Changes in Maternal Platelet Physiology during Gestation and Their Interaction with Trophoblasts
Abstract
1. Introduction
2. Platelet-Derived Factors
2.1. Types of Granules
2.2. Platelet Releasate
2.3. Platelet-Derived Extracellular Vesicles
3. Interaction of Maternal Platelets and Trophoblasts
3.1. Implantation and Development of the Placenta
3.2. Remodeling of Uterine Spiral Arteries
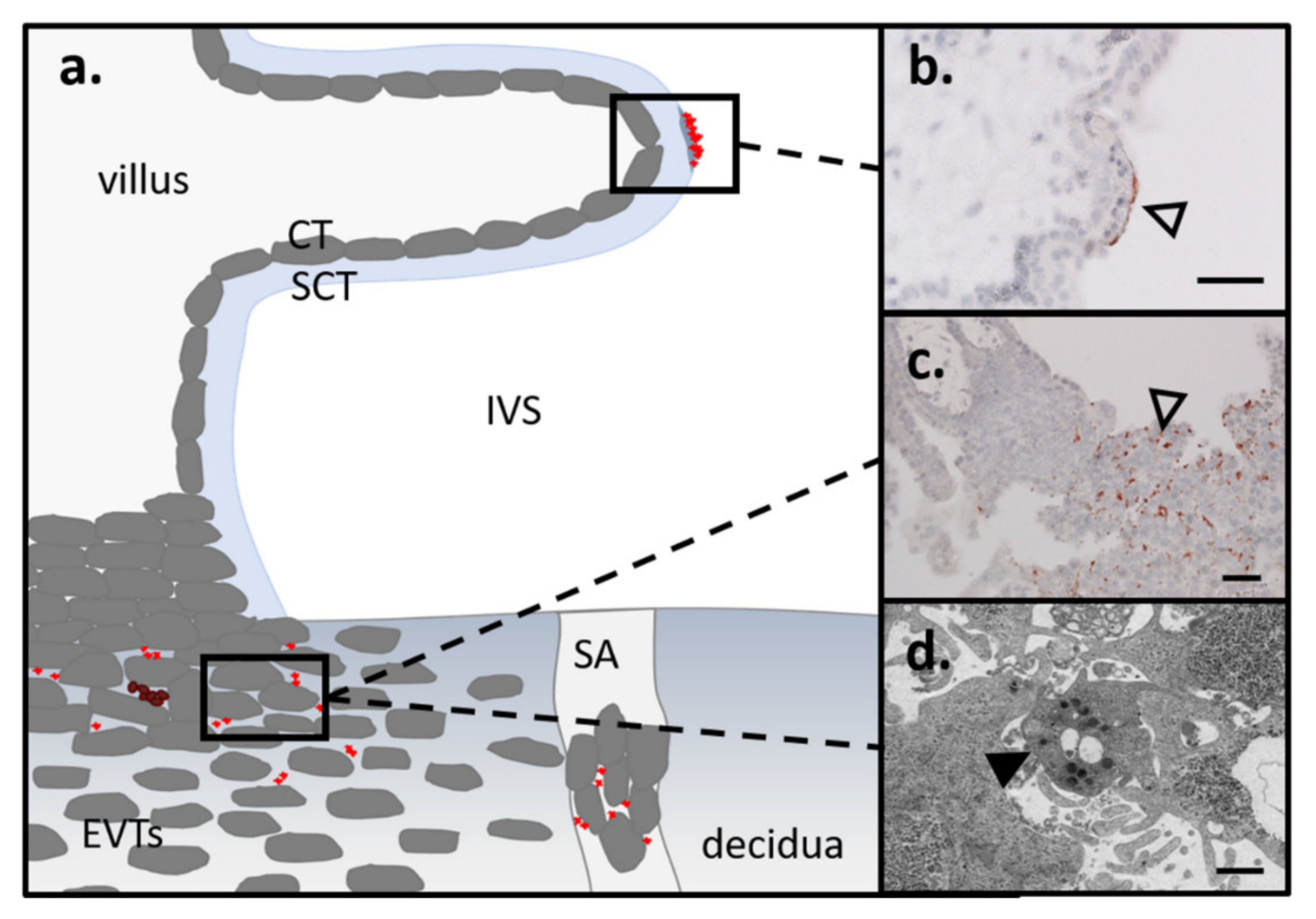
3.3. Route of Platelets into the Intervillous Space/EVTs in First and Term
4. Mechanisms of Platelet Activation
4.1. Agonist-Induced Activation
4.2. Platelet Activation Due to Mechanical Stimuli (Shear Stress)
4.3. Platelets in Pregnancy
5. Pro and Anticoagulatory Mechanisms of the Placenta
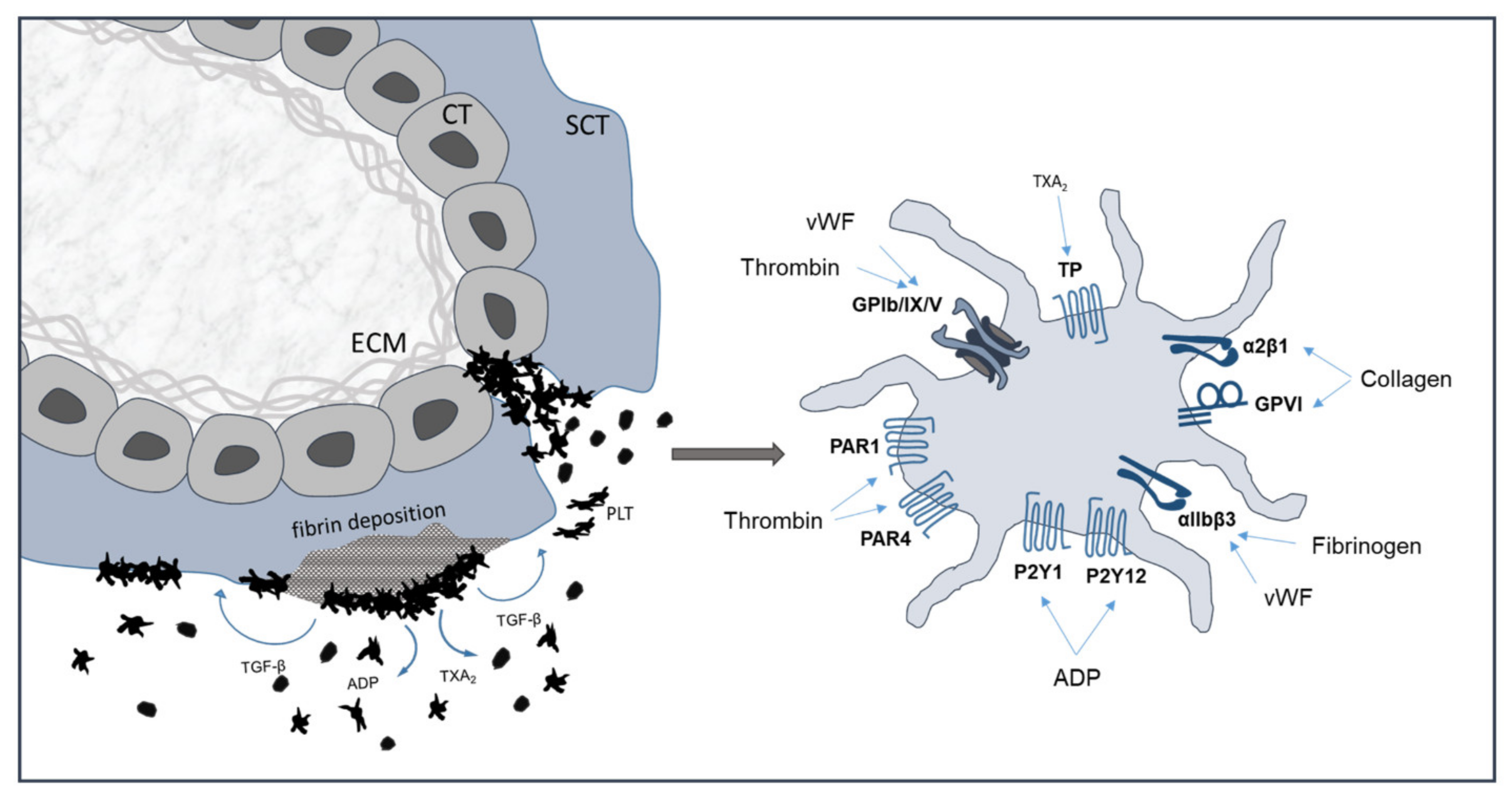
5.1. Coagulatory Mechanism of the Trophoblasts
5.2. Subepithelial Extracellular Matrix Exposed upon Damaged Syncytiotrophoblast
5.3. Coagulatory Factors Released by Trophoblasts into the Maternal Circulation
6. Platelet Activation in Pregnancy Complications
6.1. Preeclampsia
6.2. Treatments
7. Outlook/Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| α-G | α-granules |
| β TG | β-thromboglobulin |
| BDNF | Brain Derived Neurotrophic Factor |
| bFGF | Basic Fibroblast Growth Factor; |
| BMP | Bone Morphogenetic Protein; |
| C | Complement |
| CTGF | Connective Tissue Growth Factor |
| circRNA | Circular RNA |
| CT | Cytotrophoblast |
| DG | Dense granules |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EV | Extracellular vesicle |
| EVT | Extravillous trophoblast |
| eEVT | Endovascular trophoblast |
| ESC | Endometrial stromal cells |
| GA | Gestational age |
| Gly | Glycogen |
| GP | Glycoprotein |
| GPIIb/IIIa | Glycoproteins IIb/IIIa |
| HGF | Hepatocyte growth factor |
| hCG | Human chorionic gonadotropin |
| iEVT | Interstitial extravillous trophoblast |
| IGF-1 | Insulin-like Growth Factor-1 |
| IL-8 | Interleukin 8 |
| IUGR | Intrauterine growth restriction |
| IVS | Intervillous space |
| Lys | Lysosomes |
| lncRNA | Noncoding RNA |
| MCP-1 | Monocyte Chemotactic Protein-1 |
| MCP-3 | Monocyte Chemotactic Protein-3 |
| MIP-1α | Macrophage Inflammatory Protein 1α |
| MMP | Matrix Metalloproteinase |
| MF | Microfilaments |
| miRNA | MicroRNA |
| Mit | Mitochondria |
| MPV | Mean platelet volume |
| mRNA | Messenger RNA |
| NAP-2 | Neutrophil-Activating Protein-2 |
| OCS | Open canicular system |
| PAI-1 | Plasminogen Activator Inhibitor |
| PDGF | Platelet-derived growth factor |
| PE | Preeclampsia |
| PF4 | Platelet factor 4 |
| PLT | Platelet |
| P-MVs | Platelet-derived microvesicles |
| PR | Platelet releasate |
| PSE | Phosphatidylserine externalization |
| PSG | Pregnancy-Specific Glycoprotein |
| RANTES | Regulated on Activation, Normal T Cell Expressed and Secreted |
| SDF-1 | Stromal Cell-Derived Factor-1 |
| TFPI | Tissue Factor Pathway Inhibitor |
| SA | Spiral artery |
| SS | Shear stress |
| SCT | Syncytiotrophoblast |
| STBEV | Syncytiotrophoblast-derived extracellular vesicles |
| TE | Trophectoderm |
| TGF-β | Transforming Growth Factor-β |
| TIMP | Tissue Inhibitor of Metalloproteinases |
| TM | Thrombomodulin |
| TMA | Thrombotic microangiopathy |
| tPA | Tissue-type plasminogen activator |
| TXA2 | Thromboxane A2 |
| uPA | Urokinase-type plasminogen activator |
| VEGF | Vascular endothelial growth factor |
| vWF | Von Willebrand factor |
References
- Michelson, A.D. (Ed.) Platelets, 3rd ed.; Academic Press: Waltham, MA, USA, 2013; ISBN 978-0-12-387837-3. [Google Scholar]
- Reese, J.A.; Peck, J.D.; Yu, Z.; Scordino, T.A.; Deschamps, D.R.; McIntosh, J.J.; Terrell, D.R.; Vesely, S.K.; George, J.N. Platelet sequestration and consumption in the placental intervillous space contribute to lower platelet counts during pregnancy. Am. J. Hematol. 2019, 94, E8–E11. [Google Scholar] [CrossRef] [PubMed]
