Global Proteotoxicity Caused by Human β2 Microglobulin Variants Impairs the Unfolded Protein Response in C. elegans
Abstract
:1. Introduction
2. Results
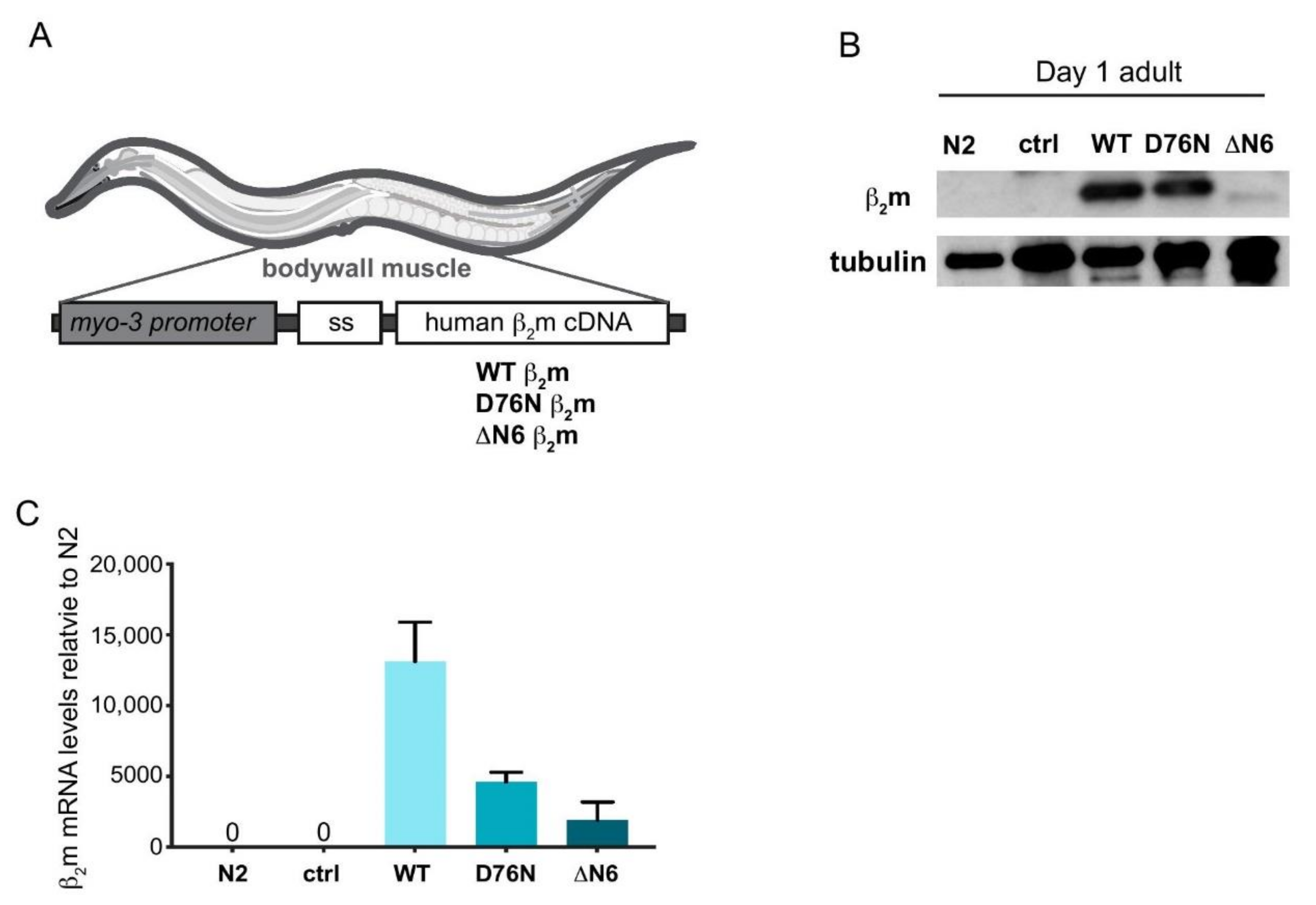
2.1. Generation of C. elegans Models Expressing Human β2m Variants
2.2. Expression of D76N β2m and ΔN6 β2m Variants Delays Development and Reduces Lifespan
2.3. Expression of β2m Variants in the C. elegans Body Wall Muscle Reduces Motility
2.4. Expression of the D76N β2m and ΔN6 β2m Variants Leads to Wide-Spread Endogenous Protein Aggregation
2.5. Expression of Amyloidogenic β2m Variants Impair Cellular Stress Responses
2.6. Expression of β2m Variants Impacts Protein Secretion
3. Discussion
4. Material and Methods
4.1. C. elegans Strains and Maintenance
4.1.1. Strains Generated in This Study:
- PVH69 pccEx007[myo3::WT hβ2m::GFP::unc-54 3′UTR + myo2::RFP];
- PVH177 pccEx009[myo-3p::WT hβ2m::unc-54 3′UTR + myo-2p::RFP];
- PVH178 pccEx010[myo-3p::D76N β2m::unc-54 3′UTR + myo-2p::RFP];
- PVH179 pccEx011[myo-3p::ΔN6 β2m::unc-54 3′UTR + myo-2p::RFP];
- PVH182 pccEx024[myo-2p::RFP].
