Congestive Hepatopathy Secondary to Right Ventricular Hypertrophy Related to Monocrotaline-Induced Pulmonary Arterial Hypertension
Abstract
:1. Introduction
2. Results
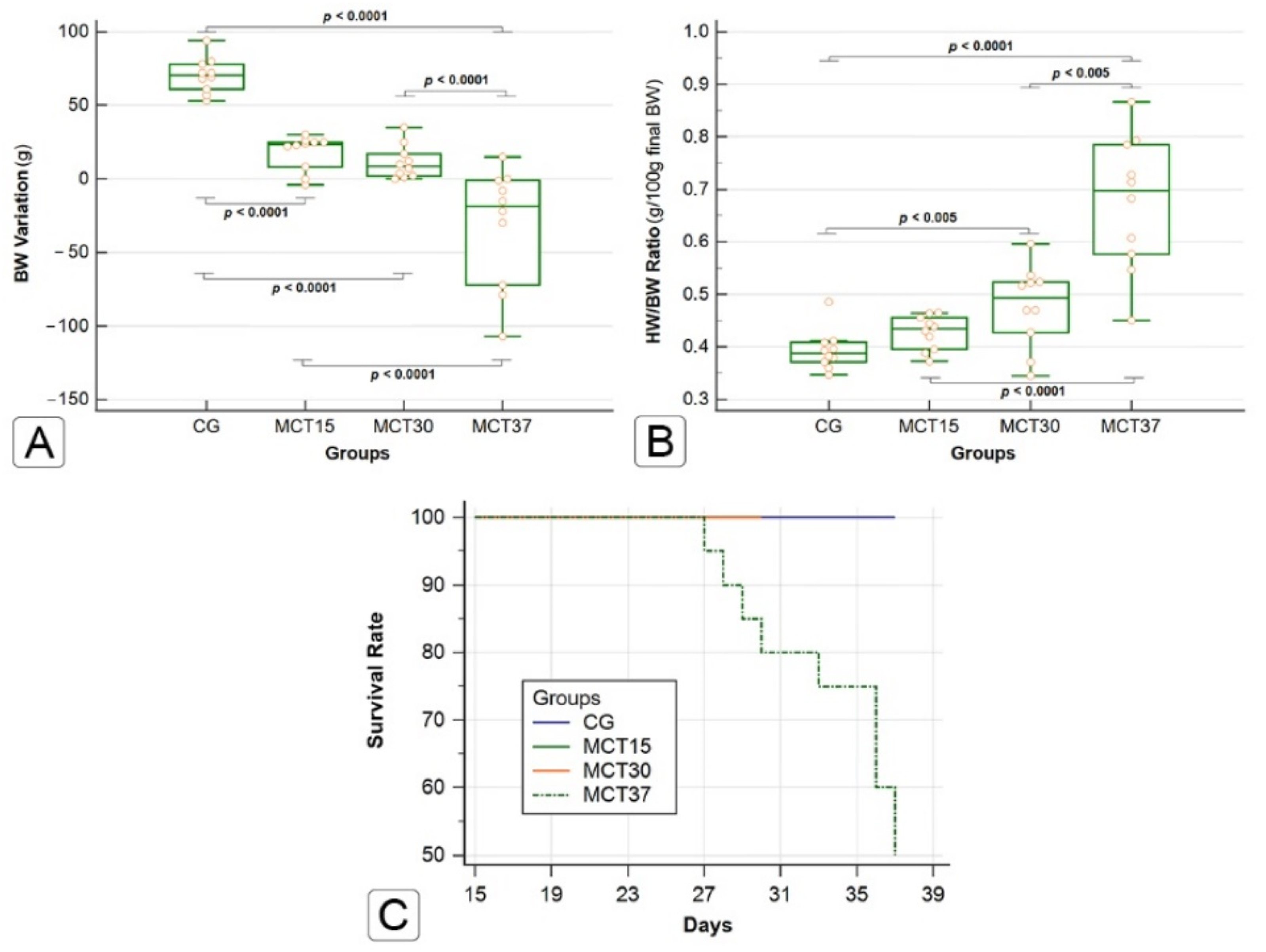
2.1. Effects of Monocrotaline on Physical Characteristics and Survival
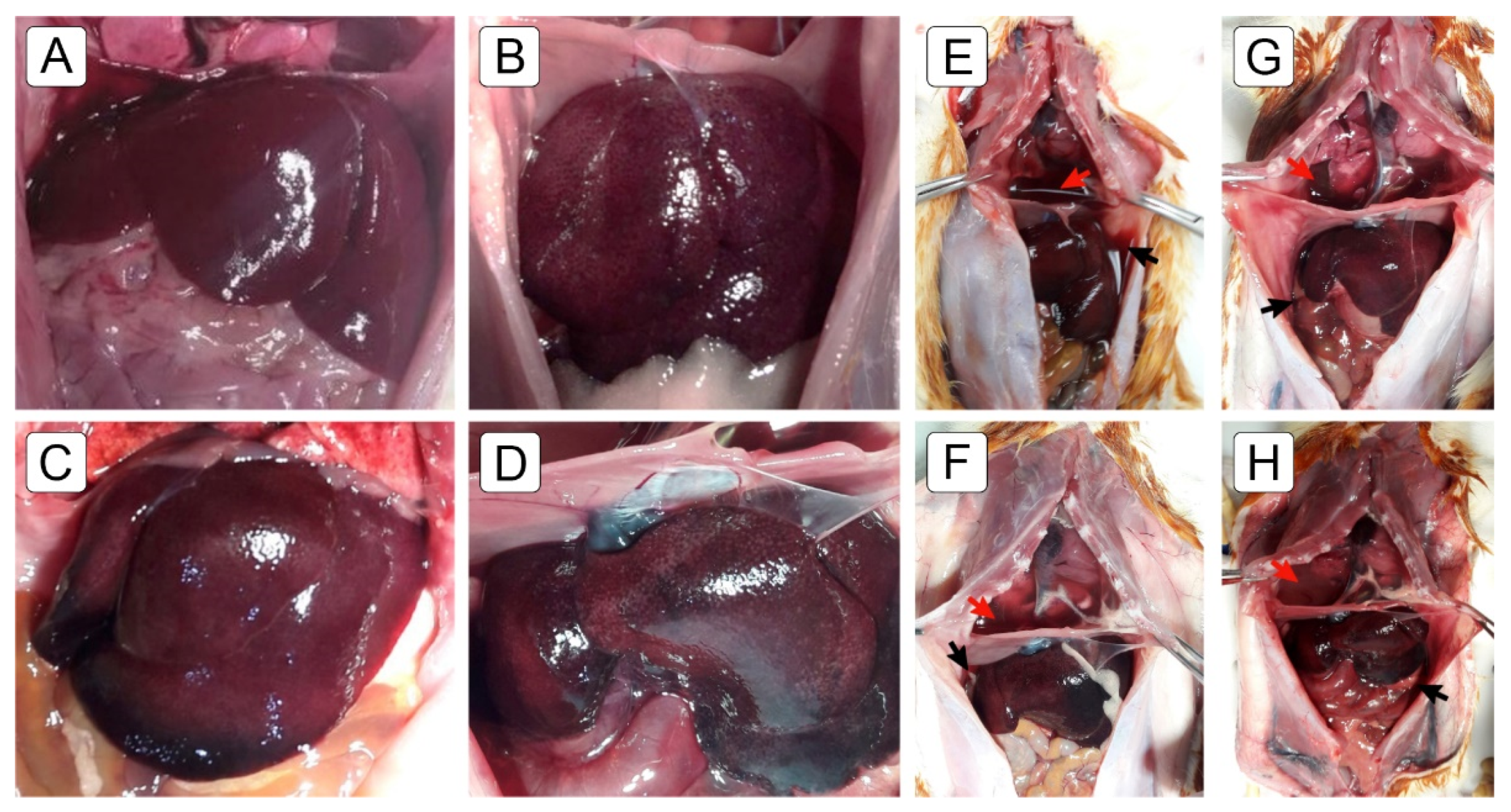
2.2. Macroscopic Findings
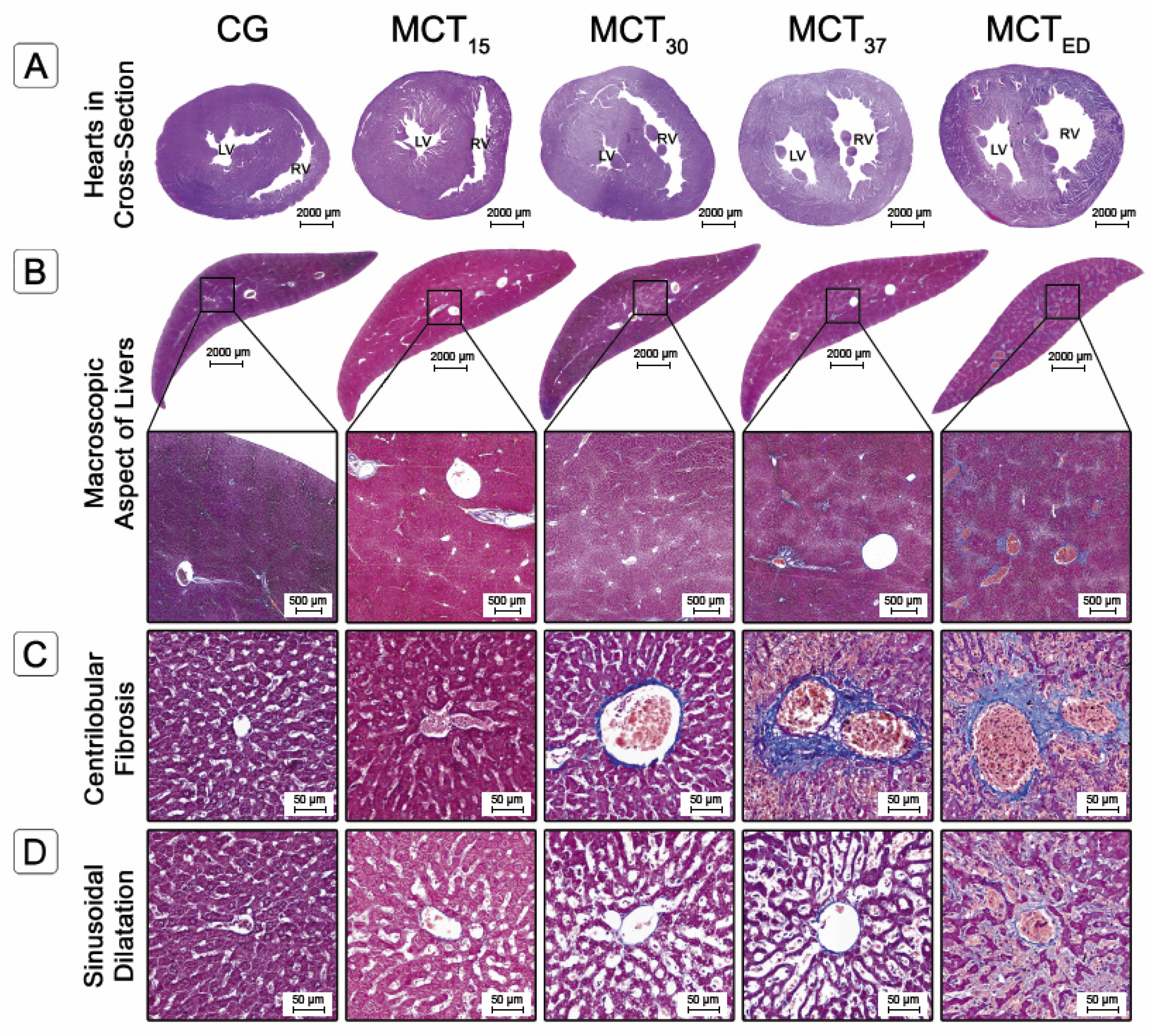
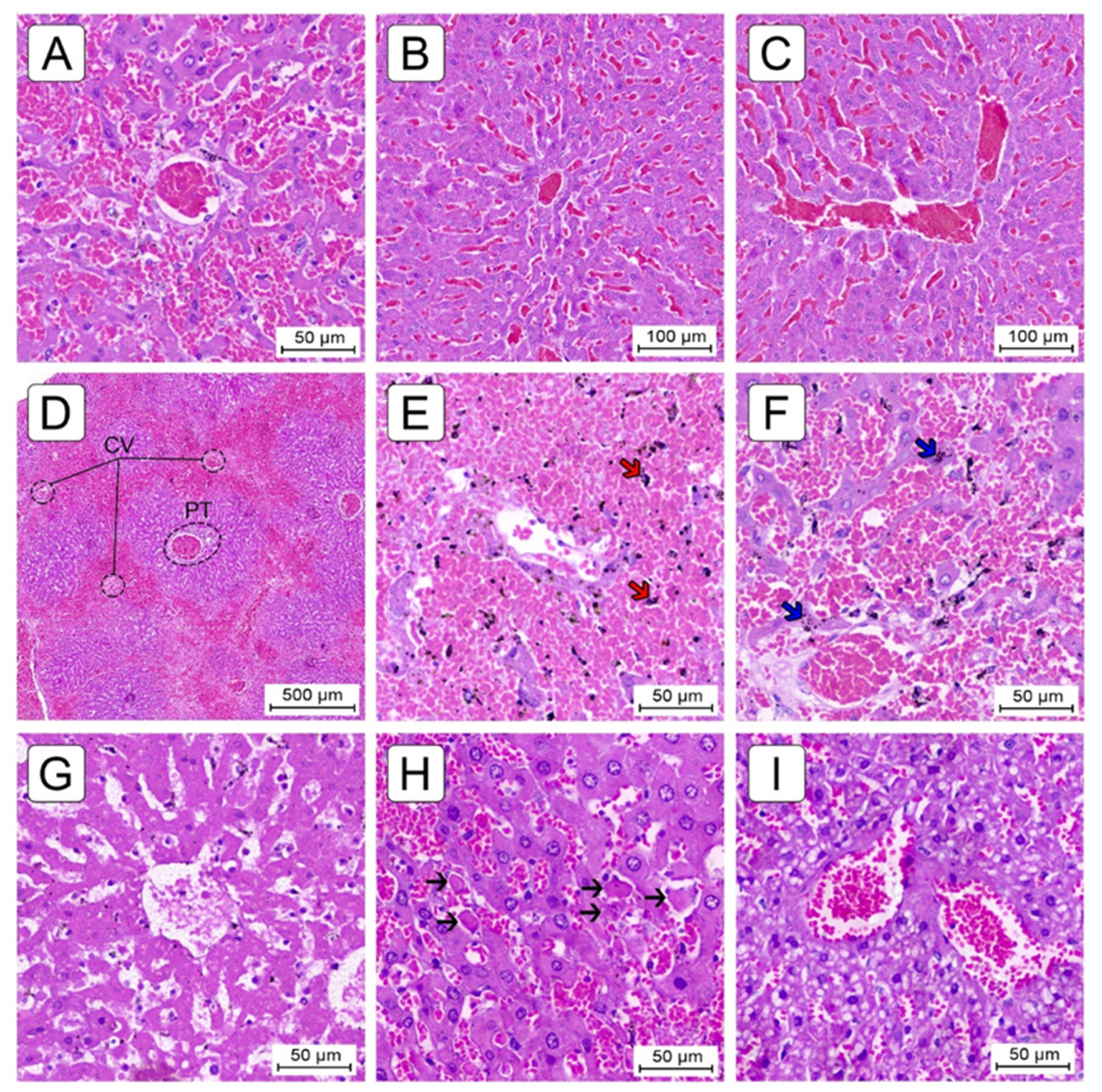
2.3. Histological Evaluation
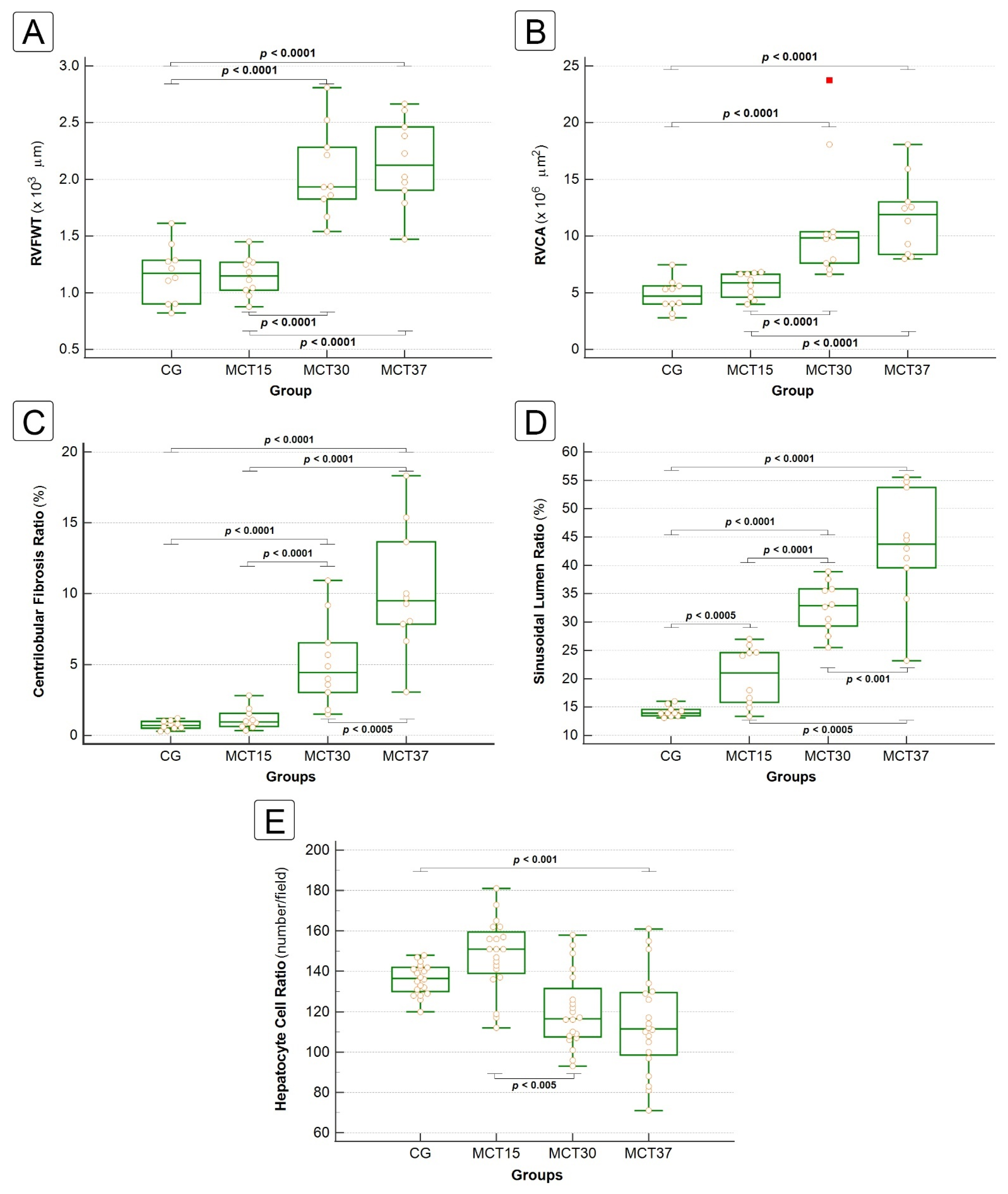
2.3.1. Liver Fibrosis
2.3.2. Sinusoidal Dilatation and Hepatocyte Cell Count
2.3.3. Congestion and Centrilobular Hemorrhagic Necrosis
2.3.4. Miscellaneous Lesions
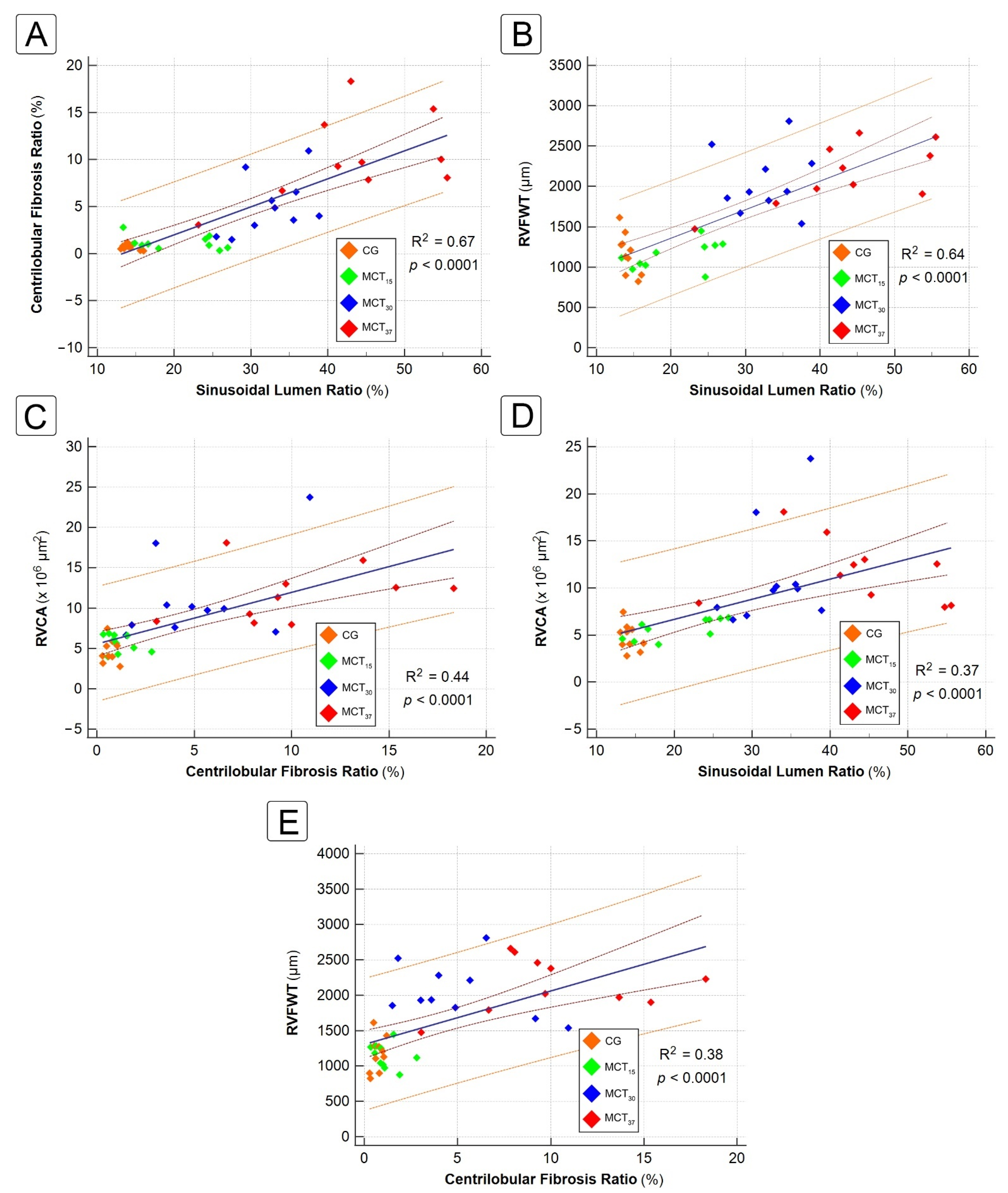
2.4. Correlation between Hepatic and Cardiac Morphometric Parameters
3. Discussion
4. Materials and Methods
4.1. Ethical Aspects
4.2. Experimental Animals
4.3. Drugs and Chemicals
4.4. Experimental Design—Induction of Pulmonary Arterial Hypertension
4.5. Histopathology
4.5.1. Histological Preparation
4.5.2. Digital Slide Scanner and Image Acquisition
4.5.3. Histological Evaluation
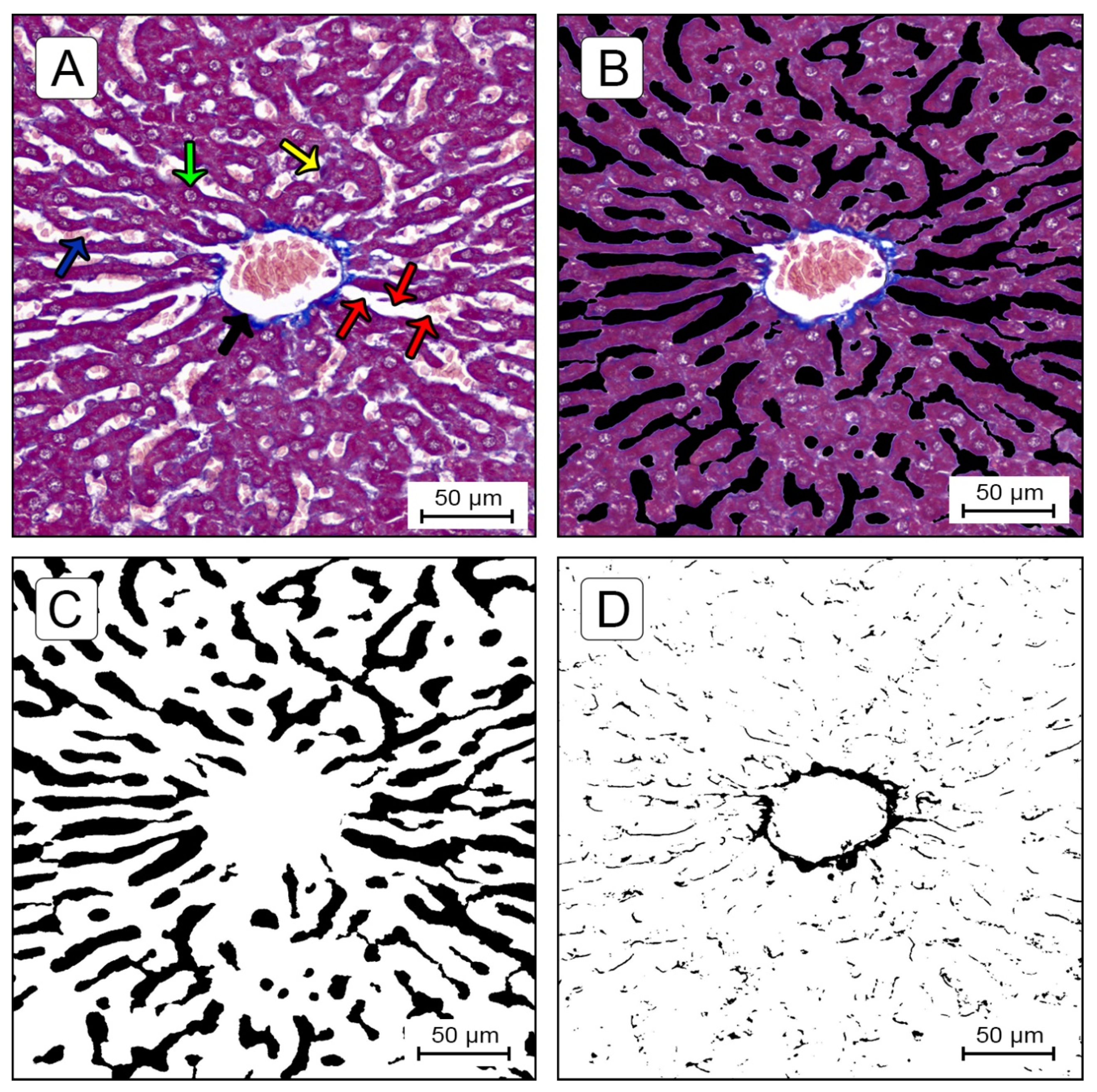
Quantification of Sinusoidal Lumen Ratio (SLR) and Centrilobular Fibrosis Ratio (CFR)
Quantification of Hepatocyte Nuclei
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef]
