The Autophagy-Related Organelle Autophagoproteasome Is Suppressed within Ischemic Penumbra
Abstract
1. Introduction
2. Results
2.1. The Approach and the Outcomes of Middle Cerebral Artery (MCA) Occlusion on Various Brain Regions to Be Analyzed at Light and Electron Microscopy
2.2. Effects of MCA Occlusion on HSP70 and LC3 Expression within Area Penumbra (General Features)
2.3. Effects of MCA Occlusion on LC3 and P20S Expression within Area Penumbra at Light Microscopy
2.4. Effects of MCA Occlusion on the Amount of LC3, P20S and HSP70 within Area Penumbra at Western Blotting
2.5. Effects of MCA Occlusion within Area Penumbra at Electron Microscopy (General Features)
2.6. The Amount and Compartmentalization of LC3 within Area Penumbra
2.7. The Amount and Compartmentalization of P20S within Area Penumbra
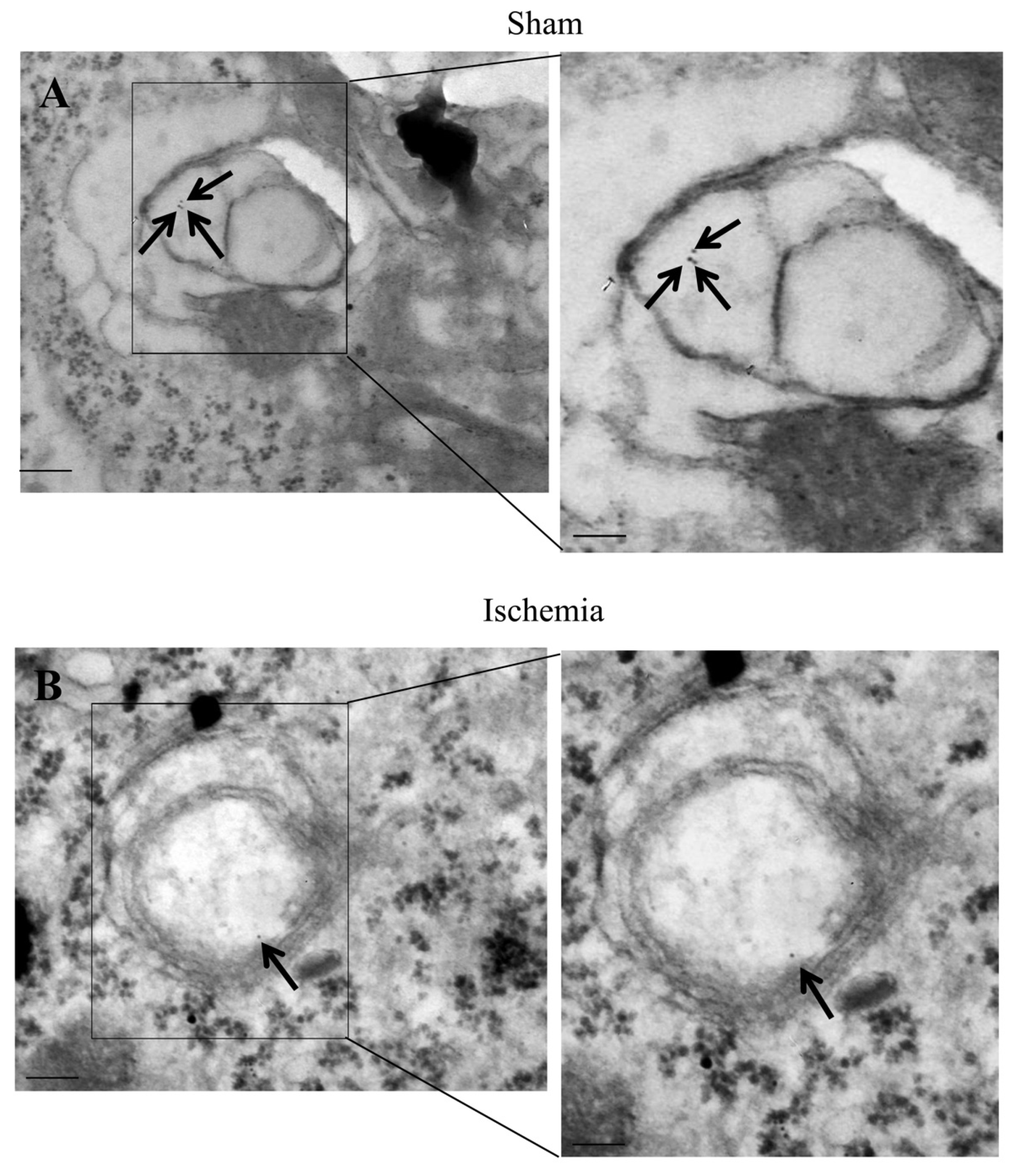
2.8. The Amount and Compartmentalization of P20S + LC3 Vacuoles (Autophagoproteasomes) within Area Penumbra
3. Discussion
4. Materials and Methods
4.1. Permanent Focal Ischemia in Mice
4.2. Tissue Preparation
4.3. Histology
4.4. Immune-Histochemical Analysis
4.5. SDS Page Immunoblotting
4.6. Transmission Electron Microscopy (TEM)
4.7. Post-Embedding Immuno-Gold Microscopy
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leigh, R.; Knutsson, L.; Zhou, J.; van Zijl, P.C. Imaging the physiological evolution of the ischemic penumbra in acute ischemic stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1500–1516. [Google Scholar] [CrossRef]
- Moseley, M.E.; Kucharczyk, J.; Mintorovitch, J.; Cohen, Y.; Kurhanewicz, J.; Derugin, N.; Asgari, H.; Norman, D. Diffusion-weighted MR imaging of acute stroke: Correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am. J. Neuroradiol. 1990, 11, 423–429. [Google Scholar]
- Detre, J.A.; Zager, E.L.; Alsop, D.C.; Harris, V.A.; Welsh, F.A. Correlation of diffusion MRI and heat shock protein in a rat embolic stroke model. J. Neurol. Sci. 1997, 148, 163–169. [Google Scholar] [CrossRef]
- Rami, A. Upregulation of Beclin 1 in the ischemic penumbra. Autophagy 2008, 4, 227–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rami, A.; Kögel, D. Apoptosis meets autophagy-like cell death in the ischemic penumbra: Two sides of the same coin? Autophagy 2008, 4, 422–426. [Google Scholar] [CrossRef]
- Rami, A.; Langhagen, A.; Steiger, S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol. Dis. 2008, 29, 132–141. [Google Scholar] [CrossRef]
