miR-29a-3p/THBS2 Axis Regulates PAH-Induced Cardiac Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Hemodynamic Analysis
2.2. Echocardiography
2.3. Monocrotaline (MCT)-Induced PAH Animal Model
2.4. Micro-Computed Tomography (MicroCT)
2.5. Cell Culture
2.6. Masson’s Trichrome Staining and Immunostaining
2.7. Transmission Electron Microscopy (TEM)
2.8. Second-Harmonic Generation (SHG) Microscopy
2.9. MiRNA Extraction
2.10. RNA Isolation
2.11. MiRNA Reverse Transcription and Real-Time PCR
2.12. Nano Ultra-Performance Liquid Chromatographic System with Tandem Mass Spectrometry (nanoUPLC-MS/MS)
2.13. Proteomic Data Analysis
2.14. Human MiR-29a-3p Mimic and Inhibitor Transfection
2.15. Western Blot Analysis
2.16. MiR-29a-3p-Specific In Situ Hybridization (ISH) Assay
2.17. Immunohistochemistry (IHC) Staining
2.18. Enzyme-Linked Immunosorbent Assay (ELISA)
2.19. Exosome Isolation
2.20. Statistical Analysis
3. Results
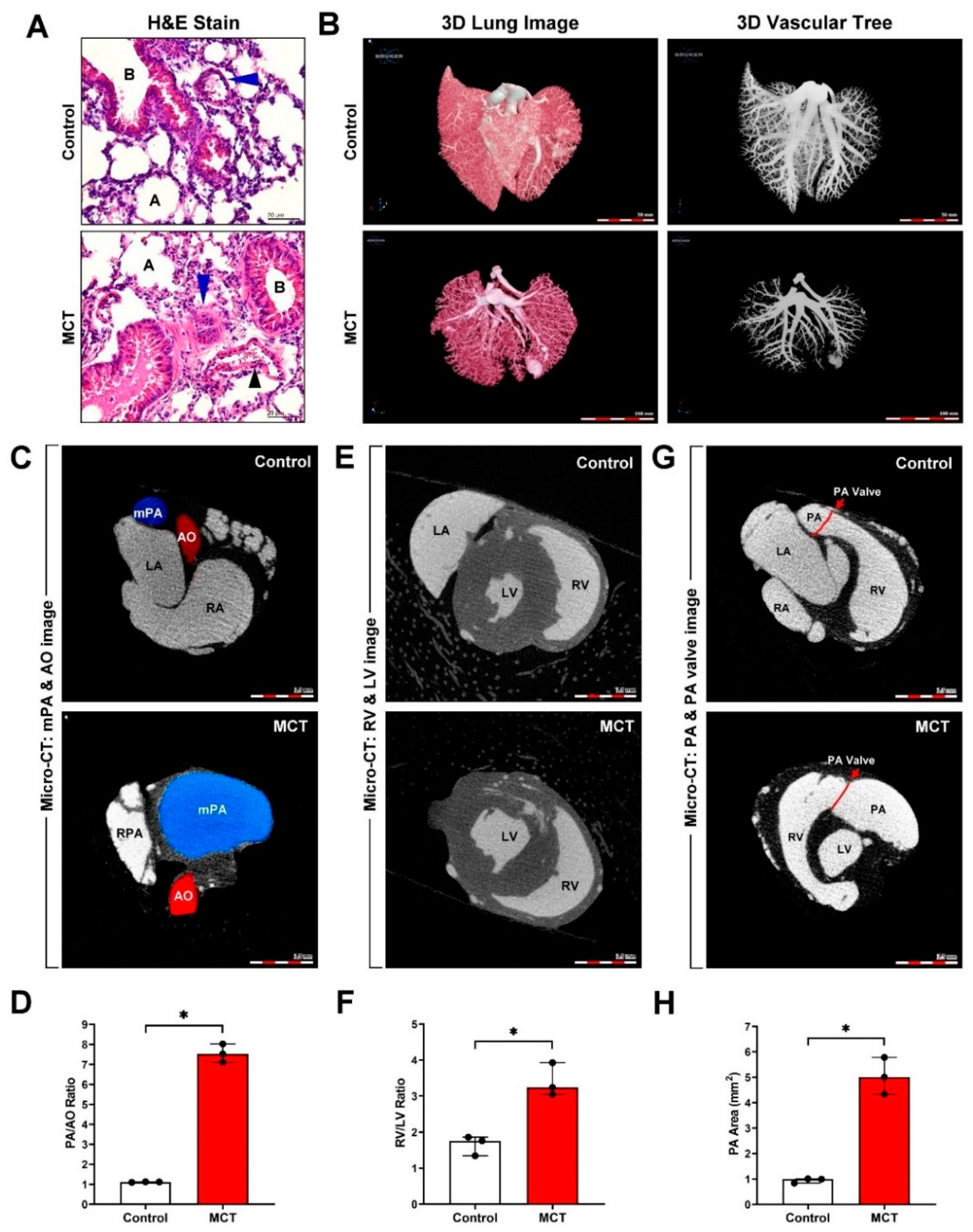
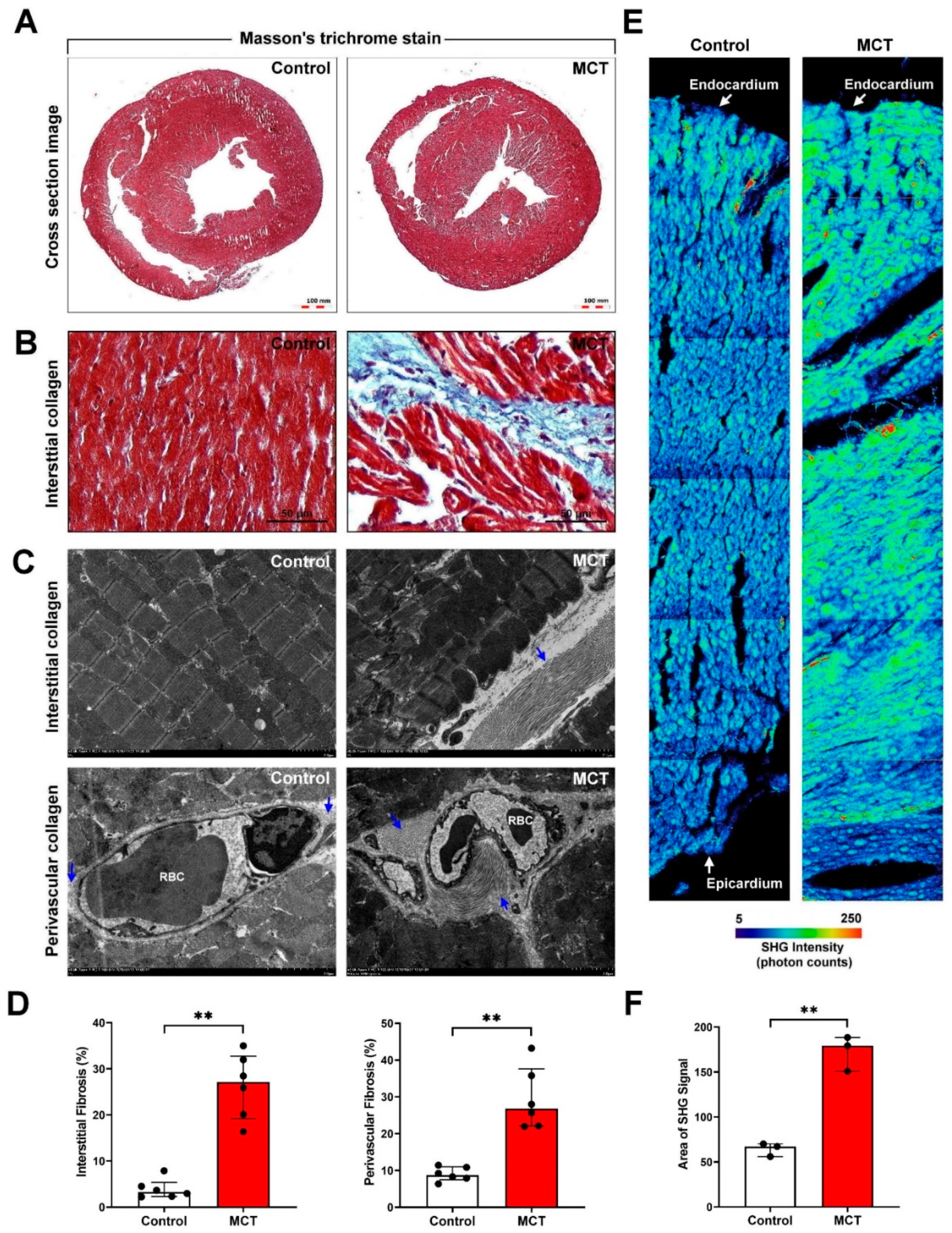
3.1. Successful Establishment and Evaluation of an MCT-Induced PAH and Cardiac Remodeling Mouse Model
3.2. Cardiomyocyte-Derived Exosomes Exist in the Cardiac Microenvironment In Vitro and In Vivo
3.3. Bioinformatics Analyses of MCT-Treated HCM-Derived Exosomes
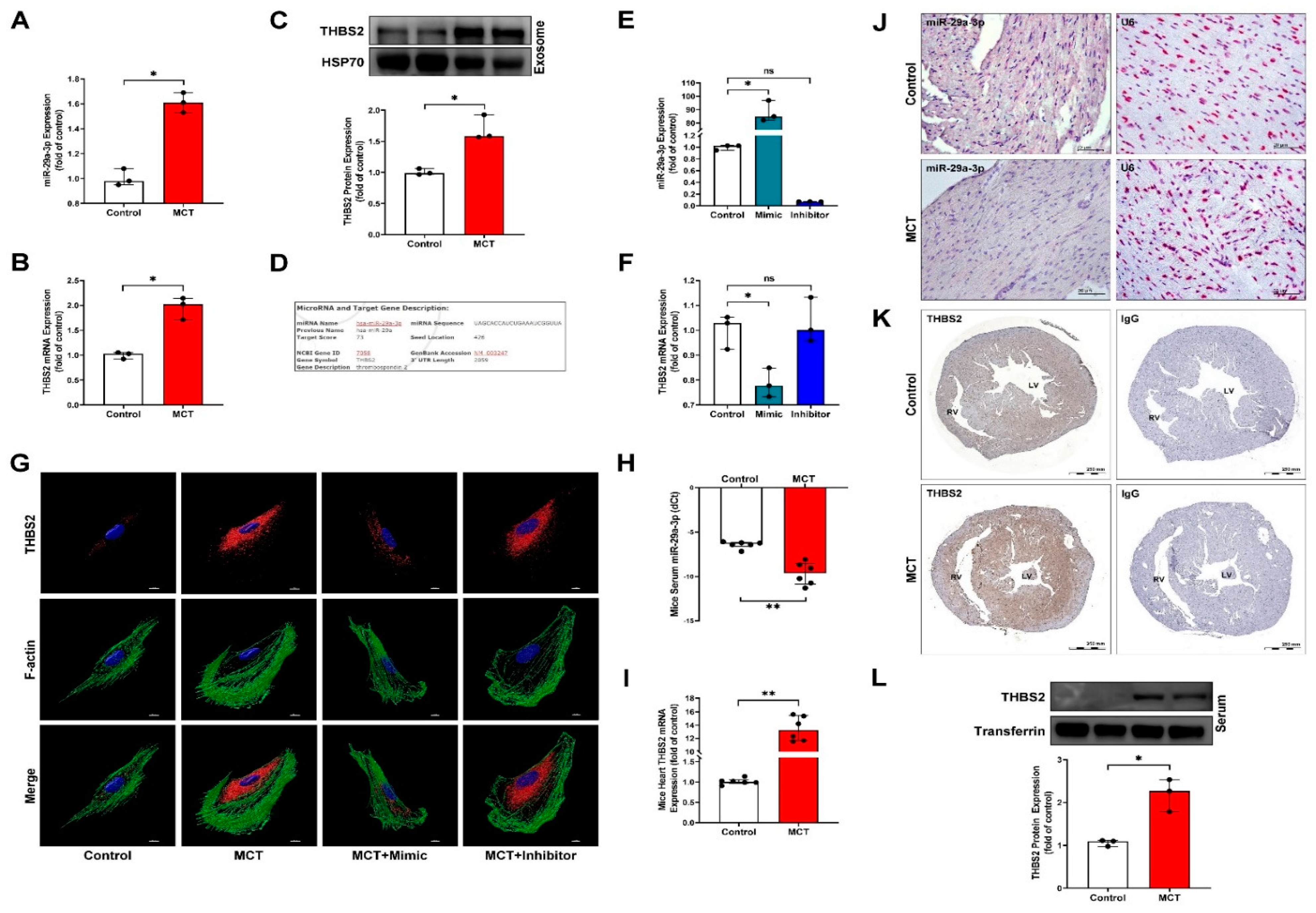
3.4. MiR-29a-3p Modulates THBS2 Expression In Vitro and In Vivo
3.5. Cardiomyocyte-Derived Exosomal THBS2 Regulates Activation and Transformation of Cardiac Fibroblasts and Induces Cardiac Fibrosis
3.6. Circulating MiR-29a-3p and THBS2 Levels Are Prognostic Markers for Disease Severity of PAH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damico, R.; Kolb, T.M.; Valera, L.; Wang, L.; Housten, T.; Tedford, R.J.; Kass, D.A.; Rafaels, N.; Gao, L.; Barnes, K.C.; et al. Serum endostatin is a genetically determined predictor of survival in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2015, 191, 208–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zungu-Edmondson, M.; Shults, N.V.; Wong, C.M.; Suzuki, Y.J. Modulators of right ventricular apoptosis and contractility in a rat model of pulmonary hypertension. Cardiovasc. Res. 2016, 110, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thenappan, T.; Chan, S.Y.; Weir, E.K. Role of extracellular matrix in the pathogenesis of pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1322–H1331. [Google Scholar] [CrossRef]
