Vitamin D and Rheumatic Diseases: A Review of Clinical Evidence
Abstract
:1. Introduction
2. Physiology of Vitamin D
3. General Concepts of Vitamin D for Skeletal and Immune Health
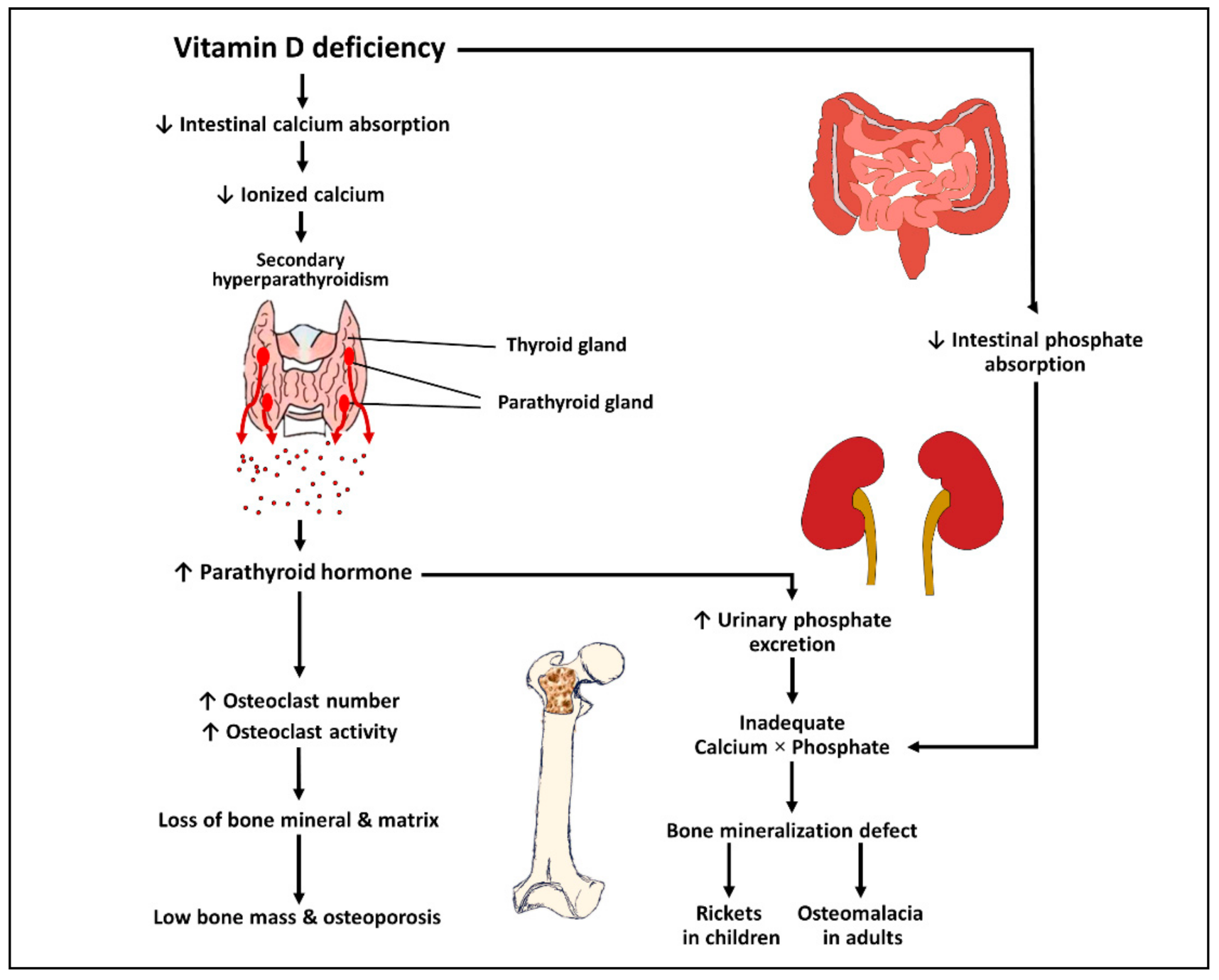
3.1. Effects of Vitamin D on Bone and Mineral Metabolism
3.2. Effects of Vitamin D on the Immune System
3.3. Defining Optimal Serum 25-hydroxyvitamin D
3.4. Recommended Vitamin D Intake
3.5. Vitamin D from Sunlight Exposure and Diets
3.6. Screening for Vitamin D Status
4. Evidence on Vitamin D for Prevention and Treatment of Rheumatic Diseases
4.1. Rheumatoid Arthritis
4.2. Systemic Lupus Erythematosus
4.3. Spondyloarthropathies
4.4. Gout and Hyperuricemia
4.5. Osteoarthritis
4.6. Other Rheumatic Diseases
| Disease | First Author and Year of Publication | Study Design/Intervention/Studied Participants | Main Results |
|---|---|---|---|
| RA | Guan et al., 2020 [72] | A meta-analysis of 6 randomized controlled trial investigating the efficacy of 8000–50,000 IUs per week of oral vitamin D or equivalent or oral 1,25(OH)2D on disease activity in patients with RA (total n = 438). | Vitamin D supplementation resulted in a significant improvement in the DAS28 (WMD −0.41, 95%CI: −0.59–−0.23), ESR (WMD −3.40, 95%CI: −6.62–−0.18) and tender joint count (WMD −1.44, 95%CI: −2.74–−0.14) but not in pain VAS. |
| Nguyen et al., 2020 [73] | A subgroup meta-analysis of 2 randomized controlled trial investigating the efficacy of oral 50,000 IUs/week of vitamin D3 or 0.5 mcg/day of alfacalcidol [1α-hydroxyvitamin D3] on the risk of flare in RA patients in remission (total n = 252). | Vitamin D supplementation resulted in an insignificant reduction in RA flares defined by the DAS28 of >3.2 (risk difference −0.10, 95% CI: −0.21–0.00). | |
| SLE | Zheng et al., 2019 [89] | A meta-analysis of 5 randomized controlled trials investigating the efficacy of 200–50,000 IUs/week of vitamin D3 or equivalent on disease activity in patients with SLE (total n = 490). | Vitamin D3 supplementation resulted in a decrease in the fatigue severity scale scores in patients with SLE in a meta-analysis of 2 trials (n = 79; SMD −1.179, 95% CI: −1.90–−0.46). However, no significant changes in the SLEDAI and positivity of anti-dsDNA was observed. |
| Hyperuricemia | Nimitphong et al., 2021 [111] | A randomized controlled trial giving either 20,000 IUs/week of vitamin D2, 15,000 IUs/week of of vitamin D3 or placebo to patients with prediabetes (n = 71). | Among patients with baseline serum uric >6 mg/dL (n = 36), associated with a reduction in mean serum uric acid level by 0.6 mg/dL. |
| OA | Sanghi et al., 2013 [125] | A randomized controlled trial giving either 60,000 IUs of vitamin D3/day for 10 days followed by 60,000 IUs/month for 12 months or placebo to patients with knee OA with serum 25(OH)D <20 ng/mL (n = 107). | Vitamin D3 supplementation results in a significant decrease in visual analog scale pain (between-group MD −0.39, 95%CI: −0.71–−0.08) and total WOMAC (MD −3.53, 95%CI: −4.39–−2.71). |
| Jin et al., 2016 [126] | A randomized controlled trial giving either 50,000 IUs/week of vitamin D3 per day for 2 years or placebo to patients with knee OA with serum 25(OH)D <24 ng/mL (n = 413). | Vitamin D3 supplementation resulted in significant improvements in WOMAC function (MD −72.9, 95%CI: −126.4–−19.4) and total WOMAC (MD −91.4, 95%CI: −165.1–−17.7). However, no significant difference between groups in tibial cartilage volume or WOMAC pain was observed. | |
| Chronic widespread pain/fibromyalgia | Yong et al., 2017 [136] | A meta-analysis of 4 randomized controlled trials investigating the efficacy of 25,000–75,000 IUs/week of oral or intramuscular vitamin D on pain VAS in patients with chronic widespread pain or fibromyalgia (total n = 287) | Vitamin D supplementation resulted in a significantly lower pain VAS (pooled MD 0.46, 95%CI 0.09–0.89). |
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B.; Lee, S.-M.; Onal, M.; Benkusky, N.A. The vitamin D receptor: Contemporary genomic approaches reveal new basic and translational insights. J. Clin. Investig. 2017, 127, 1146–1154. [Google Scholar] [CrossRef] [Green Version]