- Ferrucci, M.; Biagioni, F.; Ryskalin, L.; Limanaqi, F.; Gambardella, S.; Frati, A.; Fornai, F. Ambiguous Effects of Autophagy Activation Following Hypoperfusion/Ischemia. Int. J. Mol. Sci. 2018, 19, 2756. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shao, B.Z.; Deng, Z.; Chen, S.; Yue, Z.; Miao, C.Y. Autophagy in ischemic stroke. Prog. Neurobiol. 2018, 163–164, 98–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Hu, G.; Zhang, G.; Zhao, G.; Shi, M. Ginsenoside Rd attenuates blood-brain barrier damage by suppressing proteasome-mediated signaling after transient forebrain ischemia. Neuroreport 2020, 31, 466–472. [Google Scholar] [CrossRef]
- Tadokoro, K.; Fukui, Y.; Yamashita, T.; Liu, X.; Tsunoda, K.; Shang, J.; Morihara, R.; Nakano, Y.; Tian, F.; Sasaki, R.; et al. Bone Marrow Stromal Cell Transplantation Drives Molecular Switch from Autophagy to the Ubiquitin-Proteasome System in Ischemic Stroke Mice. J. Stroke Cerebrovasc. Dis. 2020, 29, 104743. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yamashita, T.; Shang, J.; Shi, X.; Morihara, R.; Huang, Y.; Sato, K.; Takemoto, M.; Hishikawa, N.; Ohta, Y.; et al. Molecular switching from ubiquitin-proteasome to autophagy pathways in mice stroke model. J. Cereb. Blood Flow Metab. 2020, 40, 214–224. [Google Scholar] [CrossRef]
- He, G.Q.; Chen, Y.; Liao, H.J.; Xu, W.M.; Zhang, W.; He, G.L. Associations between Huwe1 and autophagy in rat cerebral neuron oxygen-glucose deprivation and reperfusion injury. Mol. Med. Rep. 2020, 22, 5083–5094. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, P.; Lazzeri, G.; Biagioni, F.; Busceti, C.L.; Gambardella, S.; Salvetti, A.; Fornai, F. The autophagoproteasome a novel cell clearing organelle in baseline and stimulated conditions. Front. Neuroanat. 2016, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, G.; Biagioni, F.; Fulceri, F.; Busceti, C.L.; Scavuzzo, M.C.; Ippolito, C.; Salvetti, A.; Lenzi, P.; Fornai, F. mTOR Modulates Methamphetamine-Induced Toxicity through Cell Clearing Systems. Oxid. Med. Cell. Longev. 2018, 2018, 6124745. [Google Scholar] [CrossRef] [PubMed]
- Limanaqi, F.; Biagioni, F.; Salvetti, A.; Puglisi-Allegra, S.; Lenzi, P.; Fornai, F. Morphology, clearing efficacy, and mTOR dependency of the organelle autophagoproteasome. Eur. J. Histochem. 2021, 65, 3220. [Google Scholar] [CrossRef]
- Cohen-Kaplan, V.; Livneh, I.; Avni, N.; Fabre, B.; Ziv, T.; Kwon, Y.T.; Ciechanover, A. p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc. Natl. Acad. Sci. USA 2016, 113, E7490–E7499. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Ab-udu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Kinouchi, H.; Sharp, F.R.; Hill, M.P.; Koistinaho, J.; Sagar, S.M.; Chan, P.H. Induction of 70-kDa heat shock protein and hsp70 mRNA following transient focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 1993, 13, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Matson, G.B.; Liu, J.; Mancuso, A.; Kayama, T.; Sharp, F.R.; Weinstein, P.R. Correlation between changes in apparent diffusion coefficient and induction of heat shock protein, cell-specific injury marker expression and protein synthesis reduction on diffusion-weighted magnetic resonance images after temporary focal cerebral ischemia in rats. J. Neurosurg. 2002, 96, 1084–1093. [Google Scholar]
- Weinstein, P.R.; Hong, S.; Sharp, F.R. Molecular identification of the ischemic penumbra. Stroke 2004, 35, 2666–26670. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.; Yoon, B.W.; Kim, Y.J.; Ma, S.J.; Roh, J.K.; Lee, J.S.; Seo, J.S. Targeted hsp70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke 2001, 32, 2905–2912. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, H.M.; Kim, Y.J.; Lee, K.M.; Kim, M.; Yoon, B.W. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke 2004, 35, 2195–2199. [Google Scholar] [CrossRef]
- Giffard, R.G.; Yenari, M.A. Many mechanisms for hsp70 protection fromcerebralischemia. J. Neurosurg. Anesthesiol. 2004, 16, 53–61. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, Y.; Lee, J.E.; Yenari, M.A. The 70-kDa heat shock protein (Hsp70) as a therapeutic target for stroke. Expert Opin. Ther. Targets 2018, 22, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, G.; Busceti, C.L.; Biagioni, F.; Fabrizi, C.; Morucci, G.; Giorgi, F.S.; Ferrucci, M.; Lenzi, P.; Puglisi-Allegra, S.; Fornai, F. Norepinephrine Protects against Methamphetamine Toxicity through β2-Adrenergic Receptors Promoting LC3 Compartmentalization. Int. J. Mol. Sci. 2021, 22, 7232. [Google Scholar] [CrossRef]
- Backhauss, C.; Karkoutly, C.; Welsch, M.; Krieglstein, J. A mouse model of focal cerebral ischemia for screening neuroprotective drug effects. J. Pharmacol. Toxicol. Methods 1992, 27, 27–32. [Google Scholar] [CrossRef]
- Mastroiacovo, F.; Busceti, C.L.; Biagioni, F.; Moyanova, S.G.; Meisler, M.H.; Battaglia, G.; Caricasole, A.; Bruno, V.; Nicoletti, F. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Osborne, K.A.; Shigeno, T.; Balarsky, A.M.; Ford, I.; McCulloch, J.; Teasdale, G.M.; Graham, D.I. Quantitative assessment of early brain damage in a rat model of focal cerebral ischemia. J. Neurol. Neurosurg. Psychiatry 1987, 50, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, G.; Lenzi, P.; Busceti, C.L.; Ferrucci, M.; Falleni, A.; Bruno, V.; Paparelli, A.; Fornai, F. Mechanisms involved in the formation of dopamine-induced intracellular bodies within striatal neurons. J. Neurochem. 2007, 101, 1414–1427. [Google Scholar] [CrossRef]