- Klinke, A.; Schubert, T.; Müller, M.; Legchenko, E.; Zelt, J.G.E.; Shimauchi, T.; Napp, L.C.; Rothman, A.M.K.; Bonnet, S.; Stewart, D.J.; et al. Emerging therapies for right ventricular dysfunction and failure. Cardiovasc. Diagn. Ther. 2020, 10, 1735–1767. [Google Scholar] [CrossRef]
- Sun, L.; Lin, P.; Chen, Y.; Yu, H.; Ren, S.; Wang, J.; Zhao, L.; Du, G. miR-182-3p/Myadm contribute to pulmonary artery hypertension vascular remodeling via a KLF4/p21-dependent mechanism. Theranostics 2020, 10, 5581–5599. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The extracellular matrix in ischemic and nonischemic heart failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Matricellular proteins in cardiac adaptation and disease. Physiol. Rev. 2012, 92, 635–688. [Google Scholar] [CrossRef] [Green Version]
- Calabro, N.E.; Barrett, A.; Chamorro-Jorganes, A.; Tam, S.; Kristofik, N.J.; Xing, H.; Loye, A.M.; Sessa, W.C.; Hansen, K.; Kyriakides, T.R. Thrombospondin-2 regulates extracellular matrix production, LOX levels, and cross-linking via downregulation of miR-29. Matrix Biol. 2019, 82, 71–85. [Google Scholar] [CrossRef]
- Schellings, M.W.; Pinto, Y.M.; Heymans, S. Matricellular proteins in the heart: Possible role during stress and remodeling. Cardiovasc. Res. 2004, 64, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Swinnen, M.; Vanhoutte, D.; Van Almen, G.C.; Hamdani, N.; Schellings, M.W.; D’Hooge, J.; Van der Velden, J.; Weaver, M.S.; Sage, E.H.; Bornstein, P.; et al. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation 2009, 120, 1585–1597. [Google Scholar] [CrossRef] [Green Version]
- Pohjolainen, V.; Mustonen, E.; Taskinen, P.; Näpänkangas, J.; Leskinen, H.; Ohukainen, P.; Peltonen, T.; Aro, J.; Juvonen, T.; Satta, J.; et al. Increased thrombospondin-2 in human fibrosclerotic and stenotic aortic valves. Atherosclerosis 2012, 220, 66–71. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Thrombospondins: A role in cardiovascular disease. Int. J. Mol. Sci. 2017, 18, 1540. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Sturdivant, J.; Ebrahimi, A.; Ganguly, S.; Elbayoumi, T. Novel pharmaceutical strategy for selective abrogation of TSP1-induced vascular dysfunction by decoy recombinant CD47 soluble receptor in prophylaxis and treatment models. Biomedicines 2021, 9, 642. [Google Scholar] [CrossRef]