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017, 69, 1521–1537. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Leung, P.S.C.; Adamopoulos, I.E.; Gershwin, M.E. The implication of vitamin D and autoimmunity: A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, D.R.; Ding, N.; Parnell, G.P.; Shahijanian, F.; Coulter, S.; Schibeci, S.D.; Atkins, A.R.; Stewart, G.J.; Evans, R.M.; Downes, M.; et al. Cistromic and genetic evidence that the vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes Immun. 2016, 17, 213–219. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Spira, A.; Holick, M.F. Influence of Vitamin D Status and Vitamin D3 Supplementation on Genome Wide Expression of White Blood Cells: A Randomized Double-Blind Clinical Trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blau, J.E.; Collins, M.T. The PTH-Vitamin D-FGF23 axis. Rev. Endocr. Metab. Disord. 2015, 16, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014, 144 (Pt A), 22–27. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef]
- Baldock, P.A.; Thomas, G.P.; Hodge, J.M.; Baker, S.U.; Dressel, U.; O’Loughlin, P.D.; Nicholson, G.C.; Briffa, K.H.; Eisman, J.A.; Gardiner, E.M. Vitamin D action and regulation of bone remodeling: Suppression of osteoclastogenesis by the mature osteoblast. J. Bone Miner. Res. 2006, 21, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Udagawa, N.; Suda, T. Vitamin D endocrine system and osteoclasts. Bonekey Rep. 2014, 3, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Charoenngam, N.; Rujirachun, P.; Holick, M.F.; Ungprasert, P. Oral vitamin D3 supplementation increases serum fibroblast growth factor 23 concentration in vitamin D-deficient patients: A systematic review and meta-analysis. Osteoporos. Int. 2019, 30, 2183–2193. [Google Scholar] [CrossRef]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [Green Version]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Adams, J.S.; Ren, S.; Liu, P.T.; Chun, R.F.; Lagishetty, V.; Gombart, A.F.; Borregaard, N.; Modlin, R.L.; Hewison, M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 2009, 182, 4289–4295. [Google Scholar] [CrossRef] [Green Version]
- Verway, M.; Bouttier, M.; Wang, T.-T.; Carrier, M.; Calderon, M.; An, B.-S.; Devemy, E.; McIntosh, F.; Divangahi, M.; Behr, M.A.; et al. Vitamin D induces interleukin-1β expression: Paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 2013, 9, e1003407. [Google Scholar] [CrossRef] [Green Version]
- Gombart, A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef] [Green Version]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef]
- Szeles, L.; Keresztes, G.; Torocsik, D.; Balajthy, Z.; Krenacs, L.; Poliska, S.; Steinmeyer, A.; Zuegel, U.; Pruenster, M.; Rot, A.; et al. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J. Immunol. 2009, 182, 2074–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adorini, L.; Penna, G. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. In Dendritic Cells; Lombardi, G., Riffo-Vasquez, Y., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin, Germany, 2009; Volume 188. [Google Scholar] [CrossRef]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urry, Z.; Xystrakis, E.; Richards, D.F.; McDonald, J.; Sattar, Z.; Cousins, D.J.; Corrigan, C.J.; Hickman, E.; Brown, Z.; Hawrylowicz, C.M. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J. Clin. Investig. 2009, 119, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickie, L.J.; Church, L.D.; Coulthard, L.R.; Mathews, R.J.; Emery, P.; McDermott, M.F. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology 2010, 49, 1466–1471. [Google Scholar] [CrossRef] [Green Version]