- Mattii, L.; Bianchi, F.; Falleni, A.; Frascarelli, S.; Masini, M.; Alì, G.; Chiellini, G.; Sabbatini, A.R.M. Ultrastructural Localization of Histidine-rich Glycoprotein in Skeletal Muscle Fibers: Colocalization With AMP Deaminase. J. Histochem. Cytochem. 2020, 68, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bendayan, M.; Zollinger, M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J. Histochem. Cytochem. 1983, 31, 101–109. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.; Mattii, L.; Moscato, S.; Bernardini, N.; Segnani, C.; Dolfi, A.; Bianchi, F. Immunohistochemical demonstration of the small GTPase RhoA-A on epoxyresin embedded sections. Micron 2004, 35, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Falleni, A.; Lucchesi, P.; Ghezzani, C.; Brogger, M.I. Ultrastructural and cytochemical aspects of the germarium and the vitellarium in Syndesmis patagonica (Platyhelminthes, Rhabdocoela, Umagillidae). J. Morphol. 2014, 275, 703–719. [Google Scholar] [CrossRef]
- Moscato, S.; Cabiati, M.; Bianchi, F.; Vaglini, F.; Morales, M.A.; Burchielli, S.; Botta, L.; Sabbatini, A.R.M.; Falleni, A.; Del Ry, S.; et al. Connexin 26 expression in mammalian cardiomyocytes. Sci. Rep. 2018, 8, 13975–13985. [Google Scholar] [CrossRef]
- Lucocq, J.M.; Habermann, A.; Watt, S.; Backer, J.M.; Mayhew, T.M.; Griffiths, G. A rapid method for assessing the distribution of gold labeling on thin sections. J. Histochem. Cytochem. 2004, 52, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Lucocq, J.M.; Hacker, C. Cutting a fine figure: On the use of thin sections in electron microscopy to quantify autophagy. Autophagy 2013, 9, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Swanlund, J.M.; Kregel, K.C.; Oberley, T.D. Investigating autophagy: Quantitative morphometric analysis using electron microscopy. Autophagy 2010, 6, 270–277. [Google Scholar] [CrossRef]
















| Antibody | Distributor | Catalog Number | RRID | Concentration |
|---|---|---|---|---|
| Rabbit polyclonal anti LC3B | Santa Cruz Biotechnology, Dallas, TX, USA | Cod. SC28266 | AB_2137719 | 1:50 |
| Monoclonal mouse anti P20S | Abcam, Cambridge, UK | Cod. AB22674 | AB_2171376 | 1:100 l.m. 1:20 e.m. 1:1000 w.b. |
| Monoclonal mouse anti HSP70 | Thermo Fisher Scientific, Waltham, MA, USA | Cod. 33-3800 | AB_2533116 | 1:100 |
| Rabbit polyclonal anti LC3B | Abcam, Cambridge, UK | Cod. AB128025 | AB_11143008 | 1:50 |
| Rabbit polyclonal anti Beclin 1 | Abcam, Cambridge, UK | Cod. AB62557 | AB_955699 | 1:50 |