- Boehlen, F.; Hohlfeld, P.; Extermann, P.; Perneger, T.V.; de Moerloose, P. Platelet count at term pregnancy: A reappraisal of the threshold. Obstet. Gynecol. 2000, 95, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Sainio, S.; Kekomäki, R.; Riikonen, S.; Teramo, K. Maternal thrombocytopenia at term: A population-based study. Acta Obstet. Gynecol. Scand. 2000, 79, 744–749. [Google Scholar] [CrossRef]
- Shehata, N.; Burrows, R.; Kelton, J.G. Gestational thrombocytopenia. Clin. Obstet. Gynecol. 1999, 42, 327–334. [Google Scholar] [CrossRef]
- Cines, D.B.; Levine, L.D. Thrombocytopenia in pregnancy. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 144–151. [Google Scholar] [CrossRef]
- Sato, Y.; Fujiwara, H.; Zeng, B.-X.; Higuchi, T.; Yoshioka, S.; Fujii, S. Platelet-derived soluble factors induce human extravillous trophoblast migration and differentiation: Platelets are a possible regulator of trophoblast infiltration into maternal spiral arteries. Blood 2005, 106, 428–435. [Google Scholar] [CrossRef]
- Bass, K.E.; Morrish, D.; Roth, I.; Bhardwaj, D.; Taylor, R.; Zhou, Y.; Fisher, S.J. Human cytotrophoblast invasion is up-regulated by epidermal growth factor: Evidence that paracrine factors modify this process. Dev. Biol. 1994, 164, 550–561. [Google Scholar] [CrossRef]
- Lash, G.E.; Warren, A.Y.; Underwood, S.; Baker, P.N. Vascular endothelial growth factor is a chemoattractant for trophoblast cells. Placenta 2003, 24, 549–556. [Google Scholar] [CrossRef]
- Forstner, D.; Maninger, S.; Nonn, O.; Guettler, J.; Moser, G.; Leitinger, G.; Pritz, E.; Strunk, D.; Schallmoser, K.; Marsche, G.; et al. Platelet-derived factors impair placental chorionic gonadotropin beta-subunit synthesis. J. Mol. Med. Berl. Ger. 2020, 98, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Huppertz, B.; Frank, H.-G. The fibrinoids of the human placenta: Origin, composition and functional relevance. Ann. Anat. Anat. Anz. 1996, 178, 485–501. [Google Scholar] [CrossRef]
- Nelson, D.M.; Crouch, E.C.; Curran, E.M.; Farmer, D.R. Trophoblast interaction with fibrin matrix. Epithelialization of perivillous fibrin deposits as a mechanism for villous repair in the human placenta. Am. J. Pathol. 1990, 136, 855–865. [Google Scholar]
- Guettler, J.; Forstner, D.; Cvirn, G.; Maninger, S.; Brugger, B.A.; Nonn, O.; Kupper, N.; Pritz, E.; Wernitznig, S.; Dohr, G.; et al. Maternal platelets pass interstices of trophoblast columns and are not activated by HLA-G in early human pregnancy. J. Reprod. Immunol. 2021, 144, 103280. [Google Scholar] [CrossRef]
- Moser, G.; Guettler, J.; Forstner, D.; Gauster, M. Maternal Platelets—Friend or Foe of the Human Placenta? Int. J. Mol. Sci. 2019, 20, 5639. [Google Scholar] [CrossRef]
- Holinstat, M. Normal platelet function. Cancer Metastasis Rev. 2017, 36, 195–198. [Google Scholar] [CrossRef]
- Coppinger, J.A.; Maguire, P.B. Insights into the platelet releasate. Curr. Pharm. Des. 2007, 13, 2640–2646. [Google Scholar] [CrossRef]
- De Meyer, S.F. Platelet granules in vascular integrity. Blood 2017, 129, 1573–1574. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burnouf, T.; Strunk, D.; Koh, M.B.C.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef]
- Neumüller, J.; Ellinger, A.; Wagner, T. Transmission Electron Microscopy of Platelets FROM Apheresis and Buffy-Coat-Derived Platelet Concentrates; IntechOpen: London, UK, 2015; ISBN 978-953-51-2150-3. [Google Scholar]
- McNicol, A.; Israels, S.J. Platelet dense granules: Structure, function and implications for haemostasis. Thromb. Res. 1999, 95, 1–18. [Google Scholar] [CrossRef]