- Poelzl, G.; Auer, J. Cardiohepatic Syndrome. Curr. Heart Fail. Rep. 2015, 12, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Sessa, A.; Allaire, M.; Lebray, P.; Medmoun, M.; Tiritilli, A.; Iaria, P.; Cadranel, J.F. From congestive hepatopathy to hepatocellular carcinoma, how can we improve patient management? JHEP Rep. 2021, 3, 100249. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.P.; Cerini, R.; Sayegh, R.; Moreau, R.; Degott, C.; Lebrec, D.; Lee, S.S. Cardiac hepatopathy: Clinical, hemodynamic, and histologic characteristics and correlations. Hepatology 2003, 37, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Swanson, P.E.; Krieger, E.V.; Liou, I.W.; Carithers, R.L.; Yeh, M.M. Congestive hepatic fibrosis score: A novel histologic assessment of clinical severity. Mod. Pathol. 2014, 27, 1552–1558. [Google Scholar] [CrossRef] [Green Version]
- Koehne de Gonzalez, A.K.; Lefkowitch, J.H. Heart Disease and the Liver: Pathologic Evaluation. Gastroenterol. Clin. North Am. 2017, 46, 421–435. [Google Scholar]
- Laribi, S.; Mebazaa, A. Cardiohepatic syndrome: Liver injury in decompensated heart failure. Curr. Heart Fail. Rep. 2014, 11, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Gewehr, D.M.; Salgueiro, G.R.; de Noronha, L.; Kubrusly, F.B.; Kubrusly, L.F.; Coltro, G.A.; Preto, P.C.; Bertoldi, A.d.S.; Vieira, H.I. Plexiform lesions in an experimental model of monocrotalin-induced pulmonary arterial hypertension. Arq. Bras. Cardiol. 2020, 115, 480–490. [Google Scholar] [CrossRef]