- Hanatani, S.; Izumiya, Y.; Takashio, S.; Kimura, Y.; Araki, S.; Rokutanda, T.; Tsujita, K.; Yamamoto, E.; Tanaka, T.; Yamamuro, M.; et al. Circulating thrombospondin-2 reflects disease severity and predicts outcome of heart failure with reduced ejection fraction. Circ. J. 2014, 78, 903–910. [Google Scholar] [CrossRef] [Green Version]
- van Almen, G.C.; Swinnen, M.; Carai, P.; Verhesen, W.; Cleutjens, J.P.; D’Hooge, J.; Verheyen, F.K.; Pinto, Y.M.; Schroen, B.; Carmeliet, P.; et al. Absence of thrombospondin-2 increases cardiomyocyte damage and matrix disruption in doxorubicin-induced cardiomyopathy. J. Mol. Cell. Cardiol. 2011, 51, 318–328. [Google Scholar] [CrossRef]
- Zhang, K.; Li, M.; Yin, L.; Fu, G.; Liu, Z. Role of thrombospondin-1 and thrombospondin-2 in cardiovascular diseases (Review). Int. J. Mol. Med. 2020, 45, 1275–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, T.; Morita, H. Thrombospondin-2. Int. Heart J. 2019, 60, 235–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, J.; Wilhelm, J.; Marsh, L.M.; Ghanim, B.; Klepetko, W.; Kovacs, G.; Olschewski, H.; Olschewski, A.; Kwapiszewska, G. Distinct differences in gene expression patterns in pulmonary arteries of patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis with pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2014, 190, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Z. Cardiomyocyte-derived exosomes: Biological functions and potential therapeutic implications. Front. Physiol. 2019, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Waldenström, A.; Gennebäck, N.; Hellman, U.; Ronquist, G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS ONE 2012, 7, e34653. [Google Scholar]