- Parsons, M.E.M.; Szklanna, P.B.; Guerrero, J.A.; Wynne, K.; Dervin, F.; O’Connell, K.; Allen, S.; Egan, K.; Bennett, C.; McGuigan, C.; et al. Platelet Releasate Proteome Profiling Reveals a Core Set of Proteins with Low Variance between Healthy Adults. Proteomics 2018, 18, e1800219. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef]
- Guerrero, J.A.; Bennett, C.; van der Weyden, L.; McKinney, H.; Chin, M.; Nurden, P.; McIntyre, Z.; Cambridge, E.L.; Estabel, J.; Wardle-Jones, H.; et al. Gray platelet syndrome: Proinflammatory megakaryocytes and α-granule loss cause myelofibrosis and confer metastasis resistance in mice. Blood 2014, 124, 3624–3635. [Google Scholar] [CrossRef]
- Deppermann, C.; Cherpokova, D.; Nurden, P.; Schulz, J.-N.; Thielmann, I.; Kraft, P.; Vögtle, T.; Kleinschnitz, C.; Dütting, S.; Krohne, G.; et al. Gray platelet syndrome and defective thrombo-inflammation in Nbeal2-deficient mice. J. Clin. Investig. 2013, 123, 69210. [Google Scholar] [CrossRef]
- Tao, S.-C.; Guo, S.-C.; Zhang, C.-Q. Platelet-derived Extracellular Vesicles: An Emerging Therapeutic Approach. Int. J. Biol. Sci. 2017, 13, 828–834. [Google Scholar] [CrossRef]
- Taus, F.; Meneguzzi, A.; Castelli, M.; Minuz, P. Platelet-Derived Extracellular Vesicles as Target of Antiplatelet Agents. What Is the Evidence? Front. Pharmacol. 2019, 10, 1256. [Google Scholar] [CrossRef]
- Szatanek, R.; Baran, J.; Siedlar, M.; Baj-Krzyworzeka, M. Isolation of extracellular vesicles: Determining the correct approach (Review). Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Aatonen, M.; Grönholm, M.; Siljander, P.R.-M. Platelet-derived microvesicles: Multitalented participants in intercellular communication. Semin. Thromb. Hemost. 2012, 38, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef] [PubMed]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2020, 11, 604274. [Google Scholar] [CrossRef]
- Heijnen, H.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. What is the placenta? Am. J. Obstet. Gynecol. 2015, 213, S6.e1–S6.e4. [Google Scholar] [CrossRef]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Dev. Camb. Engl. 2019, 146, dev163428. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B.; Gauster, M.; Orendi, K.; König, J.; Moser, G. Oxygen as modulator of trophoblast invasion. J. Anat. 2009, 215, 14–20. [Google Scholar] [CrossRef]
- Moser, G.; Gauster, M.; Orendi, K.; Glasner, A.; Theuerkauf, R.; Huppertz, B. Endoglandular trophoblast, an alternative route of trophoblast invasion? Analysis with novel confrontation co-culture models. Hum. Reprod. Oxf. Engl. 2010, 25, 1127–1136. [Google Scholar] [CrossRef]
- Roberts, V.H.J.; Morgan, T.K.; Bednarek, P.; Morita, M.; Burton, G.J.; Lo, J.O.; Frias, A.E. Early first trimester uteroplacental flow and the progressive disintegration of spiral artery plugs: New insights from contrast-enhanced ultrasound and tissue histopathology. Hum. Reprod. Oxf. Engl. 2017, 32, 2382–2393. [Google Scholar] [CrossRef]
- Weiss, G.; Sundl, M.; Glasner, A.; Huppertz, B.; Moser, G. The trophoblast plug during early pregnancy: A deeper insight. Histochem. Cell Biol. 2016, 146, 749–756. [Google Scholar] [CrossRef] [PubMed]
- James, J.L.; Stone, P.R.; Chamley, L.W. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum. Reprod. Update 2006, 12, 137–144. [Google Scholar] [CrossRef]
- Brugger, B.A.; Guettler, J.; Gauster, M. Go with the Flow-Trophoblasts in Flow Culture. Int. J. Mol. Sci. 2020, 21, 4666. [Google Scholar] [CrossRef]