- Lemire, J.M.; Archer, D.C.; Beck, L.; Spiegelberg, H.L. Immunosuppressive Actions of 1,25-Dihydroxyvitamin D3: Preferential Inhibition of Th1 Functions. J. Nutr. 1995, 125, 1704S–1708S. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.J.; O’Garra, A. 1α,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4; T Cells to Enhance the Development of Th2 Cells. J. Immunol. 2001, 167, 4974. [Google Scholar] [CrossRef] [Green Version]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; von Essen, M.R. The vitamin d receptor and T cell function. Front. Immunol. 2013, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Eckard, A.R.; O’Riordan, M.A.; Rosebush, J.C.; Lee, S.T.; Habib, J.G.; Ruff, J.H.; Labbato, D.; Daniels, J.E.; Uribe-Leitz, M.; Tangpricha, V.; et al. Vitamin D supplementation decreases immune activation and exhaustion in HIV-1-infected youth. Antivir. Ther. 2018, 23, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Stallings, V.A.; Schall, J.I.; Hediger, M.L.; Zemel, B.S.; Tuluc, F.; Dougherty, K.A.; Samuel, J.L.; Rutstein, R.M. High-dose vitamin D3 supplementation in children and young adults with HIV: A randomized, placebo-controlled trial. Pediatr. Infect. Dis. J. 2015, 34, e32–e40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemire, J.M.; Adams, J.S.; Sakai, R.; Jordan, S.C. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Investig. 1984, 74, 657–661. [Google Scholar] [CrossRef] [Green Version]
- Rolf, L.; Muris, A.-H.; Hupperts, R.; Damoiseaux, J. Illuminating vitamin D effects on B cells—The multiple sclerosis perspective. Immunology 2016, 147, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heine, G.; Niesner, U.; Chang, H.D.; Steinmeyer, A.; Zugel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef]
- Shirakawa, A.K.; Nagakubo, D.; Hieshima, K.; Nakayama, T.; Jin, Z.; Yoshie, O. 1,25-dihydroxyvitamin D3 induces CCR10 expression in terminally differentiating human B cells. J. Immunol. 2008, 180, 2786–2795. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, E.A.; Nguyen, J.K.; Liu, J.; Keller, E.; Campbell, N.; Zhang, C.J.; Smith, H.R.; Li, X.; Jorgensen, T.N. Low Levels of Vitamin D Promote Memory B Cells in Lupus. Nutrients 2020, 12, 291. [Google Scholar] [CrossRef] [Green Version]
- Infante, M.; Ricordi, C.; Sanchez, J.; Clare-Salzler, M.J.; Padilla, N.; Fuenmayor, V.; Chavez, C.; Alvarez, A.; Baidal, D.; Alejandro, R.; et al. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients 2019, 11, 2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.K.; Stehle, P.; Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Haddad Kashani, H.; Seyed Hosseini, E.; Nikzad, H.; Soleimani, A.; Soleimani, M.; Tamadon, M.R.; Keneshlou, F.; Asemi, Z. The Effects of Vitamin D Supplementation on Signaling Pathway of Inflammation and Oxidative Stress in Diabetic Hemodialysis: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pharmacol. 2018, 9, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 17685. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Allen, R.; Charoenngam, N.; Lewanczuk, R.; Holick, M.F. Variable Genomic and Metabolomic Responses to Varying Doses of Vitamin D Supplementation. Anticancer Res. 2020, 40, 535–543. [Google Scholar] [CrossRef]
- Carlberg, C.; Seuter, S.; de Mello, V.D.; Schwab, U.; Voutilainen, S.; Pulkki, K.; Nurmi, T.; Virtanen, J.; Tuomainen, T.P.; Uusitupa, M. Primary vitamin D target genes allow a categorization of possible benefits of vitamin D(3) supplementation. PLoS ONE 2013, 8, e71042. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: What dietetics practitioners need to know. J. Am. Diet. Assoc. 2011, 111, 524–527. [Google Scholar] [CrossRef]
- Luxwolda, M.F.; Kuipers, R.S.; Kema, I.P.; van der Veer, E.; Dijck-Brouwer, D.A.; Muskiet, F.A. Vitamin D status indicators in indigenous populations in East Africa. Eur. J. Nutr. 2013, 52, 1115–1125. [Google Scholar] [CrossRef]
- Dudenkov, D.V.; Mara, K.C.; Petterson, T.M.; Maxson, J.A.; Thacher, T.D. Serum 25-Hydroxyvitamin D Values and Risk of All-Cause and Cause-Specific Mortality: A Population-Based Cohort Study. Mayo Clin. Proc. 2018, 93, 721–730. [Google Scholar] [CrossRef]