- Gomez-Arroyo, J.G.; Farkas, L.; Alhussaini, A.A.; Farkas, D.; Kraskauskas, D.; Voelkel, N.F.; Bogaard, H.J. The monocrotaline model of pulmonary hypertension in perspective. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L363–L369. [Google Scholar] [CrossRef]
- Hill, N.S.; Gillespie, M.N.; McMurtry, I.F. Fifty Years of Monocrotaline-Induced Pulmonary Hypertension: What Has It Meant to the Field? Chest 2017, 152, 1106–1108. [Google Scholar] [CrossRef] [Green Version]
- Henrion, J. Hypoxic hepatitis. Liver Int. 2012, 32, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Fortea, J.I.; Puente, Á.; Cuadrado, A.; Huelin, P.; Pellón, R.; González Sánchez, F.J.; Mayorga, M.; Cagigal, M.L.; García Carrera, I.; Cobreros, M.; et al. Congestive Hepatopathy. Int. J. Mol. Sci. 2020, 21, 9420. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, U.D.; Seren, S.; Bayraktar, Y. Hepatic venous outflow obstruction: Three similar syndromes. World J. Gastroenterol. 2007, 13, 1912–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz, S.J.; Manickavasagan, H.R.; Amirjazil, I. The Liver in Circulatory Failure. In Schiff’s Diseases of the Liver; Schiff, E.R., Maddrey, W.C., Rajender Reddy, K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 935–946. [Google Scholar]
- Tanaka, M.; Wanless, I.R. Pathology of the liver in Budd-Chiari syndrome: Portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology 1998, 27, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef]