- Allerkamp, H.H.; Clark, A.R.; Lee, T.C.; Morgan, T.K.; Burton, G.J.; James, J.L. Something old, something new: Digital quantification of uterine vascular remodelling and trophoblast plugging in historical collections provides new insight into adaptation of the utero-placental circulation. Hum. Reprod. Oxf. Engl. 2021, 36, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Blaschitz, A.; Siwetz, M.; Schlenke, P.; Gauster, M. Adhering maternal platelets can contribute to the cytokine and chemokine cocktail released by human first trimester villous placenta. Placenta 2015, 36, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Brass, L.F. Thrombin and platelet activation. Chest 2003, 124, S18–S25. [Google Scholar] [CrossRef]
- Davì, G.; Patrono, C. Platelet activation and atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494. [Google Scholar] [CrossRef]
- Varga-Szabo, D.; Pleines, I.; Nieswandt, B. Cell adhesion mechanisms in platelets. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Offermanns, S. Activation of platelet function through G protein-coupled receptors. Circ. Res. 2006, 99, 1293–1304. [Google Scholar] [CrossRef]
- Jennings, L.K. Mechanisms of platelet activation: Need for new strategies to protect against platelet-mediated atherothrombosis. Thromb. Haemost. 2009, 102, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Pleines, I.; Bender, M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J. Thromb. Haemost. 2011, 9 (Suppl. 1), 92–104. [Google Scholar] [CrossRef]
- Mann, K.G. Thrombin formation. Chest 2003, 124, S4–S10. [Google Scholar] [CrossRef]
- Brummel, K.E.; Paradis, S.G.; Butenas, S.; Mann, K.G. Thrombin functions during tissue factor-induced blood coagulation. Blood 2002, 100, 148–152. [Google Scholar] [CrossRef]
- Vu, T.K.; Hung, D.T.; Wheaton, V.I.; Coughlin, S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64, 1057–1068. [Google Scholar] [CrossRef]
- Coughlin, S.R. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 2005, 3, 1800–1814. [Google Scholar] [CrossRef]
- Leger, A.J.; Covic, L.; Kuliopulos, A. Protease-activated receptors in cardiovascular diseases. Circulation 2006, 114, 1070–1077. [Google Scholar] [CrossRef]
- Landis, R.C. Protease activated receptors: Clinical relevance to hemostasis and inflammation. Hematol. Oncol. Clin. N. Am. 2007, 21, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Martorell, L.; Martínez-González, J.; Rodríguez, C.; Gentile, M.; Calvayrac, O.; Badimon, L. Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb. Haemost. 2008, 99, 305–315. [Google Scholar] [CrossRef]
- De Candia, E.; Hall, S.W.; Rutella, S.; Landolfi, R.; Andrews, R.K.; De Cristofaro, R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J. Biol. Chem. 2001, 276, 4692–4698. [Google Scholar] [CrossRef] [PubMed]
- Roka-Moiia, Y.; Walk, R.; Palomares, D.E.; Ammann, K.R.; Dimasi, A.; Italiano, J.E.; Sheriff, J.; Bluestein, D.; Slepian, M.J. Platelet Activation via Shear Stress Exposure Induces a Differing Pattern of Biomarkers of Activation versus Biochemical Agonists. Thromb. Haemost. 2020, 120, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, H.; Alexandridis, P.; Neelamegham, S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood 2003, 101, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Moake, J.L.; Turner, N.A.; Stathopoulos, N.A.; Nolasco, L.H.; Hellums, J.D. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J. Clin. Invest. 1986, 78, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Salomon, D.R.; Ikeda, Y.; Ruggeri, Z.M. Characterization of the unique mechanism mediating the shear-dependent binding of soluble von Willebrand factor to platelets. J. Biol. Chem. 1995, 270, 23352–23361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kelkar, A.; Neelamegham, S. von Willebrand factor self-association is regulated by the shear-dependent unfolding of the A2 domain. Blood Adv. 2019, 3, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Pushin, D.M.; Salikhova, T.Y.; Zlobina, K.E.; Guria, G.T. Platelet activation via dynamic conformational changes of von Willebrand factor under shear. PLoS ONE 2020, 15, e0234501. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.G.; Alpoim, P.N.; Komatsuzaki, F.; das Carvalho, M.G.; Dusse, L.M.S. Preeclampsia: Are platelet count and indices useful for its prognostic? Hematol. Amst. Neth. 2013, 18, 360–364. [Google Scholar] [CrossRef]
