Selection and Validation of Candidate Reference Genes for Gene Expression Analysis by RT-qPCR in Rubus
Abstract
:1. Introduction
2. Results
2.1. Selection and Expression Levels of Reference Genes
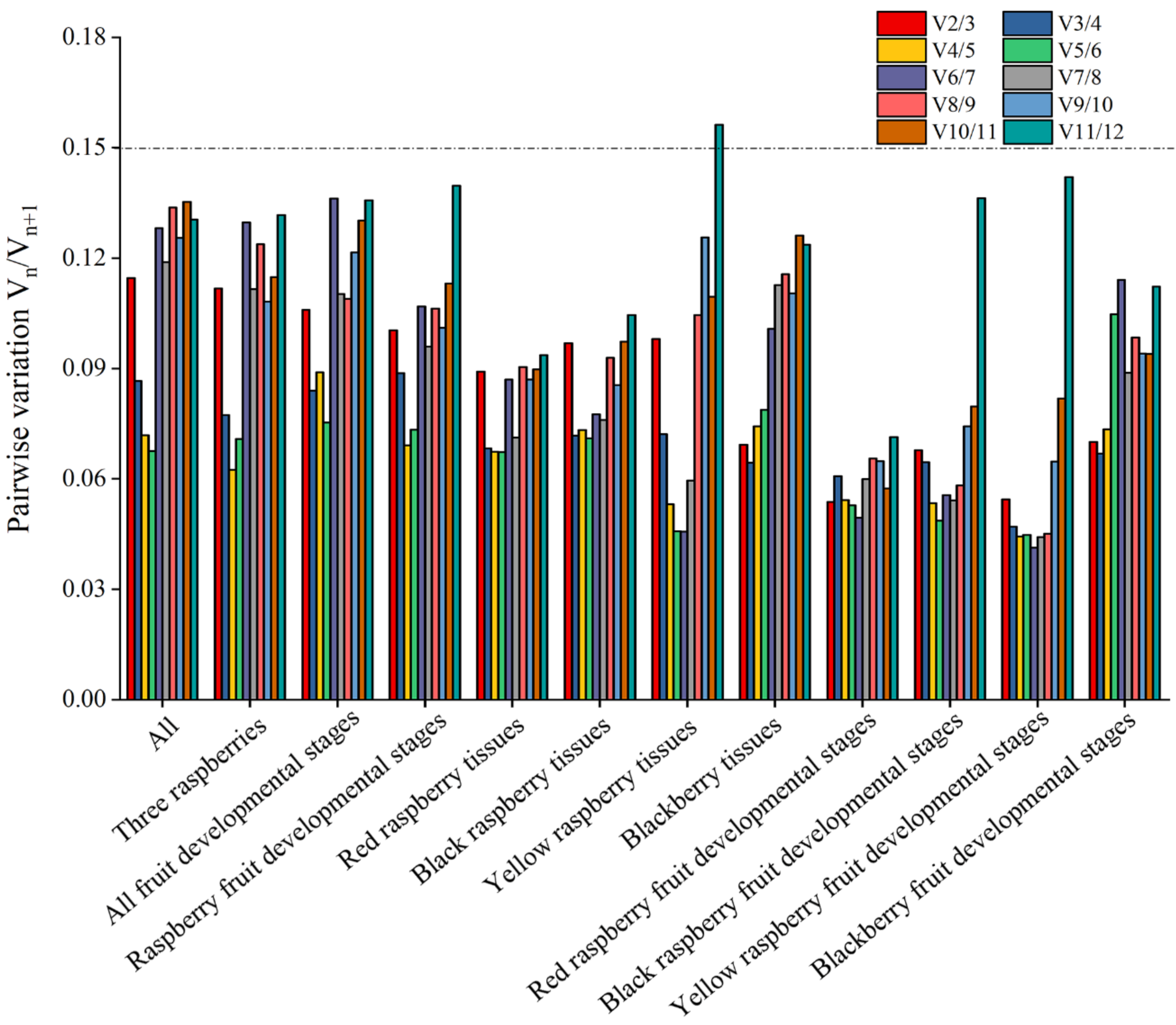
2.2. Estimation of Stability by geNorm Analysis
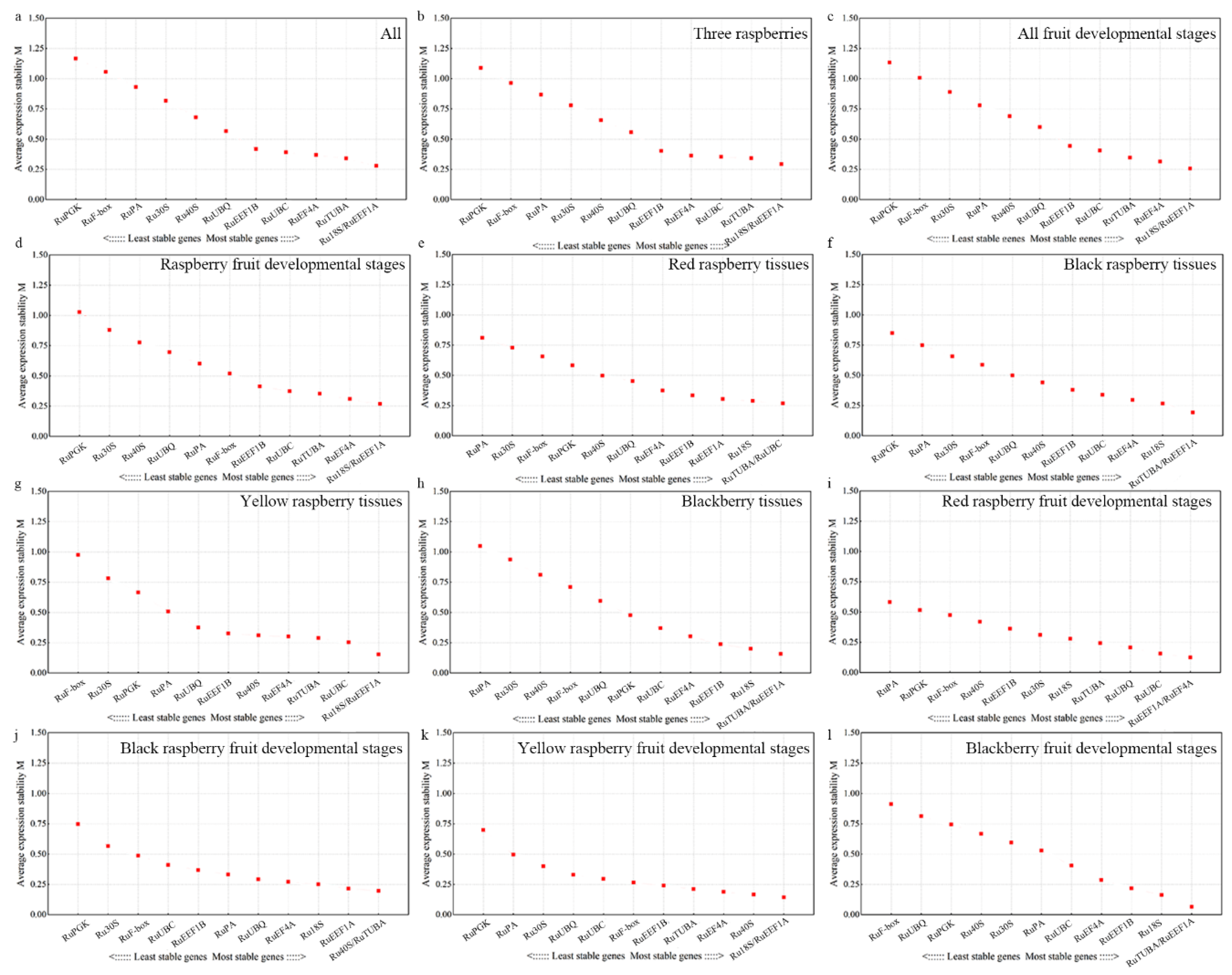
2.3. Estimation of Stability by NormFinder
2.4. Estimation of Stability by BestKeeper
2.5. Comprehensive Ranking Analysis
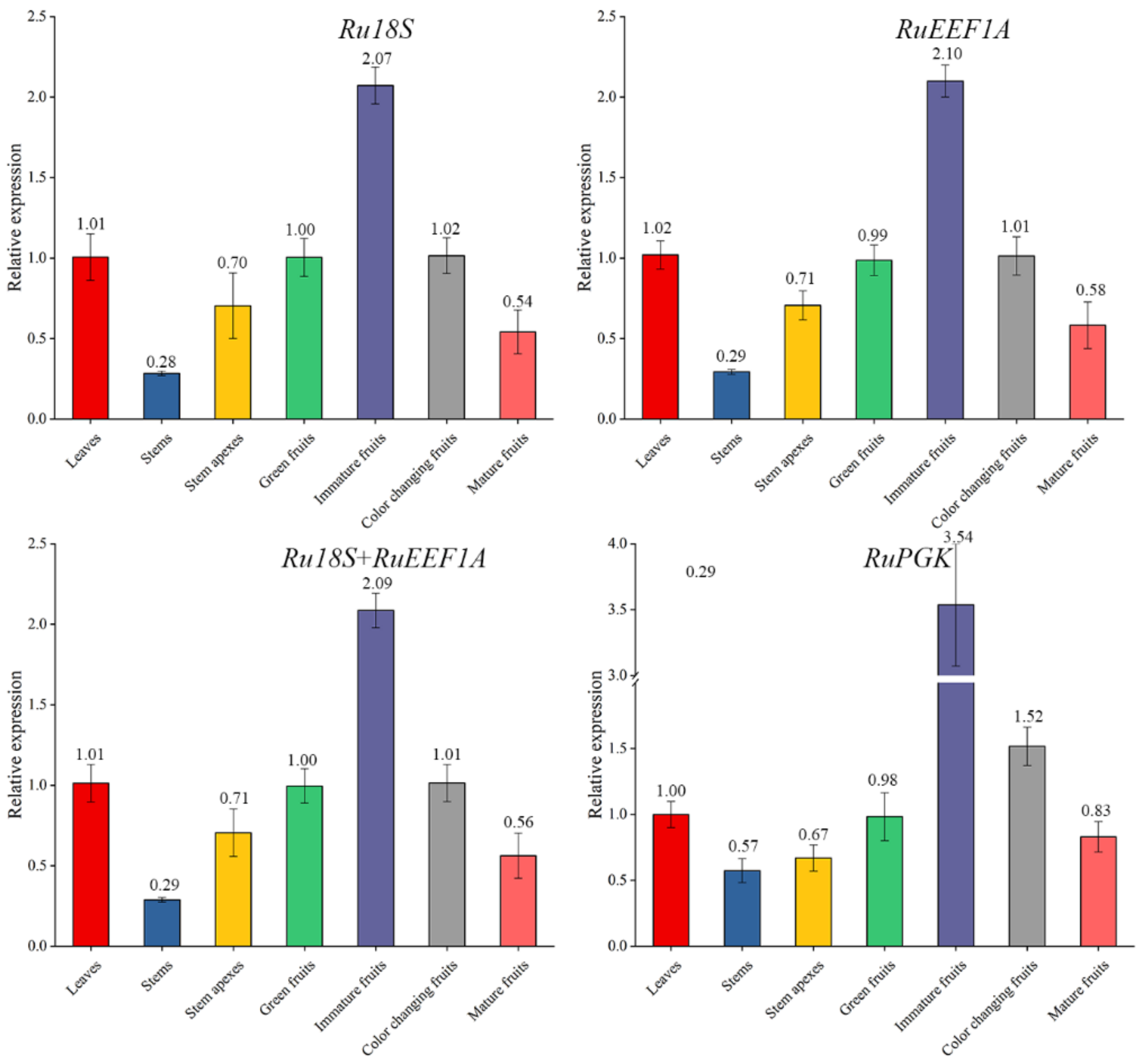
2.6. Validation of the Selected Candidate Reference Genes
3. Discussion
4. Material and Methods
4.1. Plant Materials
4.2. Primer Design for Candidate Reference Genes
4.3. Total RNA Extraction and cDNA Synthesis
4.4. RT-qPCR Amplification
4.5. Data Processing and Analysis
4.6. Validation of Selected Reference Genes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Seco, D.; Zhang, Y.; Gutierrez-Mañero, F.J.; Martin, C.; Ramos-Solano, B. RNA-Seq analysis and transcriptome assembly for blackberry (Rubus sp. Var. Lochness) fruit. BMC Genom. 2015, 16, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travisany, D.; Ayala-Raso, A.; Di Genova, A.; Monsalve, L.; Bernales, M.; Martínez, J.P.; González-Agüero, M.; Defilippi, B.; Cherian, S.; Maass, A.; et al. RNA-Seq analysis and transcriptome assembly of raspberry fruit (Rubus idaeus ¨Heritage¨) revealed several candidate genes involved in fruit development and ripening. Sci. Hortic. 2019, 254, 26–34. [Google Scholar] [CrossRef]
- Zafra-Rojas, Q.Y.; González-Martínez, B.E.; Cruz-Cansino, N.D.S.; López-Cabanillas, M.; Suárez-Jacobo, A.; Cervantes-Elizarrarás, A.; Ramírez-Moreno, E. Effect of Ultrasound on In Vitro Bioaccessibility of Phenolic Compounds and Antioxidant Capacity of Blackberry (Rubus fruticosus) Residues cv. Tupy. Plant Foods Hum. Nutr. 2020, 75, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Strik, B.C.; Clark, J.R.; Finn, C.E.; Bañados, M.P. Worldwide Blackberry Production. HortTechnology 2007, 17, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Vlad, I.A.; Goji, G.; Dinulică, F.; Bartha, S.; Vasilescu, M.M.; Mihaiescu, T. Consuming Blackberry as a Traditional Nutraceutical Resource from an Area with High Anthropogenic Impact. Forests 2019, 10, 246. [Google Scholar] [CrossRef] [Green Version]