- Yang, Y.; Li, Y.; Chen, X.; Cheng, X.; Liao, Y.; Yu, X. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J. Mol. Med. 2016, 94, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Das, S.; Rodosthenous, R.S.; Holvoet, P.; Vanhaverbeke, M.; Monteiro, M.C.; Monteiro, V.V.S.; Radosinska, J.; Bartekova, M.; Jansen, F.; et al. Extracellular vesicles in cardiovascular theranostics. Theranostics 2017, 7, 4168–4182. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.F.; Zhu, H.; Millard, R.W.; Fan, G.C. Exosomes function in pro- and anti-angiogenesis. Curr. Angiogenesis 2013, 2, 54–59. [Google Scholar]
- Hu, M.; Guo, G.; Huang, Q.; Cheng, C.; Xu, R.; Li, A.; Liu, N.; Liu, S. The harsh microenvironment in infarcted heart accelerates transplanted bone marrow mesenchymal stem cells injury: The role of injured cardiomyocytes-derived exosomes. Cell Death Dis. 2018, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Racchetti, G.; Meldolesi, J. Extracellular vesicles of mesenchymal stem cells: Therapeutic properties discovered with extraordinary success. Biomedicines 2021, 9, 667. [Google Scholar] [CrossRef]
- Chouvarine, P.; Geldner, J.; Giagnorio, R.; Legchenko, E.; Bertram, H.; Hansmann, G. Trans-right-ventricle and transpulmonary MicroRNA gradients in human pulmonary arterial hypertension. Pediatr. Crit. Care Med. 2020, 21, 340–349. [Google Scholar] [CrossRef]
- Luo, Y.; Dong, H.Y.; Zhang, B.; Feng, Z.; Liu, Y.; Gao, Y.Q.; Dong, M.Q.; Li, Z.C. miR-29a-3p attenuates hypoxic pulmonary hypertension by inhibiting pulmonary adventitial fibroblast activation. Hypertension 2015, 65, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Moraes, L.N.; Fernandez, G.J.; Vechetti-Júnior, I.J.; Freire, P.P.; Souza, R.W.A.; Villacis, R.A.R.; Rogatto, S.R.; Reis, P.P.; Dal-Pai-Silva, M.; Carvalho, R.F. Integration of miRNA and mRNA expression profiles reveals microRNA-regulated networks during muscle wasting in cardiac cachexia. Sci. Rep. 2017, 7, 6998. [Google Scholar] [CrossRef]
- Jafarinejad-Farsangi, S.; Gharibdoost, F.; Farazmand, A.; Kavosi, H.; Jamshidi, A.; Karimizadeh, E.; Noorbakhsh, F.; Mahmoudi, M. MicroRNA-21 and microRNA-29a modulate the expression of collagen in dermal fibroblasts of patients with systemic sclerosis. Autoimmunity 2019, 52, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Cushing, L.; Kuang, P.P.; Qian, J.; Shao, F.; Wu, J.; Little, F.; Thannickal, V.J.; Cardoso, W.V.; Lü, J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Assmann, T.S.; Recamonde-Mendoza, M.; de Souza, B.M.; Bauer, A.C.; Crispim, D. MicroRNAs and diabetic kidney disease: Systematic review and bioinformatic analysis. Mol. Cell. Endocrinol. 