- Janes, S.L.; Goodall, A.H. Flow cytometric detection of circulating activated platelets and platelet hyper-responsiveness in pre-eclampsia and pregnancy. Clin. Sci. Lond. Engl. 1994, 86, 731–739. [Google Scholar] [CrossRef]
- Sheu, J.-R.; Hsiao, G.; Lin, W.-Y.; Chen, T.-F.; Chien, Y.-Y.; Lin, C.-H.; Tzeng, C.-R. Mechanisms involved in agonist-induced hyperaggregability of platelets from normal pregnancy. J. Biomed. Sci. 2002, 9, 17–25. [Google Scholar] [CrossRef]
- Lok, C.A.R.; Nieuwland, R.; Sturk, A.; Hau, C.M.; Boer, K.; Vanbavel, E.; Vanderpost, J.A.M. Microparticle-associated P-selectin reflects platelet activation in preeclampsia. Platelets 2007, 18, 68–72. [Google Scholar] [CrossRef]
- Ayhan, A.; Akkök, E.; Urman, B.; Yarali, H.; Dündar, S.; Kirazli, S. Beta-thromboglobulin and platelet factor 4 levels in pregnancy and preeclampsia. Gynecol. Obstet. Invest. 1990, 30, 12–14. [Google Scholar] [CrossRef]
- Hayashi, M.; Kiumi, F.; Mitsuya, K. Changes in platelet ATP secretion and aggregation during pregnancy and in preeclampsia. Am. J. Med. Sci. 1999, 318, 115–121. [Google Scholar] [CrossRef]
- Yoneyama, Y.; Suzuki, S.; Sawa, R.; Otsubo, Y.; Power, G.G.; Araki, T. Plasma adenosine levels increase in women with normal pregnancies. Am. J. Obstet. Gynecol. 2000, 182, 1200–1203. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Mayo, G.; Catella, F.; Entman, S.S.; FitzGerald, G.A. Increased thromboxane biosynthesis in normal pregnancy is mainly derived from platelets. Am. J. Obstet. Gynecol. 1987, 157, 325–330. [Google Scholar] [CrossRef]
- Shanley, D.K.; Kiely, P.A.; Golla, K.; Allen, S.; Martin, K.; O’Riordan, R.T.; Ball, M.; Aplin, J.D.; Singer, B.B.; Caplice, N.; et al. Pregnancy-specific glycoproteins bind integrin αIIbβ3 and inhibit the platelet-fibrinogen interaction. PLoS ONE 2013, 8, e57491. [Google Scholar] [CrossRef] [PubMed]
- Szklanna, P.B.; Parsons, M.E.; Wynne, K.; O’Connor, H.; Egan, K.; Allen, S.; Ní Áinle, F.; Maguire, P.B. The Platelet Releasate is Altered in Human Pregnancy. Proteomics Clin. Appl. 2019, 13, e1800162. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, M. Hemostasis during normal pregnancy and puerperium. Semin. Thromb. Hemost. 2003, 29, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Vattai, A.; Zhang, X.; Zhu, J.; Thaler, C.J.; Mahner, S.; Jeschke, U.; von Schönfeldt, V. Role of Plasminogen Activator Inhibitor Type 1 in Pathologies of Female Reproductive Diseases. Int. J. Mol. Sci. 2017, 18, 1651. [Google Scholar] [CrossRef] [PubMed]
- Lanir, N.; Aharon, A.; Brenner, B. Procoagulant and anticoagulant mechanisms in human placenta. Semin. Thromb. Hemost. 2003, 29, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.; Singh, K.K.; Gupta, A.; Markmeyer, P.; Lochmann, F.; Gupta, D.; Rana, R.; Elwakiel, A.; Huebner, H.; Ruebner, M.; et al. Placental thromboinflammation impairs embryonic survival by reducing placental thrombomodulin expression. Blood 2021, 137, 977–982. [Google Scholar] [CrossRef]
- Mackman, N. The role of tissue factor and factor VIIa in hemostasis. Anesth. Analg. 2009, 108, 1447–1452. [Google Scholar] [CrossRef]