- Galli, V.; Borowski, J.M.; Perin, E.C.; Messias, R.D.S.; Labonde, J.; Pereira, I.D.S.; Silva, S.D.D.A.; Rombaldi, C.V. Validation of reference genes for accurate normalization of gene expression for real time-quantitative PCR in strawberry fruits using different cultivars and osmotic stresses. Gene 2015, 554, 205–214. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, A.; Yang, S.; Huang, L. Reference gene selection for real-time quantitative PCR normalization in Hemarthria compressa and Hemarthria altissima leaf tissue. Mol. Biol. Rep. 2019, 46, 4763–4769. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Y.; Sun, H. Selection of reference genes for RT-qPCR normalization in blueberry (Vaccinium corymbosum × angustifolium) under various abiotic stresses. FEBS Open Bio 2020, 10, 1418–1435. [Google Scholar] [CrossRef]
- Chen, C.; Yuan, M.; Song, J.; Liu, Y.; Xia, Z.; Yuan, Y.; Wang, W.; Xie, Q.; Guan, X.; Chen, Q.; et al. Genome-wide identification and testing of superior reference genes for transcript normalization during analyses of flesh development in Asian pear cultivars. Sci. Hortic. 2020, 271, 109459. [Google Scholar] [CrossRef]
- Joseph, J.T.; Poolakkalody, N.J.; Shah, J.M. Plant reference genes for development and stress response studies. J. Biosci. 2018, 43, 173–187. [Google Scholar] [CrossRef]
- Hwang, J.; Oh, C.-S.; Kang, B.-C. Translation elongation factor 1B (eEF1B) is an essential host factor for Tobacco mosaic virus infection in plants. Virology 2013, 439, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, J.; Xu, J.; Jin, W.; Yang, L.; Yin, T.; Shi, J. Identification of Reference Genes for Quantitative Gene Expression Studies in Pinus massoniana and Its Introgression Hybrid. Forests 2019, 10, 787. [Google Scholar] [CrossRef] [Green Version]
- Karuppaiya, P.; Yan, X.-X.; Liao, W.; Wu, J.; Chen, F.; Tang, L. Identification and validation of superior reference gene for gene expression normalization via RT-qPCR in staminate and pistillate flowers of Jatropha curcas—A biodiesel plant. PLoS ONE 2017, 12, e0172460. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Chiluisa-Utreras, V.; Vaca, I.; Chicaiza, O.; Peñaherrera, S. Analysis of the expression of β-actin, RuANS and RuMYB10 genes involved in the biosynthesis of anthocyanin using RT-qPCR in Rubus niveus in the Rumiñahui Canton. J. Berry Res. 2017, 7, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Gotame, T.P.; Cullen, D.W.; Graham, J.; Hedley, P.E.; Smith, K.; Morris, J.; Andersen, L.; Petersen, K.K. Effect of short-term exposure to high-temperature on total gene expression in the leaves of four raspberry (Rubus idaeus L.) cultivars. J. Hortic. Sci. Biotech. 2014, 89, 532–541. [Google Scholar] [CrossRef]