- Amrein, K.; Quraishi, S.A.; Litonjua, A.A.; Gibbons, F.K.; Pieber, T.R.; Camargo, C.A., Jr.; Giovannucci, E.; Christopher, K.B. Evidence for a U-Shaped Relationship Between Prehospital Vitamin D Status and Mortality: A Cohort Study. J. Clin. Endocrinol. Metab. 2014, 99, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Aleksova, A.; Beltrami, A.P.; Belfiore, R.; Barbati, G.; Di Nucci, M.; Scapol, S.; De Paris, V.; Carriere, C.; Sinagra, G. U-shaped relationship between vitamin D levels and long-term outcome in large cohort of survivors of acute myocardial infarction. Int. J. Cardiol. 2016, 223, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [Green Version]
- Dowdy, J.C.; Sayre, R.M.; Holick, M.F. Holick’s rule and vitamin D from sunlight. J. Steroid Biochem. Mol. Biol. 2010, 121, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Force, U.S.P.S.T. Screening for Vitamin D Deficiency in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1436–1442. [Google Scholar] [CrossRef]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Therneau, T.M.; Gabriel, S.E. Is the incidence of rheumatoid arthritis rising?: Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010, 62, 1576–1582. [Google Scholar] [CrossRef] [Green Version]
- Schulze-Koops, H.; Kalden, J.R. The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2001, 15, 677–691. [Google Scholar] [CrossRef]
- Paradowska-Gorycka, A.; Wajda, A.; Romanowska-Próchnicka, K.; Walczuk, E.; Kuca-Warnawin, E.; Kmiolek, T.; Stypinska, B.; Rzeszotarska, E.; Majewski, D.; Jagodzinski, P.P.; et al. Th17/Treg-Related Transcriptional Factor Expression and Cytokine Profile in Patients with Rheumatoid Arthritis. Front. Immunol. 2020, 11, 3189. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.-g.; Li, Y.-h.; Qi, L.; Liu, X.-g.; Yuan, C.-z.; Hu, N.-w.; Ma, D.-x.; Li, Z.-f.; Yang, Q.; et al. Increased Frequencies of Th22 Cells as well as Th17 Cells in the Peripheral Blood of Patients with Ankylosing Spondylitis and Rheumatoid Arthritis. PLoS ONE 2012, 7, e31000. [Google Scholar] [CrossRef]
- Aho, K.; Heliovaara, M. Risk factors for rheumatoid arthritis. Ann. Med. 2004, 36, 242–251. [Google Scholar] [CrossRef]
- Aslam, M.M.; John, P.; Bhatti, A.; Jahangir, S.; Kamboh, M.I. Vitamin D as a Principal Factor in Mediating Rheumatoid Arthritis-Derived Immune Response. Biomed. Res. Int. 2019, 2019, 3494937. [Google Scholar] [CrossRef] [Green Version]
- Bagheri-Hosseinabadi, Z.; Imani, D.; Yousefi, H.; Abbasifard, M. Vitamin D receptor (VDR) gene polymorphism and risk of rheumatoid arthritis (RA): Systematic review and meta-analysis. Clin. Rheumatol. 2020, 39, 3555–3569. [Google Scholar] [CrossRef]
- Rozmus, D.; Ciesielska, A.; Plominski, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieslinska, A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms-The Risk of Malignant Tumors and Other Diseases. Int. J. Mol. Sci. 2020, 21, 7822. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.C. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: A meta-analysis. Clin. Exp. Rheumatol. 2016, 34, 827–833. [Google Scholar]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G.; Iowa Women’s Health, S. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef]
- Arkema, E.V.; Hart, J.E.; Bertrand, K.A.; Laden, F.; Grodstein, F.; Rosner, B.A.; Karlson, E.W.; Costenbader, K.H. Exposure to ultraviolet-B and risk of developing rheumatoid arthritis among women in the Nurses’ Health Study. Ann. Rheum. Dis. 2013, 72, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Staples, J.A.; Ponsonby, A.-L.; Lim, L.L.Y.; McMichael, A.J. Ecologic analysis of some immune-related disorders, including type 1 diabetes, in Australia: Latitude, regional ultraviolet radiation, and disease prevalence. Environ. Health Perspect. 2003, 111, 518–523. [Google Scholar] [CrossRef]