2018, 477, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Urban, G.W.; Bettermann, K.; Vucur, M.; Zimmermann, H.; Schmidt, S.; Janssen, J.; Koppe, C.; Knolle, P.; Castoldi, M.; et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 2011, 53, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Sassi, Y.; Avramopoulos, P.; Ramanujam, D.; Grüter, L.; Werfel, S.; Giosele, S.; Brunner, A.D.; Esfandyari, D.; Papadopoulou, A.S.; De Strooper, B.; et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat. Commun. 2017, 8, 1614. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Pinto, Y.M.; Creemers, E.E. Non-cardiomyocyte microRNAs in heart failure. Cardiovasc. Res. 2012, 93, 573–582. [Google Scholar] [CrossRef]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 277–313. [Google Scholar] [CrossRef]
- Weyers, J.J.; Carlson, D.D.; Murry, C.E.; Schwartz, S.M.; Mahoney, W.M., Jr. Retrograde perfusion and filling of mouse coronary vasculature as preparation for micro computed tomography imaging. J. Vis. Exp. 2012, 10, e3740. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.C.; Li, C.Y.; Hsu, C.H.; Chen, H.L.; Chen, Y.H.; Liu, Y.P.; Liu, Y.R.; Kuo, H.F.; Liu, P.L. Mitochondrial protection by simvastatin against angiotensin II-mediated heart failure. Br. J. Pharmacol. 2019, 176, 3791–3804. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.F.; Hsieh, C.C.; Wang, S.C.; Chang, C.Y.; Hung, C.H.; Kuo, P.L.; Liu, Y.R.; Li, C.Y.; Liu, P.L. Simvastatin attenuates cardiac fibrosis via regulation of cardiomyocyte-derived exosome secretion. J. Clin. Med. 2019, 8, 794. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Izumiya, Y.; Hanatani, S.; Yamamoto, E.; Kusaka, H.; Tokitsu, T.; Takashio, S.; Sakamoto, K.; Tsujita, K.; Tanaka, T.; et al. High serum levels of thrombospondin-2 correlate with poor prognosis of patients with heart failure with preserved ejection fraction. Heart Vessels 2016, 31, 52–59. [Google Scholar] [CrossRef]