- Jain, N.; Vergish, S.; Khurana, J.P. Validation of house-keeping genes for normalization of gene expression data during diurnal/circadian studies in rice by RT-qPCR. Sci. Rep. 2018, 8, 3203. [Google Scholar] [CrossRef] [Green Version]
- Shabrangy, A.; Roustan, V.; Reipert, S.; Weidinger, M.; Roustan, P.-J.; Stoeger, E.; Weckwerth, W.; Ibl, V. Using RT-qPCR, Proteomics, and Microscopy to Unravel the Spatio-Temporal Expression and Subcellular Localization of Hordoindolines Across Development in Barley Endosperm. Front. Plant Sci. 2018, 9, 775. [Google Scholar] [CrossRef]
- Kumar, D.; Das, P.K.; Sarmah, B.K. Reference gene validation for normalization of RT-qPCR assay associated with germination and survival of rice under hypoxic condition. J. Appl. Genet. 2018, 59, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, P.F.V.; Núñez, M.M.; Rivas, M.R.; Portillo, R.E.V.; Bibbins-Martínez, M.D.; Suárez, S.L.; Cárdenas, F.F.R. Reference genes for RT-qPCR normalisation in different tissues, developmental stages and stress conditions of amaranth. Plant Biol. 2018, 20, 713–721. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, X.; Sun, M.; Cong, H.; Qiao, F. Evaluation of reference genes for normalizing RT-qPCR in leaves and suspension cells of Cephalotaxus hainanensis under various stimuli. Plant Methods 2019, 15, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huggett, J.F.; Dheda, K.; Bustin, S.; Zumla, P.S.A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005, 6, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamori, L.M.; Pereira, A.V.C.; Souza, G.M.; Vieira, L.G.E.; Ribas, A.F. Identification of Endogenous Reference Genes for RT-qPCR Expression Analysis in Urochloa brizantha Under Abiotic Stresses. Sci. Rep. 2017, 7, 8502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Niu, J.; Quan, S. Identification of appropriate reference genes for RT-qPCR analysis in Juglans regia L. PLoS ONE 2018, 13, e0209424. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.M.; Yakovlev, I.A.; Strauss, S.H. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol. 2004, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.; Tao, Y.-B.; Chen, M.-S.; Fu, Q.; Li, C.; Dong, Y.; Wang, X.; He, H.; Xu, Z.-F. Selection of Reliable Reference Genes for Gene Expression Studies of a Promising Oilseed Crop, Plukenetia volubilis, by Real-Time Quantitative PCR. Int. J. Mol. Sci. 2015, 16, 12513–12530. [Google Scholar] [CrossRef] [Green Version]