4.1.2. Cloning and Generation of Transgenic C. elegans Strains
4.2. Primers Used
- BamHI-B2m-for
- 5′ AAAGGATCCATTCAAAGAACTCCAAAAATTC 3′
- MscI-B2m-rev
- 5′ AAATGGCCATTACATGTCTCGATCCCACTTA 3′
- SalI-signalpeptide B2m-for
- 5′ TCGACAAAAATGTCTCGCTCCGTGGCCTTAGCTGTGGCCTTAGCTGTGCTC GCGCTACTCTCTCTTTCTGGCCTGGAGGCTG 3′
- BamHI-signalpeptide B2m-rev
- 5′ GATCCAGCCTCCAGGCCAGAAAGAGAGAGTAGCGCGAGCACAGCTAAG GCCACGGAGCGAGACATTTTTG 3′
4.3. Western Blot Analysis and SDS-PAGE Silver Staining
4.4. Soluble/Insoluble Protein Fractionation
4.5. Developmental Delay Assay
4.6. Fecundity Assay
4.7. Lifespan Assay
4.8. Paralysis Assay
4.9. Thrashing Assay
4.10. Thermotolerance
4.11. ER Stress Assay
4.12. Quantitative RT-PCR
4.13. Quantification of Extracellular Aggregation Using LBP-2::tagRFP as a Reporter
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Hipp, M.S.; Park, S.-H.; Hartl, F.U. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 2014, 24, 506–514. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Iadanza, M.G.; Jackson, M.P.; Hewitt, E.W.; Ranson, N.A.; Radford, S.E. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 755–773. [Google Scholar] [CrossRef]
- Smith, D.P.; Radford, S.E. Role of the single disulphide bond of beta(2)-microglobulin in amyloidosis in vitro. Protein Sci. 2001, 10, 1775–1784. [Google Scholar] [CrossRef]
- Isenman, D.E.; Painter, R.H.; Dorrington, K.J. The structure and function of immunoglobulin domains: Studies with beta-2-microglobulin on the role of the intrachain disulfide bond. Proc. Natl. Acad. Sci. USA 1975, 72, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, K. Pathogenesis of beta2-microglobulin amyloidosis. Pathol Int. 2001, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gejyo, F.; Yamada, T.; Odani, S.; Nakagawa, Y.; Arakawa, M.; Kunitomo, T.; Kataoka, H.; Suzuki, M.; Hirasawa, Y.; Shirahama, T.; et al. A new form of amyloid protein associated with chronic hemodialysis was identified as β2-microglobulin. Biochem. Biophys. Res. Commun. 1985, 129, 701–706. [Google Scholar] [CrossRef]
- Esposito, G.; Michelutti, R.; Verdone, G.; Viglino, P.; Ández, H.H.; Robinson, C.; Amoresano, A.; Piaz, F.D.; Monti, M.; Pucci, P.; et al. Removal of the N-terminal hexapeptide from human β2-microglobulin facilitates protein aggregation and fibril formation. Protein Sci. 2000, 9, 831–845. [Google Scholar] [CrossRef]
- Eichner, T.; Kalverda, A.P.; Thompson, G.S.; Homans, S.W.; Radford, S.E. Conformational conversion during amyloid formation at atomic resolution. Mol. Cell 2011, 41, 161–172. [Google Scholar] [CrossRef]
- Karamanos, T.; Pashley, C.L.; Kalverda, A.P.; Thompson, G.S.; Mayzel, M.; Orekhov, V.Y.; Radford, S.E. A population shift between sparsely populated folding intermediates determines amyloidogenicity. J. Am. Chem. Soc. 2016, 138, 6271–6280. [Google Scholar] [CrossRef] [Green Version]
- Myers, S.L.; Jones, S.; Jahn, T.; Morten, I.J.; Tennent, G.A.; Hewitt, E.; Radford, S. A systematic study of the effect of physiological factors on β2-microglobulin amyloid formation at neutral pH. Biochemistry 2006, 45, 2311–2321. [Google Scholar] [CrossRef]