- Allen, L.A.; Felker, G.M.; Pocock, S.; McMurray, J.J.V.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L.; Granger, C.B. Liver function abnormalities and outcome in patients with chronic heart failure: Data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur. J. Heart Fail. 2009, 11, 170–177. [Google Scholar] [CrossRef]
- van Deursen, V.M.; Damman, K.; Hillege, H.L.; van Beek, A.P.; van Veldhuisen, D.J.; Voors, A.A. Abnormal Liver Function in Relation to Hemodynamic Profile in Heart Failure Patients. J. Card. Fail. 2010, 16, 84–90. [Google Scholar] [CrossRef]
- Gelow, J.M.; Desai, A.S.; Hochberg, C.P.; Glickman, J.N.; Givertz, M.M.; Fang, J.C. Clinical predictors of hepatic fibrosis in chronic advanced heart failure. Circ. Hear. Fail. 2010, 3, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richman, S.M.; Delman, A.J.; Grob, D. Alterations in indices of liver function in congestive heart failure with particular reference to serum enzymes. Am. J. Med. 1961, 30, 211–225. [Google Scholar] [CrossRef]
- Simonetto, D.A.; Yang, H.Y.; Yin, M.; de Assuncao, T.M.; Kwon, J.H.; Hilscher, M.; Pan, S.; Yang, L.; Bi, Y.; Beyder, A.; et al. Chronic passive venous congestion drives hepatic fibrogenesis via sinusoidal thrombosis and mechanical forces. Hepatology 2015, 61, 648–659. [Google Scholar] [CrossRef] [Green Version]
- Lemmer, A.; VanWagner, L.B.; Ganger, D. Assessment of Advanced Liver Fibrosis and the Risk for Hepatic Decompensation in Patients With Congestive Hepatopathy. Hepatology 2018, 68, 1633–1641. [Google Scholar] [CrossRef] [Green Version]