- Vieira, V.M.; Hart, J.E.; Webster, T.F.; Weinberg, J.; Puett, R.; Laden, F.; Costenbader, K.H.; Karlson, E.W. Association between residences in U.S. northern latitudes and rheumatoid arthritis: A spatial analysis of the Nurses’ Health Study. Environ. Health Perspect. 2010, 118, 957–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajjaj-Hassouni, N.; Mawani, N.; Allali, F.; Rkain, H.; Hassouni, K.; Hmamouchi, I.; Dougados, M. Evaluation of Vitamin D Status in Rheumatoid Arthritis and Its Association with Disease Activity across 15 Countries: “The COMORA Study”. Int. J. Rheumatol. 2017, 2017, 5491676. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Hao, Y.; Guan, Y.; Bu, H.; Wang, H. The Effect of Vitamin D Supplementation on Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 596007. [Google Scholar] [CrossRef]
- Nguyen, Y.; Sigaux, J.; Letarouilly, J.-G.; Sanchez, P.; Czernichow, S.; Flipo, R.-M.; Soubrier, M.; Semerano, L.; Seror, R.; Sellam, J.; et al. Efficacy of Oral Vitamin Supplementation in Inflammatory Rheumatic Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 13, 107. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; Zhang, Q.; Li, M.; Wang, J. Effect of vitamin D on the recurrence rate of rheumatoid arthritis. Exp. Ther. Med. 2015, 10, 1812–1816. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, K.; Danda, D. Supplementation of 1,25 dihydroxy vitamin D3 in patients with treatment naive early rheumatoid arthritis: A randomised controlled trial. Int. J. Rheum. Dis. 2011, 14, 332–339. [Google Scholar] [CrossRef]
- Li, C.; Yin, S.; Yin, H.; Cao, L.; Zhang, T.; Wang, Y. Efficacy and Safety of 22-Oxa-Calcitriol in Patients with Rheumatoid Arthritis: A Phase II Trial. Med. Sci. Monit. 2018, 24, 9127–9135. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.C.; Ghetu, M.V.; Bieniek, M.L. Systemic Lupus Erythematosus: Primary Care Approach to Diagnosis and Management. Am. Fam. Physician 2016, 94, 284–294. [Google Scholar] [PubMed]
- Rees, F.; Doherty, M.; Grainge, M.J.; Lanyon, P.; Zhang, W. The worldwide incidence and prevalence of systemic lupus erythematosus: A systematic review of epidemiological studies. Rheumatology 2017, 56, 1945–1961. [Google Scholar] [CrossRef] [Green Version]
- Barbhaiya, M.; Costenbader, K.H. Environmental exposures and the development of systemic lupus erythematosus. Curr. Opin. Rheumatol. 2016, 28, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Fueyo, A.; Bradley, S.J.; Tsokos, G.C. T cells in Systemic Lupus Erythematosus. Curr. Opin. Immunol. 2016, 43, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Zhuang, H.; Shumyak, S.; Yang, L.; Reeves, W.H. Mechanisms of autoantibody production in systemic lupus erythematosus. Front. Immunol. 2015, 6, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postal, M.; Peliçari, K.O.; Sinicato, N.A.; Marini, R.; Costallat, L.T.L.; Appenzeller, S. Th1/Th2 cytokine profile in childhood-onset systemic lupus erythematosus. Cytokine 2013, 61, 785–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad Yusoff, F.; Wong, K.K.; Mohd Redzwan, N. Th1, Th2, and Th17 cytokines in systemic lupus erythematosus. Autoimmunity 2020, 53, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Khandker, S.S.; Alam, S.S.; Kotyla, P.; Hassan, R. Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun. Rev. 2019, 18, 102392. [Google Scholar] [CrossRef]
- Sahebari, M.; Nabavi, N.; Salehi, M. Correlation between serum 25(OH)D values and lupus disease activity: An original article and a systematic review with meta-analysis focusing on serum VitD confounders. Lupus 2014, 23, 1164–1177. [Google Scholar] [CrossRef]
- Monticielo, O.A.; Teixeira, T.d.M.; Chies, J.A.B.; Brenol, J.C.T.; Xavier, R.M. Vitamin D and polymorphisms of VDR gene in patients with systemic lupus erythematosus. Clin. Rheumatol. 2012, 31, 1411–1421. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, S.; Liu, J.S.; Gui, M.; Zhang, H. Expression of vitamin D receptor in renal tissue of lupus nephritis and its association with renal injury activity. Lupus 2019, 28, 290–294. [Google Scholar] [CrossRef]
- Costenbader, K.H.; Feskanich, D.; Holmes, M.; Karlson, E.W.; Benito-Garcia, E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann. Rheum. Dis. 2008, 67, 530–535. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.; Gonzalez, A.; Yue, J.; Wu, X.; Qiu, M.; Gui, L.; Zhu, S.; Huang, L. Efficacy and Safety of Vitamin D Supplementation in Patients with Systemic Lupus Erythematosus: A Meta-analysis of Randomized Controlled Trials. Am. J. Med. Sci. 2019, 358, 104–114. [Google Scholar] [CrossRef]