- Sarell, C.J.; Woods, L.A.; Su, Y.; Debelouchina, G.T.; Ashcroft, A.E.; Griffin, R.G.; Stockley, P.; Radford, S.E. Expanding the repertoire of amyloid polymorphs by Co-polymerization of related protein precursors. J. Biol. Chem. 2013, 288, 7327–7337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valleix, S.; Gillmore, J.D.; Bridoux, F.; Mangione, P.P.; Dogan, A.; Nedelec, B.; Boimard, M.; Touchard, G.; Goujon, J.-M.; Lacombe, C.; et al. Hereditary systemic amyloidosis due to Asp76Asn variant β2-microglobulin. N. Engl. J. Med. 2012, 366, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.-F.; Hellewell, A.; Gosal, W.S.; Homans, S.W.; Hewitt, E.; Radford, S.E. Fibril fragmentation enhances amyloid cytotoxicity. J. Biol. Chem. 2009, 284, 34272–34282. [Google Scholar] [CrossRef] [Green Version]
- Milanesi, L.; Sheynis, T.; Xue, W.-F.; Orlova, E.; Hellewell, A.; Jelinek, R.; Hewitt, E.; Radford, S.; Saibil, H.R. Direct three-dimensional visualization of membrane disruption by amyloid fibrils. Proc. Natl. Acad. Sci. USA 2012, 109, 20455–20460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodchild, S.C.; Sheynis, T.; Thompson, R.; Tipping, K.W.; Xue, W.-F.; Ranson, N.A.; Beales, P.A.; Hewitt, E.W.; Radford, S.E. β2-microglobulin amyloid fibril-induced membrane disruption is enhanced by endosomal lipids and acidic pH. PLoS ONE 2014, 9, e104492. [Google Scholar] [CrossRef] [Green Version]
- Jakhria, T.; Hellewell, A.L.; Porter, M.Y.; Jackson, M.P.; Tipping, K.W.; Xue, W.-F.; Radford, S.; Hewitt, E.W. β2-microglobulin amyloid fibrils are nanoparticles that disrupt lysosomal membrane protein trafficking and inhibit protein degradation by lysosomes. J. Biol. Chem. 2014, 289, 35781–35794. [Google Scholar] [CrossRef] [Green Version]
- Okoshi, T.; Yamaguchi, I.; Ozawa, D.; Hasegawa, K.; Naiki, H. Endocytosed β2-microglobulin amyloid fibrils induce necrosis and apoptosis of rabbit synovial fibroblasts by disrupting endosomal/Lysosomal membranes: A Novel mechanism on the cytotoxicity of amyloid fibrils. PLoS ONE 2015, 10, e0139330. [Google Scholar] [CrossRef] [Green Version]
- Balch, W.E.; Morimoto, R.I.; Dillin, A.; Kelly, J.W. Adapting proteostasis for disease intervention. Science 2008, 319, 916–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åkerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calfon, M.; Zeng, H.; Urano, F.; Till, J.H.; Hubbard, S.R.; Harding, H.P.; Clark, S.G.; Ron, D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002, 415, 92–96. [Google Scholar] [CrossRef]
- Safra, M.; Ben-Hamo, S.; Kenyon, C.; Henis-Korenblit, S. The ire-1 ER Stress-response pathway is required for normal secretory- protein metabolism in C. elegans. J. Cell Sci. 2013, 126, 4136–4146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genereux, J.C.; Qu, S.; Zhou, M.; Ryno, L.M.; Wang, S.; Shoulders, M.D.; Kaufman, R.J.; Lasmezas, C.I.; Kelly, J.W.; Wiseman, R.L. Unfolded protein response-induced ERdj3 secretion links ER stress to extracellular proteostasis. EMBO J. 2015, 34, 4–19. [Google Scholar] [CrossRef]
- Chen, J.J.; Genereux, J.C.; Suh, E.H.; Vartabedian, V.F.; Rius, B.; Qu, S.; Dendle, M.T.; Kelly, J.W.; Wiseman, R.L. Endoplasmic reticulum proteostasis influences the oligomeric state of an amyloidogenic protein secreted from mammalian cells. Cell Chem. Biol. 2016, 23, 1282–1293. [Google Scholar] [CrossRef] [Green Version]