- Zarivi, O.; Cesare, P.; Ragnelli, A.M.; Aimola, P.; Leonardi, M.; Bonfigli, A.; Colafarina, S.; Poma, A.M.; Miranda, M.; Pacioni, G. Validation of reference genes for quantitative real-time PCR in Périgord black truffle (Tuber melanosporum) developmental stages. Phytochemistry 2015, 116, 78–86. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [Green Version]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.-Q.; Guo, W.-C.; Wan, P.-J.; Zhou, L.-T.; Ren, X.-L.; Ahmat, T.; Fu, K.-Y.; Li, G.-Q. Validation of reference genes for expression analysis by quantitative real-time PCR in Leptinotarsa decemlineata (Say). BMC Res. Notes 2013, 6, 93. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xie, W.; Yu, X.; Zhang, Z.; Zhao, Y.; Wang, N.; Wang, Y. Selection of Suitable Reference Genes for RT-qPCR Gene Expression Analysis in Siberian Wild Rye (Elymus sibiricus) under Different Experimental Conditions. Genes 2019, 10, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruduś, I.; Kępczyński, J. Reference gene selection for molecular studies of dormancy in wild oat (Avena fatua L.) caryopses by RT-qPCR method. PLoS ONE 2018, 13, e0192343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, S.; Ahmed, R.; Haque, S.; Alam, M.; Islam, S. Identification and validation of reference genes for real-time quantitative RT-PCR analysis in jute. BMC Mol. Biol. 2019, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Lucho, S.R.; do Amaral, M.N.; Benitez, L.C.; Milech, C.; Kleinowski, A.M.; Bianchi, V.J.; Braga, E.J.B. Validation of reference genes for RT-qPCR studies in Stevia rebaudiana in response to elicitor agents. Physiol. Mol. Biol. Plants 2018, 24, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Shi, Q.; Yang, T.; Fei, Z.; Wei, A. Expression Stabilities of Ten Candidate Reference Genes for RT-qPCR in Zanthoxylum bungeanum Maxim. Molecules 2018, 23, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Length (bp) |
|---|---|---|---|
| Ru18S | ACGTCATCCTCCGGCAAAGC | ACGACGAAGCTCGCAAGTACAC | 103 |
| Ru30S | ACCCGACTTGCGTCCTACACT | AGCGCTTGACCCATTGGAAGC | 119 |
| Ru40S | GGGACCAAGCCATGGGTAAGC | CTAAGCTGCGGCTGTGGACTG | 105 |
| RuTUBA | ATCCTTCTCGAGGGCGGCAA | AAGCGTGCGTTTGTGCACTG | 93 |
| RuEEF1A | CCTTTCGCGCTCAGCCTTGA | AAGTCGACCACCACGGGTCA | 150 |
| RuEEF1B | CCACCATGGCCGTCACCTTC | GACGAAACGCAGCCGTACCA | 193 |
| RuEF4A | GTGCAGCAGGTCTCGCTTGT | TGGCAACACCCTTCCTCCCA | 106 |
| RuF-box | GGGATCCATTGCCAGCAGCA | GCAGCCGGAGAAGGATGTCTG | 72 |