- Kataria, R.K.; Brent, L.H. Spondyloarthropathies. Am. Fam. Physician 2004, 69, 2853–2860. [Google Scholar]
- Sharip, A.; Kunz, J. Understanding the Pathogenesis of Spondyloarthritis. Biomolecules 2020, 10, 1461. [Google Scholar] [CrossRef]
- De Martinis, M.; Ginaldi, L.; Sirufo, M.M.; Bassino, E.M.; De Pietro, F.; Pioggia, G.; Gangemi, S. IL-33/Vitamin D Crosstalk in Psoriasis-Associated Osteoporosis. Front. Immunol. 2021, 11, 3416. [Google Scholar] [CrossRef]
- Cubillos, S.; Krieg, N.; Norgauer, J. Effect of Vitamin D on Peripheral Blood Mononuclear Cells from Patients with Psoriasis Vulgaris and Psoriatic Arthritis. PLoS ONE 2016, 11, e0153094. [Google Scholar] [CrossRef] [Green Version]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A brief overview. Clin. Med. 2021, 21, 170–173. [Google Scholar] [CrossRef]
- Petho, Z.; Kulcsar-Jakab, E.; Kalina, E.; Balogh, A.; Pusztai, A.; Gulyas, K.; Horvath, A.; Szekanecz, Z.; Bhattoa, H.P. Vitamin D status in men with psoriatic arthritis: A case-control study. Osteoporos. Int. 2015, 26, 1965–1970. [Google Scholar] [CrossRef]
- Sağ, M.S.; Sağ, S.; Tekeoğlu, İ.; Solak, B.; Kamanlı, A.; Nas, K.; Harman, H.; Kantar, M. Comparison of 25-hidroksi Vitamin D serum concentrations in patients with psoriasis and psoriatic arthritis. J. Back Musculoskelet. Rehabil. 2018, 31, 37–43. [Google Scholar] [CrossRef]
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239. [Google Scholar] [CrossRef]
- McCullough, P.J.; McCullough, W.P.; Lehrer, D.; Travers, J.B.; Repas, S.J. Oral and Topical Vitamin D, Sunshine, and UVB Phototherapy Safely Control Psoriasis in Patients with Normal Pretreatment Serum 25-Hydroxyvitamin D Concentrations: A Literature Review and Discussion of Health Implications. Nutrients 2021, 13, 1511. [Google Scholar] [CrossRef]
- McCullough, P.; Amend, J. Results of daily oral dosing with up to 60,000 international units (iu) of vitamin D3 for 2 to 6 years in 3 adult males. J. Steroid Biochem. Mol. Biol. 2017, 173, 308–312. [Google Scholar] [CrossRef]
- Theodoridis, X.; Grammatikopoulou, M.G.; Stamouli, E.-M.; Talimtzi, P.; Pagkalidou, E.; Zafiriou, E.; Haidich, A.-B.; Bogdanos, D.P. Effectiveness of oral vitamin D supplementation in lessening disease severity among patients with psoriasis: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2021, 82, 111024. [Google Scholar] [CrossRef]
- Cai, G.; Wang, L.; Fan, D.; Xin, L.; Liu, L.; Hu, Y.; Ding, N.; Xu, S.; Xia, G.; Jin, X.; et al. Vitamin D in ankylosing spondylitis: Review and meta-analysis. Clin. Chim. Acta 2015, 438, 316–322. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shabat, N.; Watad, A.; Shabat, A.; Bragazzi, N.L.; Comaneshter, D.; Cohen, A.D.; Amital, H. Low Vitamin D Levels Predict Mortality in Ankylosing Spondylitis Patients: A Nationwide Population-Based Cohort Study. Nutrients 2020, 12, 1400. [Google Scholar] [CrossRef]
- Guzman-Prado, Y.; Samson, O.; Segal, J.P.; Limdi, J.K.; Hayee, B.H. Vitamin D Therapy in Adults With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, 1819–1830. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The Effect of Various Doses of Oral Vitamin D3 Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020, 40, 551–556. [Google Scholar] [CrossRef]
- Jung, K.H.; Kim, T.H.; Sheen, D.H.; Lim, M.K.; Lee, S.K.; Kim, J.Y.; Park, H.; Chae, S.C.; Shim, S.C. Associations of vitamin d binding protein gene polymorphisms with the development of peripheral arthritis and uveitis in ankylosing spondylitis. J. Rheumatol. 2011, 38, 2224–2229. [Google Scholar] [CrossRef]
- Henderson, C.M.; Fink, S.L.; Bassyouni, H.; Argiropoulos, B.; Brown, L.; Laha, T.J.; Jackson, K.J.; Lewkonia, R.; Ferreira, P.; Hoofnagle, A.N.; et al. Vitamin D–Binding Protein Deficiency and Homozygous Deletion of the GC Gene. N. Engl. J. Med. 2019, 380, 1150–1157. [Google Scholar] [CrossRef]