- Kendall, T.J.; Stedman, B.; Hacking, N.; Haw, M.; Vettukattill, J.J.; Salmon, A.P.; Cope, R.; Sheron, N.; Millward-Sadler, H.; Veldtman, G.R.; et al. Hepatic fibrosis and cirrhosis in the Fontan circulation: A detailed morphological study. J. Clin. Pathol. 2008, 61, 504–508. [Google Scholar] [CrossRef]
- Wells, M.L.; Venkatesh, S.K. Congestive hepatopathy. Abdom. Radiol. 2018, 43, 2037–2051. [Google Scholar] [CrossRef]
- Lefkowitch, J.H.; Mendez, L. Morphologic features of hepatic injury in cardiac disease and shock. J. Hepatol. 1986, 2, 313–327. [Google Scholar] [CrossRef]
- Ford, R.M.; Book, W.; Spivey, J.R. Liver disease related to the heart. Transplant. Rev. 2015, 29, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Kavoliuniene, A.; Vaitiekiene, A.; Cesnaite, G. Congestive hepatopathy and hypoxic hepatitis in heart failure: A cardiologist’s point of view. Int. J. Cardiol. 2013, 166, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Urashima, T.; Shimura, D.; Ito, R.; Kawachi, S.; Kajimura, I.; Akaike, T.; Kusakari, Y.; Fujiwara, M.; Ogawa, K.; et al. Low cardiac output leads hepatic fibrosis in right heart failure model rats. PLoS ONE 2016, 11, e0148666. [Google Scholar] [CrossRef] [PubMed]
- Arcidi, J.M.; Moore, G.W.; Hutchins, G.M. Hepatic morphology in cardiac dysfunction. A clinicopathologic study of 1000 subjects at autopsy. Am. J. Pathol. 1981, 104, 159–166. [Google Scholar]