| RuUBC | AGGGAATCCCACCGGACCAG | TCAGCCAAAGTGCGACCATCC | 73 |
| RuUBQ | GGCCGCACCCTTGCAGATTA | TGCATCCCACCACGTAGACGA | 77 |
| RuPA | GCTTCAGCAAGACTCCCATAAGGC | CAGGCAGAGCTCGTTGGTTGT | 104 |
| RuPGK | ACTAGGGTCCGTGCTGCTGT | CAGCAAATCCACCACAACCCACA | 191 |
| Rank | All Samples | Three Raspberries | All Fruit Developmental Stages | Raspberry Fruit Developmental Stages | Red Raspberry Tissues | Black Raspberry Tissues | ||||||||||||
| Gene Name | SD | CV (%) | Gene Name | SD | CV (%) | Gene Name | SD | CV (%) | Gene Name | SD | CV (%) | Gene Name | SD | CV (%) | Gene Name | SD | CV (%) | |
| 1 | RuEEF1A | 0.13 | 0.52 | RuEEF1A | 0.14 | 0.56 | RuEEF1A | 0.11 | 0.45 | Ru18S | 0.13 | 0.55 | Ru18S | 0.14 | 0.59 | RuTUBA | 0.13 | 0.53 |
| 2 | Ru18S | 0.17 | 0.73 | Ru18S | 0.15 | 0.63 | Ru18S | 0.14 | 0.59 | RuEEF1A | 0.14 | 0.56 | RuTUBA | 0.16 | 0.62 | Ru18S | 0.14 | 0.58 |
| 3 | RuTUBA | 0.22 | 0.88 | RuTUBA | 0.24 | 0.96 | RuTUBA | 0.25 | 1.01 | RuEF4A | 0.22 | 0.87 | RuEEF1A | 0.17 | 0.66 | RuEEF1A | 0.18 | 0.73 |
| 4 | RuUBC | 0.28 | 1.16 | RuUBC | 0.24 | 1.02 | RuEF4A | 0.25 | 1.00 | RuTUBA | 0.29 | 1.15 | RuUBC | 0.24 | 1.00 | RuEF4A | 0.24 | 0.94 |
| 5 | RuEF4A | 0.3 | 1.18 | RuEF4A | 0.29 | 1.14 | RuUBC | 0.36 | 1.48 | RuUBC | 0.31 | 1.28 | RuEEF1B | 0.30 | 1.21 | RuUBC | 0.28 | 1.17 |
| 6 | RuEEF1B | 0.33 | 1.34 | RuEEF1B | 0.37 | 1.48 | RuEEF1B | 0.39 | 1.55 | RuEEF1B | 0.41 | 1.63 | RuEF4A | 0.31 | 1.25 | RuEEF1B | 0.29 | 1.16 |
| 7 | RuUBQ | 0.81 | 2.99 | RuUBQ | 0.84 | 3.08 | RuPA | 0.71 | 2.67 | RuF-box | 0.50 | 1.64 | Ru40S | 0.48 | 1.95 | Ru40S | 0.34 | 1.28 |
| 8 | Ru40S | 0.98 | 3.89 | Ru40S | 0.96 | 3.85 | RuUBQ | 0.89 | 3.25 | RuPA | 0.55 | 2.11 | RuUBQ | 0.52 | 1.95 | RuUBQ | 0.53 | 1.86 |
| 9 | RuPA | 1.15 | 4.35 | RuPA | 1.01 | 1.25 | Ru40S | 0.96 | 3.81 | RuUBQ | 0.87 | 3.18 | RuPGK | 0.73 | 3.06 | RuF-box | 0.81 | 2.71 |
| 10 | Ru30S | 1.21 | 4.91 | RuF-box | 1.05 | 3.54 | RuF-box | 0.98 | 3.26 | Ru40S | 1.09 | 4.32 | RuF-box | 0.76 | 2.52 | Ru30S | 0.83 | 3.31 |
| 11 | RuPGK | 1.23 | 4.86 | Ru30S | 1.19 | 4.85 | Ru30S | 1.17 | 4.68 | RuPGK | 1.17 | 4.68 | Ru30S | 1.06 | 4.34 | RuPA | 0.91 | 3.42 |
| 12 | RuF-box | 1.30 | 4.44 | RuPGK | 1.25 | 5.01 | RuPGK | 1.21 | 4.78 | Ru30S | 1.27 | 5.18 | RuPA | 1.09 | 4.25 | RuPGK | 1.23 | 4.97 |
| Rank | Yellow Raspberry Tissues | Blackberry Tissues | Red Raspberry Fruit Developmental Stages | Black Raspberry Fruit Developmental Stages | Yellow Raspberry Fruit Developmental Stages | Blackberry Fruit Developmental Stages | ||||||||||||