| Rabbit anti Caspase 9, active (cleaved) | Millipore, Burlington, MA, USA | Cod. AB3629 | AB_91558 | 1:50 |
| Alexafluor 488 anti-mouse | Thermo Fisher Scientific, Waltham, MA, USA | Cod. A21202 | AB_141607 | 1:100 |
| Donkey x Rabbit CY3 | Millipore, Burlington, MA, USA | Cod. AP182C | AB_92588 | 1:300 |
| Goat Anti-Rabbit IgG Antibody, 20 nm Gold Conjugated | Bbi solutions, Edinburgh, UK | Cod. EM GAR20/0.25 | AB_1769136 | 1:50 |
| Goat Anti-Mouse IgG Antibody, 10 nm Gold Conjugated | Bbi solutions, Edinburgh, UK | Cod. EM GAR10/0.25 | AB_1769128 | 1:50 |
| Horse Anti-Mouse IgG Antibody (H + L), Biotinylated | Vector Labs | BA-2000-1.5 | AB_2313581 | 1:200 |
| Fluorescein anti-Rabbit | Vector Labs | FI-1000-1.5 | AB_2336197 | 1:100 |
| Rabbit polyclonal anti HSP70 | Cell signaling, Danvers, MA, USA | 4872S | AB_2279841 | 1:1000 |
| Mouse monoclonal anti LC3 | MBL International, Woburn, MA, USA | M186-3 | AB_10897859 | 1:1000 |
| Mouse anti beta actin | Sigma Aldrich | A1978 | AB_476692 | 1:25,000 |
| Goat Anti-Rabbit | Millipore | 401-393 | AB_437797 | 1:3000 |
| Millipore | 401-215 | AB_10682749 | 1:3000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biagioni, F.; Mastroiacovo, F.; Lenzi, P.; Puglisi-Allegra, S.; Busceti, C.L.; Ryskalin, L.; Ferese, R.; Bucci, D.; Frati, A.; Nicoletti, F.; et al. The Autophagy-Related Organelle Autophagoproteasome Is Suppressed within Ischemic Penumbra. Int. J. Mol. Sci. 2021, 22, 10364. https://doi.org/10.3390/ijms221910364
Biagioni F, Mastroiacovo F, Lenzi P, Puglisi-Allegra S, Busceti CL, Ryskalin L, Ferese R, Bucci D, Frati A, Nicoletti F, et al. The Autophagy-Related Organelle Autophagoproteasome Is Suppressed within Ischemic Penumbra. International Journal of Molecular Sciences. 2021; 22(19):10364. https://doi.org/10.3390/ijms221910364
Chicago/Turabian StyleBiagioni, Francesca, Federica Mastroiacovo, Paola Lenzi, Stefano Puglisi-Allegra, Carla L. Busceti, Larisa Ryskalin, Rosangela Ferese, Domenico Bucci, Alessandro Frati, Ferdinando Nicoletti, and et al. 2021. "The Autophagy-Related Organelle Autophagoproteasome Is Suppressed within Ischemic Penumbra" International Journal of Molecular Sciences 22, no. 19: 10364. https://doi.org/10.3390/ijms221910364
APA StyleBiagioni, F., Mastroiacovo, F., Lenzi, P., Puglisi-Allegra, S., Busceti, C. L., Ryskalin, L., Ferese, R., Bucci, D., Frati, A., Nicoletti, F., & Fornai, F. (2021). The Autophagy-Related Organelle Autophagoproteasome Is Suppressed within Ischemic Penumbra. International Journal of Molecular Sciences, 22(19), 10364. https://doi.org/10.3390/ijms221910364









