell Biol. 2017, 216, 1015–1034. [Google Scholar] [CrossRef] [Green Version]
- Krukowski, K.; Ma, J.; Golonzhka, O.; Laumet, G.O.; Gutti, T.; Van Duzer, J.H.; Mazitschek, R.; Jarpe, M.B.; Heijnen, C.J.; Kavelaars, A. HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain 2017, 158, 1126–1137. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.-J. Tubulin acetylation: Responsible enzymes, biological functions and human diseases. Cell Mol. Life Sci. 2015, 72, 4237–4255. [Google Scholar] [CrossRef]
- Morley, S.J.; Qi, Y.; Iovino, L.; Andolfi, L.; Guo, D.; Kalebic, N.; Castaldi, L.; Tischer, C.; Portulano, C.; Bolasco, G.; et al. Acetylated tubulin is essential for touch sensation in mice. eLife 2016, 5, e20813. [Google Scholar] [CrossRef]
- Goswami, C. TRPV1-tubulin complex: Involvement of membrane tubulin in the regulation of chemotherapy-induced peripheral neuropathy. J. Neurochem. 2012, 123, 1–13. [Google Scholar] [CrossRef]
- Goswami, C.; Hucho, T.B.; Hucho, F. Identification and characterisation of novel tubulin-binding motifs located within the C-terminus of TRPV1. J. Neurochem. 2007, 101, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Schmidt, H.; Hucho, F. TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J. 2007, 274, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Prager-Khoutorsky, M.; Khoutorsky, A.; Bourque, C.W. Unique interweaved microtubule scaffold mediates osmosensory transduction via physical interaction with TRPV1. Neuron 2014, 83, 866–878. [Google Scholar] [CrossRef] [Green Version]
- Schwab, M.E. Functions of Nogo proteins and their receptors in the nervous system. Nat. Rev. Neurosci. 2010, 11, 799–811. [Google Scholar] [CrossRef]
- Iobbi, C.; Korte, M.; Zagrebelsky, M. Nogo-66 Restricts Synaptic Strengthening via Lingo1 and the ROCK2–Cofilin Pathway to Control Actin Dynamics. Cereb. Cortex 2016, 27, 2276–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, Y.; Gao, X.; Ma, Y.; Gao, J.; Wang, Z.; Jin, W. A novel centrosome and microtubules associated subcellular localization of Nogo-A: Implications for neuronal development. Int. J. Biochem. Cell Biol. 2014, 57, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mimura, F.; Yamagishi, S.; Arimura, N.; Fujitani, M.; Kubo, T.; Kaibuchi, K.; Yamashita, T. Myelin-associated Glycoprotein Inhibits Microtubule Assembly by a Rho-kinase-dependent Mechanism. J. Biol. Chem. 2006, 281, 15970–15979. [Google Scholar] [CrossRef] [Green Version]
- Schmandke, A.; E Schwab, M. Nogo-A: Multiple Roles in CNS Development, Maintenance, and Disease. Neuroscience 2014, 20, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Pathak, G.P.; Shah, R.; Kennedy, B.E.; Murphy, J.P.; Clements, D.; Konda, P.; Giacomantonio, M.; Xu, Z.; Schlaepfer, I.R.; Gujar, S. RTN4 Knockdown Dysregulates the AKT Pathway, Destabilizes the Cytoskeleton, and Enhances Paclitaxel-Induced Cytotoxicity in Cancers. Mol. Ther. 2018, 26, 2019–2033. [Google Scholar] [CrossRef] [Green Version]
- Sekine, Y.; Algarate, P.T.; Cafferty, W.B.J.; Strittmatter, S.M. Plexina2 and CRMP2 Signaling Complex Is Activated by Nogo-A-Liganded Ngr1 to Restrict Corticospinal Axon Sprouting after Trauma. J. Neurosci. 2019, 39, 3204–3216. [Google Scholar] [CrossRef] [Green Version]
- Fukata, Y.; Itoh, T.J.; Kimura, T.; Ménager, C.; Nishimura, T.; Shiromizu, T.; Watanabe, H.; Inagaki, N.; Iwamatsu, A.; Hotani, H.; et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nature 2002, 4, 583–591. [Google Scholar] [CrossRef]
- Yoshimura, T.; Kawano, Y.; Arimura, N.; Kawabata, S.; Kikuchi, A.; Kaibuchi, K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 2005, 120, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Oláh, J.; Szenasi, T.; Szabó, A.; Kovács, K.; Lőw, P.; Štifanić, M.; Orosz, F. Tubulin Binding and Polymerization Promoting Properties of Tubulin Polymerization Promoting Proteins Are Evolutionarily Conserved. Biochemistry 2017, 56, 1017–1024. [Google Scholar] [CrossRef]