- Lopes, F.C.; Traina, F.; Almeida, C.B.; Leonardo, F.C.; Franco-Penteado, C.F.; Garrido, V.T.; Colella, M.P.; Soares, R.; Olalla-Saad, S.T.; Costa, F.F.; et al. Key endothelial cell angiogenic mechanisms are stimulated by the circulating milieu in sickle cell disease and attenuated by hydroxyurea. Haematologica 2015, 100, 730–739. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Yu, J.; Jing, Y.; Zhang, J. MiR-106a aggravates sepsis-induced acute kidney injury by targeting THBS2 in mice model. Acta Cir. Bras. 2019, 34, e201900602. [Google Scholar] [CrossRef] [Green Version]
- Egerstedt, A.; Berntsson, J.; Smith, M.L.; Gidlöf, O.; Nilsson, R.; Benson, M.; Wells, Q.S.; Celik, S.; Lejonberg, C.; Farrell, L.; et al. Profiling of the plasma proteome across different stages of human heart failure. Nat. Commun. 2019, 10, 5830. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Robey, T.E.; Mignone, J.L.; Muskheli, V.; Bornstein, P.; Murry, C.E. Lack of thrombospondin-2 reduces fibrosis and increases vascularity around cardiac cell grafts. Cardiovasc. Pathol. 2013, 22, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, S.G.; Lee, W.H.; Huang, M.; Dey, D.; Kodo, K.; Sanchez-Freire, V.; Gold, J.D.; Wu, J.C. Cross talk of combined gene and cell therapy in ischemic heart disease: Role of exosomal microRNA transfer. Circulation 2014, 130, S60–S69. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Gao, Y.; Zhang, X.; Lughmani, H.Y.; Kennedy, D.J.; Haller, S.T.; Pierre, S.V.; Shapiro, J.I.; Tian, J. A strategic expression method of miR-29b and its anti-fibrotic effect based on RNA-sequencing analysis. PLoS ONE 2020, 15, e0244065. [Google Scholar]
- Baptista, R.; Marques, C.; Catarino, S.; Enguita, F.J.; Costa, M.C.; Matafome, P.; Zuzarte, M.; Castro, G.; Reis, A.; Monteiro, P.; et al. MicroRNA-424(322) as a new marker of disease progression in pulmonary arterial hypertension and its role in right ventricular hypertrophy by targeting SMURF1. Cardiovasc. Res. 2018, 114, 53–64. [Google Scholar] [CrossRef]
- Rhodes, C.J.; Wharton, J.; Boon, R.A.; Roexe, T.; Tsang, H.; Wojciak-Stothard, B.; Chakrabarti, A.; Howard, L.S.; Gibbs, J.S.; Lawrie, A.; et al. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2013, 187, 294–302. [Google Scholar] [CrossRef]