- Vasconcelos, L.A.B.A.; De Almeida, E.A.; Bachur, L.F. Clinical evaluation and hepatic laboratory assessment in individuals with congestive heart failure. Arq. Bras. Cardiol. 2007, 88, 590–595. [Google Scholar] [CrossRef] [Green Version]
- Nikolaou, M.; Parissis, J.; Yilmaz, M.B.; Seronde, M.F.; Kivikko, M.; Laribi, S.; Paugam-Burtz, C.; Cai, D.; Pohjanjousi, P.; Laterre, P.F.; et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur. Heart J. 2013, 34, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Ohtani, T.; Kioka, H.; Tsukamoto, Y.; Onishi, T.; Nakamoto, K.; Katsimichas, T.; Sengoku, K.; Chimura, M.; Hashimoto, H.; et al. Liver Stiffness Reflecting Right-Sided Filling Pressure Can Predict Adverse Outcomes in Patients With Heart Failure. JACC Cardiovasc. Imaging 2019, 12, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Xavier Ávila, D.; Abreu Matos, P.; Quintino, G.; de Andrade Martins, W.; Machado, D.; Tinoco Mesquita, C.; Villacorta Junior, H. Diagnostic and Prognostic Role of Liver Elastography in Heart Failure. Int. J. Cardiovasc. Sci. 2019, 33, 227–232. [Google Scholar] [CrossRef]
- Horvath, B.; Zhu, L.; Allende, D.; Xie, H.; Guirguis, J.; Cruise, M.; Patil, D.T.; O’Shea, R.; Rivas, J.; Yordanka, R.; et al. Histology and Glutamine Synthetase Immunoreactivity in Liver Biopsies From Patients With Congestive Heart Failure. Gastroenterol. Res. 2017, 10, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krings, G.; Can, B.; Ferrell, L. Aberrant centrizonal features in chronic hepatic venous outflow obstruction: Centrilobular mimicry of portal-based disease. Am. J. Surg. Pathol. 2014, 38, 205–214. [Google Scholar] [CrossRef]