| Gene Name | SD | CV (%) | Gene Name | SD | CV (%) | Gene Name | SD | CV (%) | Gene Name | SD | CV (%) | Gene Name | SD | CV (%) | Gene Name | SD | CV (%) | |
| 1 | RuEEF1A | 0.07 | 0.28 | RuEEF1A | 0.06 | 0.25 | Ru18S | 0.14 | 0.60 | RuTUBA | 0.12 | 0.46 | Ru18S | 0.08 | 0.36 | RuEEF1A | 0.02 | 0.08 |
| 2 | Ru18S | 0.09 | 0.36 | RuTUBA | 0.09 | 0.36 | RuEF4A | 0.15 | 0.58 | Ru18S | 0.13 | 0.56 | RuEEF1A | 0.10 | 0.41 | RuTUBA | 0.04 | 0.18 |
| 3 | RuEEF1B | 0.18 | 0.72 | RuEEF1B | 0.17 | 0.70 | RuEEF1A | 0.15 | 0.61 | RuEEF1A | 0.15 | 0.59 | Ru40S | 0.10 | 0.43 | Ru18S | 0.16 | 0.70 |
| 4 | RuUBC | 0.19 | 0.79 | Ru18S | 0.17 | 0.71 | RuUBQ | 0.18 | 0.68 | RuEF4A | 0.18 | 0.69 | RuEF4A | 0.14 | 0.55 | RuEEF1B | 0.22 | 0.88 |
| 5 | RuTUBA | 0.25 | 1.01 | RuEF4A | 0.31 | 1.22 | RuTUBA | 0.19 | 0.77 | Ru40S | 0.19 | 0.71 | RuTUBA | 0.15 | 0.62 | RuEF4A | 0.33 | 1.29 |
| 6 | RuEF4A | 0.25 | 1.01 | RuUBC | 0.32 | 1.32 | RuUBC | 0.21 | 0.87 | RuUBQ | 0.25 | 0.86 | RuEEF1B | 0.22 | 0.89 | RuUBC | 0.47 | 1.98 |
| 7 | Ru40S | 0.26 | 1.11 | RuPGK | 0.57 | 2.17 | Ru30S | 0.24 | 0.96 | RuPA | 0.27 | 1.04 | RuF-box | 0.23 | 0.76 | Ru40S | 0.58 | 2.32 |
| 8 | RuUBQ | 0.38 | 1.41 | RuUBQ | 0.73 | 2.69 | RuEEF1B | 0.41 | 1.65 | RuEEF1B | 0.38 | 1.50 | RuUBC | 0.24 | 1.00 | RuPA | 0.66 | 2.42 |
| 9 | RuPA | 0.83 | 3.23 | RuF-box | 0.74 | 2.64 | RuF-box | 0.44 | 1.42 | RuUBC | 0.39 | 1.59 | RuUBQ | 0.37 | 1.40 | Ru30S | 0.69 | 2.68 |
| 10 | RuPGK | 0.90 | 3.44 | Ru40S | 0.97 | 3.81 | RuPGK | 0.50 | 2.07 | RuF-box | 0.58 | 1.94 | Ru30S | 0.57 | 2.49 | RuPGK | 0.73 | 2.79 |
| 11 | Ru30S | 1.19 | 5.00 | Ru30S | 1.04 | 4.14 | Ru40S | 0.51 | 2.04 | Ru30S | 0.76 | 2.98 | RuPA | 0.71 | 2.74 | RuUBQ | 0.81 | 2.92 |
| 12 | RuF-box | 1.49 | 5.11 | RuPA | 1.24 | 4.57 | RuPA | 0.61 | 2.29 | RuPGK | 1.35 | 5.45 | RuPGK | 1.33 | 5.16 | RuF-box | 1.08 | 3.80 |
| Rank | All Samples | Three Raspberries | All Fruit Developmental Stages | Raspberry Fruit Developmental Stages | ||||||||||||||||
| Gene | G | N | B | S | Gene | G | N | B | S | Gene | G | N | B | S | Gene | G | N | B | S | |
| 1 | RuEEF1A | 1 | 2 | 1 | 4 | Ru18S | 1 | 3 | 2 | 6 | RuEEF1A | 1 | 3 | 1 | 5 | RuEF4A | 2 | 1 | 3 | 6 |
| 2 | Ru18S | 1 | 3 | 2 | 6 | RuEEF1A | 1 | 4 | 1 | 6 | Ru18S | 1 | 4 | 2 | 7 | Ru18S | 1 | 5 | 1 | 7 |
| 3 | RuTUBA | 2 | 1 | 3 | 6 | RuTUBA | 2 | 1 | 3 | 6 | RuEF4A | 2 | 1 | 4 | 7 | RuEEF1A | 1 | 4 | 2 | 7 |