- Sood, R.; Kalloway, S.; Mast, A.E.; Hillard, C.J.; Weiler, H. Fetomaternal cross talk in the placental vascular bed: Control of coagulation by trophoblast cells. Blood 2006, 107, 3173–3180. [Google Scholar] [CrossRef]
- Loghmani, H.; Conway, E.M. Exploring traditional and nontraditional roles for thrombomodulin. Blood 2018, 132, 148–158. [Google Scholar] [CrossRef]
- Urano, T.; Suzuki, Y.; Iwaki, T.; Sano, H.; Honkura, N.; Castellino, F.J. Recognition of Plasminogen Activator Inhibitor Type 1 as the Primary Regulator of Fibrinolysis. Curr. Drug Targets 2019, 20, 1695–1701. [Google Scholar] [CrossRef]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C.P. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef]
- Fitzpatrick, T.E.; Graham, C.H. Stimulation of plasminogen activator inhibitor-1 expression in immortalized human trophoblast cells cultured under low levels of oxygen. Exp. Cell Res. 1998, 245, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Pathology of the Human Placenta, 4th ed.; Springer: New York, NY, USA, 2000; ISBN 978-1-4757-4199-5. [Google Scholar]
- Oefner, C.M.; Sharkey, A.; Gardner, L.; Critchley, H.; Oyen, M.; Moffett, A. Collagen type IV at the fetal-maternal interface. Placenta 2015, 36, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Watson, S.P. Platelet-collagen interaction: Is GPVI the central receptor? Blood 2003, 102, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W. Collagen-induced platelet activation. Blood Cells. Mol. Dis. 2006, 36, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Nilsson, L.; Baranov, V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: Immune modulation for pregnancy success. Am. J. Reprod. Immunol. N. Y. 2014, 72, 440–457. [Google Scholar] [CrossRef]
- Kshirsagar, S.K.; Alam, S.M.; Jasti, S.; Hodes, H.; Nauser, T.; Gilliam, M.; Billstrand, C.; Hunt, J.S.; Petroff, M.G. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 2012, 33, 982–990. [Google Scholar] [CrossRef]
- Goswami, D.; Tannetta, D.S.; Magee, L.A.; Fuchisawa, A.; Redman, C.W.G.; Sargent, I.L.; von Dadelszen, P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta 2006, 27, 56–61. [Google Scholar] [CrossRef]
- Knight, M.; Redman, C.W.; Linton, E.A.; Sargent, I.L. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br. J. Obstet. Gynaecol. 1998, 105, 632–640. [Google Scholar] [CrossRef]
- Tong, M.; Chamley, L.W. Placental extracellular vesicles and feto-maternal communication. Cold Spring Harb. Perspect. Med. 2015, 5, a023028. [Google Scholar] [CrossRef]
- Kupper, N.; Huppertz, B. The endogenous exposome of the pregnant mother: Placental extracellular vesicles and their effect on the maternal system. Mol. Aspects Med. 2021. [Google Scholar] [CrossRef]
- Tong, M.; Kleffmann, T.; Pradhan, S.; Johansson, C.L.; DeSousa, J.; Stone, P.R.; James, J.L.; Chen, Q.; Chamley, L.W. Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: Relevance for feto-maternal communication. Hum. Reprod. Oxf. Engl. 2016, 31, 687–699. [Google Scholar] [CrossRef]
- Germain, S.J.; Sacks, G.P.; Sooranna, S.R.; Soorana, S.R.; Sargent, I.L.; Redman, C.W. Systemic inflammatory priming in normal pregnancy and preeclampsia: The role of circulating syncytiotrophoblast microparticles. J. Immunol. 2007, 178, 5949–5956. [Google Scholar] [CrossRef]
- Giacomini, E.; Alleva, E.; Fornelli, G.; Quartucci, A.; Privitera, L.; Vanni, V.S.; Viganò, P. Embryonic extracellular vesicles as informers to the immune cells at the maternal-fetal interface. Clin. Exp. Immunol. 2019, 198, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G.; Tannetta, D.S.; Dragovic, R.A.; Gardiner, C.; Southcombe, J.H.; Collett, G.P.; Sargent, I.L. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012, 33, S48–S54. [Google Scholar] [CrossRef] [PubMed]