- Long, L.; Xiao, Y.; Yin, X.; Gao, S.; Zhou, L.; Liu, H. Expression of serum miR-27b and miR-451 in patients with congenital heart disease associated pulmonary artery hypertension and risk factor analysis. Exp. Ther. Med. 2020, 20, 3196–3202. [Google Scholar] [CrossRef]
- Cai, Z.; Li, J.; Zhuang, Q.; Zhang, X.; Yuan, A.; Shen, L.; Kang, K.; Qu, B.; Tang, Y.; Pu, J.; et al. MiR-125a-5p ameliorates monocrotaline-induced pulmonary arterial hypertension by targeting the TGF-β1 and IL-6/STAT3 signaling pathways. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tang, P. Clinical diagnostic value of circulating serum miR-509-3p in pulmonary arterial hypertension with congenital heart disease. Hellenic J. Cardiol. 2020, 61, 26–30. [Google Scholar] [CrossRef]
- Jin, P.; Gu, W.; Lai, Y.; Zheng, W.; Zhou, Q.; Wu, X. The circulating MicroRNA-206 level predicts the severity of pulmonary hypertension in patients with left heart diseases. Cell. Physiol. Biochem. 2017, 41, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, Y.; Yan, X.; Chen, S.; He, M.; Lei, Y.; Zhang, J.; Gongol, B.; Gu, M.; Miao, Y.; et al. MicroRNA-483 amelioration of experimental pulmonary hypertension. EMBO Mol. Med. 2020, 12, e11303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, J.; Zheng, K.; Chen, L.; Sun, Y.; Yao, Z.; Sun, Y.; Lin, Y.; Lin, K.; Yuan, L. Exosomal miR-211 contributes to pulmonary hypertension via attenuating CaMK1/PPAR-γ axis. Vasc. Pharmacol. 2021, 136, 106820. [Google Scholar] [CrossRef]
- Deng, L.; Blanco, F.J.; Stevens, H.; Lu, R.; Caudrillier, A.; McBride, M.; McClure, J.D.; Grant, J.; Thomas, M.; Frid, M.; et al. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ. Res. 2015, 117, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Z.G.; Tang, L.; Gong, S.G.; Sun, Y.Y.; Wang, L.; Jiang, R.; Wu, W.H.; Luo, C.J.; Zhang, J.; et al. Plasma exosomal miR-596: A novel biomarker predicts survival in patients with idiopathic pulmonary artery hypertension. J. Int. Med. Res. 2021, 49, 3000605211002379. [Google Scholar] [CrossRef] [PubMed]