- Gallotta, I.; Sandhu, A.; Peters, M.; Haslbeck, M.; Jung, R.; Agilkaya, S.; Blersch, J.L.; Rödelsperger, C.; Röseler, W.; Huang, C.; et al. Extracellular proteostasis prevents aggregation during pathogenic attack. Nature 2020, 584, 410–414. [Google Scholar] [CrossRef]
- Romine, I.C.; Wiseman, R.L. PERK signaling regulates extracellular proteostasis of an amyloidogenic protein during endoplasmic reticulum stress. Sci. Rep. 2019, 9, 410. [Google Scholar] [CrossRef] [Green Version]
- Cornejo, V.H.; Hetz, C. The unfolded protein response in Alzheimer’s disease. Semin. Immunopathol. 2013, 35, 277–292. [Google Scholar] [CrossRef]
- Marcora, M.S.; Belfiori-Carrasco, L.F.; Bocai, N.I.; Morelli, L.; Castaño, E.M. Amyloid-β42 clearance and neuroprotection mediated by X-box binding protein 1 signaling decline with aging in the Drosophila brain. Neurobiol. Aging 2017, 60, 57–70. [Google Scholar] [CrossRef]
- Zhang, P.; Fu, X.; Sawashita, J.; Yao, J.; Zhang, B.; Qian, J.; Tomozawa, H.; Mori, M.; Ando, Y.; Naiki, H.; et al. Mouse model to study human A β2M amyloidosis: Generation of a transgenic mouse with excessive expression of human β2-microglobulin. Amyloid 2010, 17, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Diomede, L.; Soria, C.; Romeo, M.; Giorgetti, S.; Marchese, L.; Mangione, P.P.; Porcari, R.; Zorzoli, I.; Salmona, M.; Bellotti, V.; et al. elegans expressing human β2-microglobulin: A novel model for studying the relationship between the molecular assembly and the toxic phenotype. PLoS ONE 2012, 7, e52314. [Google Scholar] [CrossRef] [PubMed]
- Faravelli, G.; Raimondi, S.; Marchese, L.; Partridge, F.A.; Soria, C.; Mangione, P.P.; Canetti, D.; Perni, M.; Aprile, F.A.; Zorzoli, I.; et al. elegans expressing D76N β2-microglobulin: A model for in vivo screening of drug candidates targeting amyloidosis. Sci. Rep. 2019, 9, 19960. [Google Scholar] [CrossRef] [PubMed]
- Pokrzywa, M.; Dacklin, I.; Hultmark, D.; Lundgren, E. Misfolded transthyretin causes behavioral changes in a Drosophila model for transthyretin-associated amyloidosis. Eur. J. Neurosci. 2007, 26, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Ochiishi, T.; Doi, M.; Yamasaki, K.; Hirose, K.; Kitamura, A.; Urabe, T.; Hattori, N.; Kinjo, M.; Ebihara, T.; Shimura, H. Development of new fusion proteins for visualizing amyloid-β oligomers in vivo. Sci. Rep. 2016, 6, 22712. [Google Scholar] [CrossRef] [PubMed]
- Mangione, P.P.; Esposito, G.; Relini, A.; Raimondi, S.; Porcari, R.; Giorgetti, S.; Corazza, A.; Fogolari, F.; Penco, A.; Goto, Y.; et al. Structure, folding dynamics, and amyloidogenesis of D76N β2-microglobulin: Roles of shear flow, hydrophobic surfaces, and α-crystallin. J. Biol. Chem. 2013, 288, 30917–30930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyedmers, J.; Mogk, A.; Bukau, B. Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 2010, 11, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Oslowski, C.M.; Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011, 490, 71–92. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.; Dillin, A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 2013, 153, 1435–1447. [Google Scholar] [CrossRef] [Green Version]