| 4 | RuUBC | 4 | 4 | 4 | 12 | RuUBC | 3 | 2 | 4 | 9 | RuTUBA | 3 | 2 | 3 | 8 | RuTUBA | 3 | 2 | 4 | 9 |
| 5 | RuEF4A | 3 | 5 | 5 | 13 | RuEF4A | 4 | 6 | 5 | 15 | RuUBC | 4 | 5 | 5 | 14 | RuUBC | 4 | 3 | 5 | 12 |
| 6 | RuEEF1B | 5 | 6 | 6 | 17 | RuEEF1B | 5 | 5 | 6 | 16 | RuEEF1B | 5 | 6 | 6 | 17 | RuEEF1B | 5 | 6 | 6 | 17 |
| 7 | RuUBQ | 6 | 7 | 7 | 20 | RuUBQ | 6 | 7 | 7 | 20 | RuUBQ | 6 | 7 | 8 | 21 | RuF-box | 6 | 9 | 7 | 22 |
| 8 | Ru40S | 7 | 8 | 8 | 23 | Ru40S | 7 | 8 | 8 | 23 | Ru40S | 7 | 8 | 9 | 24 | RuPA | 7 | 7 | 8 | 22 |
| 9 | Ru30S | 8 | 9 | 10 | 27 | RuPA | 9 | 9 | 9 | 27 | RuPA | 8 | 9 | 7 | 24 | Ru30S | 10 | 11 | 12 | 23 |
| 10 | RuPA | 9 | 10 | 9 | 28 | Ru30S | 8 | 10 | 11 | 29 | Ru30S | 9 | 10 | 11 | 30 | RuPGK | 11 | 12 | 11 | 24 |
| 11 | RuF-box | 10 | 11 | 12 | 33 | RuF-box | 10 | 11 | 10 | 31 | RuF-box | 10 | 11 | 10 | 31 | RuUBQ | 8 | 8 | 9 | 25 |
| 12 | RuPGK | 11 | 12 | 11 | 34 | RuPGK | 11 | 12 | 12 | 35 | RuPGK | 11 | 12 | 12 | 35 | Ru40S | 9 | 10 | 10 | 29 |
| Rank | Red Raspberry Tissues | Black Raspberry Tissues | Yellow Raspberry Tissues | Blackberry Tissues | ||||||||||||||||
| Gene | G | N | B | S | Gene | G | N | B | S | Gene | G | N | B | S | Gene | G | N | B | S | |
| 1 | Ru18S | 2 | 3 | 1 | 6 | RuTUBA | 1 | 2 | 1 | 4 | RuEEF1A | 1 | 3 | 1 | 5 | RuEEF1A | 1 | 1 | 1 | 3 |
| 2 | RuUBC | 1 | 1 | 4 | 6 | RuEEF1A | 1 | 1 | 3 | 5 | RuUBC | 2 | 1 | 4 | 7 | RuTUBA | 1 | 3 | 2 | 6 |
| 3 | RuTUBA | 1 | 4 | 2 | 7 | Ru18S | 2 | 6 | 2 | 10 | Ru18S | 1 | 7 | 2 | 10 | Ru18S | 2 | 2 | 4 | 8 |
| 4 | RuEEF1A | 3 | 5 | 3 | 11 | RuEF4A | 3 | 5 | 4 | 12 | RuTUBA | 3 | 2 | 5 | 10 | RuEEF1B | 3 | 4 | 3 | 10 |
| 5 | RuEEF1B | 4 | 7 | 5 | 16 | RuUBC | 4 | 8 | 5 | 17 | RuEF4A | 4 | 4 | 6 | 14 | RuEF4A | 4 | 5 | 5 | 14 |
| 6 | RuUBQ | 6 | 2 | 8 | 16 | Ru40S | 6 | 4 | 7 | 17 | RuEEF1B | 6 | 6 | 3 | 15 | RuUBC | 5 | 6 | 6 | 17 |
| 7 | RuEF4A | 5 | 8 | 6 | 19 | RuEEF1B | 5 | 7 | 6 | 18 | RuUBQ | 7 | 8 | 8 | 15 | RuPGK | 6 | 8 | 7 | 21 |
| 8 | Ru40S | 7 | 6 | 7 | 20 | RuUBQ | 7 | 3 | 8 | 18 | Ru40S | 5 | 5 | 7 | 17 | RuUBQ | 7 | 7 | 8 | 22 |
| 9 | RuPGK | 8 | 10 | 9 | 27 | RuF-box | 8 | 9 | 9 | 26 | RuPA | 8 | 9 | 9 | 26 | RuF-box | 8 | 10 | 9 | 27 |
| 10 | RuF-box | 9 | 9 | 10 | 28 | Ru30S | 9 | 10 | 10 | 29 | RuPGK | 9 | 10 | 10 | 29 | Ru40S | 9 | 9 | 10 | 28 |
| 11 | Ru30S | 10 | 11 | 11 | 32 | RuPA | 10 | 11 | 11 | 32 | Ru30S | 10 | 11 | 11 | 32 | Ru30S | 10 | 11 | 11 | 32 |
| 12 | RuPA | 11 | 12 | 12 | 35 | RuPGK | 11 | 12 | 12 | 35 | RuF-box | 11 | 12 | 12 | 35 | RuPA | 11 | 12 | 12 | 35 |