- Khanna, R.; Wilson, S.M.; Brittain, J.M.; Weimer, J.; Sultana, R.; Butterfield, A.; Hensley, K. Opening Pandora’s jar: A primer on the putative roles of CRMP2 in a panoply of neurodegenerative, sensory and motor neuron, and central disorders. Future Neurol. 2012, 7, 749–771. [Google Scholar] [CrossRef] [Green Version]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Feo, J.A.; Gallego-Delgado, J.; Puerto, M.; Wandosell, F.; Osende, J. Reticulon-4B/Nogo-B acts as a molecular linker between microtubules and actin cytoskeleton in vascular smooth muscle cells. Bba-Mol. Cell. Res. 2016, 1863, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, E.J.; Hyun, S.; Chun, J.; Kim, Y.; Kang, S.S. Phosphorylation on the Ser 824 residue of TRPV4 prefers to bind with F-actin than with microtubules to expand the cell surface area. Cell. Signal. 2012, 24, 641–651. [Google Scholar] [CrossRef] [PubMed]
- GrandPré, T.; Li, S.; Strittmatter, S. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 2002, 417, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Oertle, T.; van der Haar, M.E.; Bandtlow, C.E.; Robeva, A.; Burfeind, P.; Buss, A.; Huber, A.B.; Simonen, M.; Schnell, L.; Brosamle, C.; et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J. Neurosci. 2003, 23, 5393–5406. [Google Scholar] [CrossRef]
- Pertin, M.; Gosselin, R.-D.; Decosterd, I. The Spared Nerve Injury Model of Neuropathic Pain. Methods Mol. Biol. 2012, 851, 205–212. [Google Scholar] [PubMed]
- Yang, Y.-R.; He, Y.; Zhang, Y.; Li, Y.; Li, Y.; Han, Y.; Zhu, H.; Wang, Y. Activation of cyclin-dependent kinase 5 (Cdk5) in primary sensory and dorsal horn neurons by peripheral inflammation contributes to heat hyperalgesia. Pain 2007, 127, 109–120. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Zhao, M.; Ko, S.-Y.; Liu, J.-H.; Chen, D.; Zhang, J.; Wang, B.; Harris, S.E.; Oyajobi, B.O.; Mundy, G.R. Inhibition of Microtubule Assembly in Osteoblasts Stimulates Bone Morphogenetic Protein 2 Expression and Bone Formation through Transcription Factor Gli2. Mol. Cell. Biol. 2009, 29, 1291–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavanaugh, D.J.; Lee, H.; Lo, L.; Shields, S.D.; Zylka, M.J.; Basbaum, A.I.; Anderson, D.J. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl. Acad. Sci. USA 2009, 106, 9075–9080. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Hu, Q.; Liu, H.; Zhao, Y.; Chan, S.-O.; Wang, J. Nogo-A Induced Polymerization of Microtubule Is Involved in the Inflammatory Heat Hyperalgesia in Rat Dorsal Root Ganglion Neurons. Int. J. Mol. Sci. 2021, 22, 10360. https://doi.org/10.3390/ijms221910360
Chen L, Hu Q, Liu H, Zhao Y, Chan S-O, Wang J. Nogo-A Induced Polymerization of Microtubule Is Involved in the Inflammatory Heat Hyperalgesia in Rat Dorsal Root Ganglion Neurons. International Journal of Molecular Sciences. 2021; 22(19):10360. https://doi.org/10.3390/ijms221910360
Chicago/Turabian StyleChen, Ling, Qiguo Hu, Huaicun Liu, Yan Zhao, Sun-On Chan, and Jun Wang. 2021. "Nogo-A Induced Polymerization of Microtubule Is Involved in the Inflammatory Heat Hyperalgesia in Rat Dorsal Root Ganglion Neurons" International Journal of Molecular Sciences 22, no. 19: 10360. https://doi.org/10.3390/ijms221910360
APA StyleChen, L., Hu, Q., Liu, H., Zhao, Y., Chan, S.-O., & Wang, J. (2021). Nogo-A Induced Polymerization of Microtubule Is Involved in the Inflammatory Heat Hyperalgesia in Rat Dorsal Root Ganglion Neurons. International Journal of Molecular Sciences, 22(19), 10360. https://doi.org/10.3390/ijms221910360