- Pillay, P.; Moodley, K.; Moodley, J.; Mackraj, I. Placenta-derived exosomes: Potential biomarkers of preeclampsia. Int. J. Nanomed. 2017, 12, 8009–8023. [Google Scholar] [CrossRef] [PubMed]
- Tannetta, D.S.; Hunt, K.; Jones, C.I.; Davidson, N.; Coxon, C.H.; Ferguson, D.; Redman, C.W.; Gibbins, J.M.; Sargent, I.L.; Tucker, K.L. Syncytiotrophoblast Extracellular Vesicles from Pre-Eclampsia Placentas Differentially Affect Platelet Function. PLoS ONE 2015, 10, e0142538. [Google Scholar] [CrossRef] [PubMed]
- Thalor, N.; Singh, K.; Pujani, M.; Chauhan, V.; Agarwal, C.; Ahuja, R. A correlation between platelet indices and preeclampsia. Hematol. Transfus. Cell Ther. 2019, 41, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. The Critical Role of Abnormal Trophoblast Development in the Etiology of Preeclampsia. Curr. Pharm. Biotechnol. 2018, 19, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B.; Schleußner, E. (Eds.) Die Plazenta: Grundlagen und Klinische Bedeutung; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-662-55621-4. [Google Scholar]
- Than, N.G.; Romero, R.; Tarca, A.L.; Kekesi, K.A.; Xu, Y.; Xu, Z.; Juhasz, K.; Bhatti, G.; Leavitt, R.J.; Gelencser, Z.; et al. Integrated Systems Biology Approach Identifies Novel Maternal and Placental Pathways of Preeclampsia. Front. Immunol. 2018, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Gauster, M.; Moser, G.; Orendi, K.; Huppertz, B. Factors involved in regulating trophoblast fusion: Potential role in the development of preeclampsia. Placenta 2009, 30, S49–S54. [Google Scholar] [CrossRef]
- Tannetta, D.; Masliukaite, I.; Vatish, M.; Redman, C.; Sargent, I. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J. Reprod. Immunol. 2017, 119, 98–106. [Google Scholar] [CrossRef]
- Ness, R.B.; Sibai, B.M. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am. J. Obstet. Gynecol. 2006, 195, 40–49. [Google Scholar] [CrossRef]
- Tallah, A.; Lecarpentier, E.; Goffinet, F.; Doret-Dion, M.; Gaucherand, P.; Tsatsaris, V. Aspirin for Prevention of Preeclampsia. Drugs 2017, 77, 1819–1831. [Google Scholar] [CrossRef]
- Preedy, V.R. Handbook of Growth and Growth Monitoring in Health and Disease; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; ISBN 978-1-4419-1795-9. [Google Scholar]
- Lokki, A.I.; Heikkinen-Eloranta, J. Pregnancy induced TMA in severe preeclampsia results from complement-mediated thromboinflammation. Hum. Immunol. 2021, 82, 371–378. [Google Scholar] [CrossRef]
- Baumwell, S.; Karumanchi, S.A. Pre-eclampsia: Clinical manifestations and molecular mechanisms. Nephron Clin. Pract. 2007, 106, c72–c81. [Google Scholar] [CrossRef]
- Stern, C.; Mayer-Pickel, K.; Weiss, E.-C.; Kutllovci-Hasani, K.; Nanda, M.; Eberhard, K.; Cervar-Zivkovic, M.; Prüller, F. Low Dose Aspirin in high-risk pregnancies: The volatile effect of acetylsalicylic acid on the inhibition of platelets uncovered by G. Born’s light transmission aggregometry. J. Reprod. Immunol. 2021, 145, 103320. [Google Scholar] [CrossRef]
- Roberge, S.; Nicolaides, K.; Demers, S.; Hyett, J.; Chaillet, N.; Bujold, E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 110–120.e6. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Bujold, E.; Nicolaides, K.H. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2018, 218, 287–293.e1. [Google Scholar] [CrossRef] [PubMed]
- Rodger, M.A.; Gris, J.-C.; de Vries, J.I.P.; Martinelli, I.; Rey, É.; Schleussner, E.; Middeldorp, S.; Kaaja, R.; Langlois, N.J.; Ramsay, T.; et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: A meta-analysis of individual patient data from randomised controlled trials. Lancet Lond. Engl. 2016, 388, 2629–2641. [Google Scholar] [CrossRef]