- Kew, R.R. The Vitamin D Binding Protein and Inflammatory Injury: A Mediator or Sentinel of Tissue Damage? Front. Endocrinol. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Ponvilawan, B.; Ungprasert, P. Vitamin D insufficiency and deficiency are associated with a higher level of serum uric acid: A systematic review and meta-analysis. Mod. Rheumatol. 2020, 30, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Nimitphong, H.; Saetung, S.; Chailurkit, L.O.; Chanprasertyothin, S.; Ongphiphadhanakul, B. Vitamin D supplementation is associated with serum uric acid concentration in patients with prediabetes and hyperuricemia. J. Clin. Transl. Endocrinol. 2021, 24, 100255. [Google Scholar] [CrossRef]
- Ponvilawan, B.; Charoenngam, N. Vitamin D and uric acid: Is parathyroid hormone the missing link? J. Clin. Transl. Endocrinol. 2021, 25, 100263. [Google Scholar] [CrossRef]
- Sugimoto, R.; Watanabe, H.; Ikegami, K.; Enoki, Y.; Imafuku, T.; Sakaguchi, Y.; Murata, M.; Nishida, K.; Miyamura, S.; Ishima, Y.; et al. Down-regulation of ABCG2, a urate exporter, by parathyroid hormone enhances urate accumulation in secondary hyperparathyroidism. Kidney Int. 2017, 91, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Ponvilawan, B.; Charoenngam, N.; Ungprasert, P. Primary hyperparathyroidism is associated with a higher level of serum uric acid: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2020, 23, 174–180. [Google Scholar] [CrossRef]
- Ishay, A.; Herer, P.; Luboshitzky, R. Effects of Successful Parathyroidectomy on Metabolic Cardiovascular Risk Factors In Patients With Severe Primary Hyperparathyroidism. Endocr. Pract. 2011, 17, 584–590. [Google Scholar] [CrossRef]
- Broulik, P.D.; Brouliková, A.; Adámek, S.; Libanský, P.; Tvrdoň, J.; Broulikova, K.; Kubinyi, J. Improvement of hypertension after parathyroidectomy of patients suffering from primary hyperparathyroidism. Int. J. Endocrinol. 2011, 2011, 309068. [Google Scholar] [CrossRef] [Green Version]
- Al-Naqeeb, J.; Saeed, M.; Dye, B.; Jeranko, M. Association of Gout with Vitamin D: A Population-Based Study. Arthritis Rheumatol. 2019, 71. Available online: https://acrabstracts.org/abstract/association-of-gout-with-vitamin-d-a-population-based-study/ (accessed on 1 September 2021).
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Shane Anderson, A.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Bergink, A.P.; Zillikens, M.C.; Van Leeuwen, J.P.T.M.; Hofman, A.; Uitterlinden, A.G.; van Meurs, J.B.J. 25-Hydroxyvitamin D and osteoarthritis: A meta-analysis including new data. Semin. Arthritis Rheum. 2016, 45, 539–546. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Gantaguru, A.; Nanda, S.N.; Velagada, S.; Srinivasan, A.; Mangaraj, M. Association of vitamin D and knee osteoarthritis in younger individuals. World J. Orthop. 2020, 11, 418–425. [Google Scholar] [CrossRef]
- Rejnmark, L. Effects of vitamin d on muscle function and performance: A review of evidence from randomized controlled trials. Ther. Adv. Chronic Dis. 2011, 2, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [Green Version]
- Sanghi, D.; Mishra, A.; Sharma, A.C.; Singh, A.; Natu, S.M.; Agarwal, S.; Srivastava, R.N. Does vitamin D improve osteoarthritis of the knee: A randomized controlled pilot trial. Clin. Orthop. Relat. Res. 2013, 471, 3556–3562. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Jones, G.; Cicuttini, F.; Wluka, A.; Zhu, Z.; Han, W.; Antony, B.; Wang, X.; Winzenberg, T.; Blizzard, L.; et al. Effect of Vitamin D Supplementation on Tibial Cartilage Volume and Knee Pain Among Patients with Symptomatic Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2016, 315, 1005–1013. [Google Scholar] [CrossRef]