- Paulin, R.; Sutendra, G.; Gurtu, V.; Dromparis, P.; Haromy, A.; Provencher, S.; Bonnet, S.; Michelakis, E.D. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ. Res. 2015, 116, 56–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, R.L.; Yu, G.; Latimer, P.A.; Stack, C.; Robinson, K.; Dalby, C.M.; Kaminski, N.; van Rooij, E. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol. Med. 2014, 6, 1347–1356. [Google Scholar] [CrossRef]
- Wu, X.G.; Zhou, C.F.; Zhang, Y.M.; Yan, R.M.; Wei, W.F.; Chen, X.J.; Yi, H.Y.; Liang, L.J.; Fan, L.S.; Liang, L.; et al. Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis 2019, 22, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, F.; Zhang, T.; Wang, W.; Xi, W.; Li, Y.; Zhang, D.; Huo, Y.; Zhang, J.; Yang, A.; et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J. Exp. Clin. Cancer Res. 2019, 38, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagnato, G.; Roberts, W.N.; Roman, J.; Gangemi, S. A systematic review of overlapping microRNA patterns in systemic sclerosis and idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2017, 26, 160125. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-H.; Liu, I.-F.; Kuo, H.-F.; Li, C.-Y.; Lian, W.-S.; Chang, C.-Y.; Chen, Y.-H.; Liu, W.-L.; Lu, C.-Y.; Liu, Y.-R.; et al. miR-29a-3p/THBS2 Axis Regulates PAH-Induced Cardiac Fibrosis. Int. J. Mol. Sci. 2021, 22, 10574. https://doi.org/10.3390/ijms221910574
Hsu C-H, Liu I-F, Kuo H-F, Li C-Y, Lian W-S, Chang C-Y, Chen Y-H, Liu W-L, Lu C-Y, Liu Y-R, et al. miR-29a-3p/THBS2 Axis Regulates PAH-Induced Cardiac Fibrosis. International Journal of Molecular Sciences. 2021; 22(19):10574. https://doi.org/10.3390/ijms221910574
Chicago/Turabian StyleHsu, Chih-Hsin, I-Fan Liu, Hsuan-Fu Kuo, Chia-Yang Li, Wei-Shiung Lian, Chia-Yuan Chang, Yung-Hsiang Chen, Wei-Lun Liu, Chi-Yu Lu, Yu-Ru Liu, and et al. 2021. "miR-29a-3p/THBS2 Axis Regulates PAH-Induced Cardiac Fibrosis" International Journal of Molecular Sciences 22, no. 19: 10574. https://doi.org/10.3390/ijms221910574
APA StyleHsu, C.-H., Liu, I.-F., Kuo, H.-F., Li, C.-Y., Lian, W.-S., Chang, C.-Y., Chen, Y.-H., Liu, W.-L., Lu, C.-Y., Liu, Y.-R., Lin, T.-C., Lee, T.-Y., Huang, C.-Y., Hsieh, C.-C., & Liu, P.-L. (2021). miR-29a-3p/THBS2 Axis Regulates PAH-Induced Cardiac Fibrosis. International Journal of Molecular Sciences, 22(19), 10574. https://doi.org/10.3390/ijms221910574