- DeLeve, L.D.; McCuskey, R.S.; Wang, X.; Hu, L.; McCuskey, M.K.; Epstein, R.B.; Kanel, G.C. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology 1999, 29, 1779–1791. [Google Scholar] [CrossRef]
- Gujral, J.S.; Bucci, T.J.; Farhood, A.; Jaeschke, H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: Apoptosis or necrosis? Hepatology 2001, 33, 397–405. [Google Scholar] [CrossRef]
| Histological Parameters | Groups | p-Value | ||||
|---|---|---|---|---|---|---|
| CG | MCT15 | MCT30 | MCT37 | MCTED | ||
| CHFS | − | − | + | + | ++ | p < 0.05 a–d |
| 0 ± 0 | 0.2 ± 0.42 | 0.6 ± 0.52 a,b | 1.1 ± 0.57 a–c | 1.9 ± 0.32 a–d | ||
| SH | − | − | + | ++ | ++ | p < 0.05 a–d |
| 0 ± 0 | 0.1 ± 0.32 | 0.8 ± 0.79 a,b | 1.3 ± 0.95 a,b | 1.8 ± 0.63 a–c | ||
| SC | − | + | ++ | +++ | +++ | p < 0.05 a–d |
| 0.1 ± 0.32 | 1.1 ± 1.10 a | 1.5 ± 0.97 a | 2.3 ± 0.67 a,b | 2.9 ± 0.32 a–c | ||
| SD | − | − | + | ++ | +++ | p < 0.05 a–d |
| 0.1 ± 0.32 | 1.4 ± 0.52 a | 1.9 ± 0.57 a,b | 2.5 ± 0.71 a–c | 3.0 ± 0.00 a–d | ||
| CHN | − | − | − | +/++ | ++ | p < 0.005 a–d |
| 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.7 ± 0.82 a-c | 1.7 ± 0.67 a–d | ||
| Steatosis | − | − | + | + | ++ | p < 0.05 a–d |