- Arden, N.K.; Cro, S.; Sheard, S.; Doré, C.J.; Bara, A.; Tebbs, S.A.; Hunter, D.J.; James, S.; Cooper, C.; O’Neill, T.W.; et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: A randomised controlled trial. Osteoarthr. Cartil. 2016, 24, 1858–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, A.E.; Arnspiger, S.A. Diffuse Musculoskeletal Pain Is Not Associated with Low Vitamin D Levels or Improved by Treatment with Vitamin D. J. Clin. Rheumatol. 2008, 14, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.B.; Thierry-Palmer, M.; Gibson, K.L.; Rabinovich, C.E. Disease activity, proteinuria, and vitamin D status in children with systemic lupus erythematosus and juvenile dermatomyositis. J. Pediatr. 2012, 160, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azali, P.; Barbasso Helmers, S.; Kockum, I.; Olsson, T.; Alfredsson, L.; Charles, P.J.; Piehl Aulin, K.; Lundberg, I.E. Low serum levels of vitamin D in idiopathic inflammatory myopathies. Ann. Rheum. Dis. 2013, 72, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, L.; Sun, M.-H.; Chen, F.; Li, J.-R. Vitamin D levels in systemic sclerosis patients: A meta-analysis. Drug Des. Devel. Ther. 2017, 11, 3119–3125. [Google Scholar] [CrossRef] [Green Version]
- Alibaz-Oner, F.; Asmaz-Haliloglu, Ö.; Gogas-Yavuz, D.; Can, M.; Haklar, G.; Direskeneli, H. Vitamin D Levels in Takayasu’s Arteritis and a Review of the Literature on Vasculitides. J. Clin. Lab. Anal. 2016, 30, 529–533. [Google Scholar] [CrossRef]
- Kriegel, M.A.; Manson, J.E.; Costenbader, K.H. Does vitamin D affect risk of developing autoimmune disease?: A systematic review. Semin. Arthritis Rheum. 2011, 40, 512–531.e518. [Google Scholar] [CrossRef] [Green Version]
- Hulshof, M.M.; Bavinck, J.N.B.; Bergman, W.; Masclee, A.A.M.; Heickendorff, L.; Breedveld, F.C.; Dijkmans, B.A.C. Double-blind, placebo-controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. J. Am. Acad. Dermatol. 2000, 43, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Perrot, S.; Sommer, C.; Shir, Y.; Fitzcharles, M.-A. Diagnostic confounders of chronic widespread pain: Not always fibromyalgia. Pain Rep. 2017, 2, e598. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.C.; Sanguankeo, A.; Upala, S. Effect of vitamin D supplementation in chronic widespread pain: A systematic review and meta-analysis. Clin. Rheumatol. 2017, 36, 2825–2833. [Google Scholar] [CrossRef] [PubMed]


| Population | The Institute of Medicine’s Recommendations [49] | The Endocrine Society Clinical Practice Guideline on Vitamin D’s Recommendations [8] | ||||
|---|---|---|---|---|---|---|
| Adequate Intake | Estimated Average Allowance Per Day | Recommended Daily Allowance | Daily Requirement | Upper Limit Per Day | Treatment for Vitamin D Deficiency | |
| 0–1 year | 400 IUs | - | - | 400–1000 IUs | 2000 IUs | 2000 IU/d or 50,000 IU/wk of vitamin D2 or D3 for at least 6 weeks to achieve serum 25(OH)D >30 ng/mL (75 nmol/L) maintenance therapy of 400–1000 IU/d |
| 1–18 years | - | 400 IUs | 600 IUs | 600–1000 IUs | 4000 IUs | 2000 IU/d or 50,000 IU/wk of vitamin D2 or D3 for at least 6 weeks to achieve serum 25(OH)D >30 ng/mL (75 nmol/L) maintenance therapy of 600–1000 IU/d |
| 18–70 years | - | 400 IUs | 600 IUs | 600–1000 IUs | 10,000 IUs | 6000 IU/d or 50,000 IU/wk of vitaminD2 or D3 for 8 weeks to achieve serum 25(OH)D >30 ng/mL (75 nmol/L) maintenance therapy of 1500–2000 IU/d |
| >70 years | - | 400 IUs | 800 IUs | 1500–2000 IUs | 10,000 IUs | |
| Adult patients with abnormal kinetics of vitamin D * | - | - | - | 6000–10,000 IUs | 10,000 IUs | Dosage should be increased by 2–3 times |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenngam, N. Vitamin D and Rheumatic Diseases: A Review of Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 10659. https://doi.org/10.3390/ijms221910659
Charoenngam N. Vitamin D and Rheumatic Diseases: A Review of Clinical Evidence. International Journal of Molecular Sciences. 2021; 22(19):10659. https://doi.org/10.3390/ijms221910659
Chicago/Turabian StyleCharoenngam, Nipith. 2021. "Vitamin D and Rheumatic Diseases: A Review of Clinical Evidence" International Journal of Molecular Sciences 22, no. 19: 10659. https://doi.org/10.3390/ijms221910659
APA StyleCharoenngam, N. (2021). Vitamin D and Rheumatic Diseases: A Review of Clinical Evidence. International Journal of Molecular Sciences, 22(19), 10659. https://doi.org/10.3390/ijms221910659