| Rank | Red Raspberry Fruit Developmental Stages | Black Raspberry Fruit Developmental Stages | Yellow Raspberry Fruit Developmental Stages | Blackberry Fruit Developmental Stages | ||||||||||||||||
| Gene | G | N | B | S | Gene | G | N | B | S | Gene | G | N | B | S | Gene | G | N | B | S | |
| 1 | RuEF4A | 1 | 1 | 2 | 4 | Ru18S | 3 | 1 | 2 | 6 | Ru18S | 1 | 4 | 1 | 6 | RuTUBA | 1 | 1 | 2 | 4 |
| 2 | RuEEF1A | 1 | 2 | 3 | 6 | RuTUBA | 1 | 4 | 1 | 6 | RuEEF1A | 1 | 5 | 2 | 8 | RuEEF1A | 1 | 2 | 1 | 4 |
| 3 | RuUBQ | 3 | 3 | 4 | 10 | RuEEF1A | 2 | 2 | 3 | 7 | Ru40S | 2 | 3 | 3 | 8 | Ru18S | 2 | 3 | 3 | 8 |
| 4 | Ru18S | 5 | 5 | 1 | 11 | Ru40S | 1 | 5 | 5 | 11 | RuEF4A | 3 | 1 | 4 | 8 | RuEEF1B | 3 | 4 | 4 | 11 |
| 5 | RuUBC | 2 | 4 | 6 | 12 | RuEF4A | 4 | 3 | 4 | 11 | RuTUBA | 4 | 2 | 5 | 11 | RuEF4A | 4 | 5 | 5 | 14 |
| 6 | RuTUBA | 4 | 6 | 5 | 15 | RuUBQ | 5 | 6 | 6 | 17 | RuEEF1B | 5 | 6 | 6 | 17 | RuUBC | 5 | 7 | 6 | 18 |
| 7 | Ru30S | 6 | 7 | 7 | 20 | RuPA | 6 | 7 | 7 | 20 | RuF-box | 6 | 9 | 7 | 22 | Ru40S | 8 | 6 | 7 | 21 |
| 8 | RuEEF1B | 7 | 11 | 8 | 26 | RuEEF1B | 7 | 8 | 8 | 23 | RuUBC | 7 | 7 | 8 | 22 | RuPA | 6 | 10 | 8 | 24 |
| 9 | Ru40S | 8 | 8 | 11 | 27 | RuUBC | 8 | 9 | 9 | 26 | RuUBQ | 8 | 8 | 9 | 25 | Ru30S | 7 | 11 | 9 | 27 |
| 10 | RuF-box | 9 | 10 | 9 | 28 | RuF-box | 9 | 10 | 10 | 29 | Ru30S | 9 | 10 | 10 | 29 | RuPGK | 9 | 8 | 10 | 27 |
| 11 | RuPGK | 10 | 9 | 10 | 29 | Ru30S | 10 | 11 | 11 | 32 | RuPA | 10 | 11 | 11 | 32 | RuUBQ | 10 | 9 | 11 | 30 |
| 12 | RuPA | 11 | 12 | 12 | 35 | RuPGK | 11 | 12 | 12 | 35 | RuPGK | 11 | 12 | 12 | 35 | RuF-box | 11 | 12 | 12 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhang, C.; Yang, H.; Lyu, L.; Li, W.; Wu, W. Selection and Validation of Candidate Reference Genes for Gene Expression Analysis by RT-qPCR in Rubus. Int. J. Mol. Sci. 2021, 22, 10533. https://doi.org/10.3390/ijms221910533
Wu Y, Zhang C, Yang H, Lyu L, Li W, Wu W. Selection and Validation of Candidate Reference Genes for Gene Expression Analysis by RT-qPCR in Rubus. International Journal of Molecular Sciences. 2021; 22(19):10533. https://doi.org/10.3390/ijms221910533
Chicago/Turabian StyleWu, Yaqiong, Chunhong Zhang, Haiyan Yang, Lianfei Lyu, Weilin Li, and Wenlong Wu. 2021. "Selection and Validation of Candidate Reference Genes for Gene Expression Analysis by RT-qPCR in Rubus" International Journal of Molecular Sciences 22, no. 19: 10533. https://doi.org/10.3390/ijms221910533