- Tello-Montoliu, A.; Seecheran, N.A.; Angiolillo, D.J. Successful pregnancy and delivery on prasugrel treatment: Considerations for the use of dual antiplatelet therapy during pregnancy in clinical practice. J. Thromb. Thrombolysis 2013, 36, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Davizon-Castillo, P.; Rowley, J.W.; Rondina, M.T. Megakaryocyte and Platelet Transcriptomics for Discoveries in Human Health and Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.H.; Di Paola, J. Genomics and transcriptomics of megakaryocytes and platelets: Implications for health and disease. Res. Pract. Thromb. Haemost. 2018, 2, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.A.; Rowley, J.W.; Campbell, R.A.; Grissom, C.K.; Brown, S.M.; Beesley, S.J.; Schwertz, H.; Kosaka, Y.; Manne, B.K.; Krauel, K.; et al. Sepsis alters the transcriptional and translational landscape of human and murine platelets. Blood 2019, 134, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Guichard, J.; Massé, J.M.; Debili, N.; Cramer, E.M. Of mice and men: Comparison of the ultrastructure of megakaryocytes and platelets. Exp. Hematol. 2001, 29, 1295–1302. [Google Scholar] [CrossRef]
- Balkenhol, J.; Kaltdorf, K.V.; Mammadova-Bach, E.; Braun, A.; Nieswandt, B.; Dittrich, M.; Dandekar, T. Comparison of the central human and mouse platelet signaling cascade by systems biological analysis. BMC Genom. 2020, 21, 897. [Google Scholar] [CrossRef]
- Ware, J. Dysfunctional platelet membrane receptors: From humans to mice. Thromb. Haemost. 2004, 92, 478–485. [Google Scholar] [CrossRef]

| α-Granules | Lysosomes | Dense Granules | |
|---|---|---|---|
| Adhesion Molecules | Coagulation Factors | α-Arabinoside | ATP |
| P-Selectin | Factor V | β-Galactosidase | ADP |
| von Willebrand factor | Factor XI | β-Glucuronidase | Serotonin |
| Vitronectin | Factor XIII | n-Acetylglucosaminidase | Ca++ |
| Fibrinogen | Prothrombin | Elastase | Epinephrine |
| Integrin αIIbβ3 | Antithrombin | Collagenase | Histamine |
| Integrin αVβ3 | α2-Macroglobulin | Cathepsin | |
| Fibronectin | α2-Antiplasmin | ||
| Plasmin, Plasminogen | |||
| Protein S | |||
| PAI-1, TFPI | |||
| Regulators of Growth and Angiogenesis | Immunological Molecules | ||
| bFGF | Complement factors | ||
| HGF | Platelet factor H | ||
| IGF-1 | β1H Globulin | ||
| VEGF-A, -C | Factor D | ||
| PDGF-AA, -AB, -BB | C1 Inhibitor | ||
| BDNF | IgG | ||
| Angiostatin | Thymosin-β4 | ||
| PF4 | |||
| Thrombospondin | |||
| EGF | |||
| CTGF | |||
| TGF-β | Chemokines | ||
| Angiopoietin-1 | IL-8 | ||
| SDF-1 | NAP-2 | ||
| MMP-1, -2, -9 | RANTES | ||
| Endostatin | MCP-1,-3 | ||
| TIMP-1, -4 | MIP-1α | ||
| BMP-2, -4, -6 | β-Thromboglobulin | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forstner, D.; Guettler, J.; Gauster, M. Changes in Maternal Platelet Physiology during Gestation and Their Interaction with Trophoblasts. Int. J. Mol. Sci. 2021, 22, 10732. https://doi.org/10.3390/ijms221910732
Forstner D, Guettler J, Gauster M. Changes in Maternal Platelet Physiology during Gestation and Their Interaction with Trophoblasts. International Journal of Molecular Sciences. 2021; 22(19):10732. https://doi.org/10.3390/ijms221910732
Chicago/Turabian StyleForstner, Désirée, Jacqueline Guettler, and Martin Gauster. 2021. "Changes in Maternal Platelet Physiology during Gestation and Their Interaction with Trophoblasts" International Journal of Molecular Sciences 22, no. 19: 10732. https://doi.org/10.3390/ijms221910732
APA StyleForstner, D., Guettler, J., & Gauster, M. (2021). Changes in Maternal Platelet Physiology during Gestation and Their Interaction with Trophoblasts. International Journal of Molecular Sciences, 22(19), 10732. https://doi.org/10.3390/ijms221910732