| 0 ± 0 | 0 ± 0 | 1.6 ± 0.63 a,b | 1.5 ± 0.53 | 1.5 ± 1.01 a,b,d | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gewehr, D.M.; Giovanini, A.F.; Mattar, B.A.; Agulham, A.P.; Bertoldi, A.d.S.; Nagashima, S.; Kubrusly, F.B.; Kubrusly, L.F. Congestive Hepatopathy Secondary to Right Ventricular Hypertrophy Related to Monocrotaline-Induced Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 11891. https://doi.org/10.3390/ijms222111891
Gewehr DM, Giovanini AF, Mattar BA, Agulham AP, Bertoldi AdS, Nagashima S, Kubrusly FB, Kubrusly LF. Congestive Hepatopathy Secondary to Right Ventricular Hypertrophy Related to Monocrotaline-Induced Pulmonary Arterial Hypertension. International Journal of Molecular Sciences. 2021; 22(21):11891. https://doi.org/10.3390/ijms222111891
Chicago/Turabian StyleGewehr, Douglas Mesadri, Allan Fernando Giovanini, Beatriz Alvarez Mattar, Anelyse Pulner Agulham, Andressa de Souza Bertoldi, Seigo Nagashima, Fernando Bermudez Kubrusly, and Luiz Fernando Kubrusly. 2021. "Congestive Hepatopathy Secondary to Right Ventricular Hypertrophy Related to Monocrotaline-Induced Pulmonary Arterial Hypertension" International Journal of Molecular Sciences 22, no. 21: 11891. https://doi.org/10.3390/ijms222111891
APA StyleGewehr, D. M., Giovanini, A. F., Mattar, B. A., Agulham, A. P., Bertoldi, A. d. S., Nagashima, S., Kubrusly, F. B., & Kubrusly, L. F. (2021). Congestive Hepatopathy Secondary to Right Ventricular Hypertrophy Related to Monocrotaline-Induced Pulmonary Arterial Hypertension. International Journal of Molecular Sciences, 22(21), 11891. https://doi.org/10.3390/ijms222111891