- Bellotti, V.; Mangione, P.; Stoppini, M. Biological activity and pathological implications of misfolded proteins. Cell. Mol. Life Sci. 1999, 55, 977–991. [Google Scholar] [CrossRef]
- Gao, B.; Adhikari, R.; Howarth, M.; Nakamura, K.; Gold, M.C.; Hill, A.B.; Knee, R.; Michalak, M.; Elliott, T. Assembly and antigen-presenting function of mhc class i molecules in cells lacking the ER chaperone calreticulin. Immunity 2002, 16, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Hodkinson, J.P.; Ashcroft, A.E.; Radford, S.E. Protein misfolding and toxicity in dialysis-related amyloidosis. In Non-fibrillar Amyloidogenic Protein Assemblies—Common Cytotoxins Underlying Degenerative Diseases; Rahimi, F., Bitan, G., Eds.; Springer: Dordrecht, The Netherlands, 2021; pp. 377–405. [Google Scholar]
- Pukkila-Worley, R.; Ausubel, F.M. Immune defense mechanisms in the Caenorhabditis elegans intestinal epithelium. Curr. Opin. Immunol. 2012, 24, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Blancas-Mejía, L.M.; Ramirez-Alvarado, M. Systemic amyloidoses. Annu. Rev. Biochem. 2013, 82, 745–774. [Google Scholar] [CrossRef] [Green Version]
- Borysik, A.J.; Morten, I.J.; Radford, S.E.; Hewitt, E.W. Specific glycosaminoglycans promote unseeded amyloid formation from beta2-microglobulin under physiological conditions. Kidney Int. 2007, 72, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benseny-Cases, N.; Karamanos, T.; Hoop, C.; Baum, J.; Radford, S.E. Extracellular matrix components modulate different stages in β2-microglobulin amyloid formation. J. Biol. Chem. 2019, 294, 9392–9401. [Google Scholar] [CrossRef] [Green Version]
- Hoop, C.L.; Zhu, J.; Bhattacharya, S.; Tobita, C.A.; Radford, S.E.; Baum, J. Collagen I weakly interacts with the β-Sheets of β2-Microglobulin and enhances conformational exchange to induce amyloid formation. J. Am. Chem. Soc. 2019, 142, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Van Die, I.; Eijnden, D.H.V.D.; Yokota, A.; Kitagawa, H.; Sugahara, K. Demonstration of glycosaminoglycans in Caenorhabditis elegans. FEBS Lett. 1999, 459, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.; Yang, J.-S.; Lee, S.-J.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147–56152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nussbaum-Krammer, C.I.; Neto, M.; Brielmann, R.M.; Pedersen, J.S.; Morimoto, R.I. Investigating the spreading and toxicity of prion-like proteins using the metazoan model organism C. elegans. J. Vis. Exp. 2015, 95, e52321. [Google Scholar] [CrossRef]
- Van Oosten-Hawle, P.; Porter, R.S.; Morimoto, R.I. Regulation of organismal proteostasis by transcellular chaperone signaling. Cell 2013, 153, 1366–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, D.; Jones, L.M.; Good, S.; Miles, J.; Vijayabaskar, M.; Aston, R.; Smith, C.E.; Westhead, D.; van Oosten-Hawle, P. A PQM-1-mediated response triggers transcellular chaperone signaling and regulates organismal proteostasis. Cell Rep. 2018, 23, 3905–3919. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Good, S.C.; Dewison, K.M.; Radford, S.E.; van Oosten-Hawle, P. Global Proteotoxicity Caused by Human β2 Microglobulin Variants Impairs the Unfolded Protein Response in C. elegans. Int. J. Mol. Sci. 2021, 22, 10752. https://doi.org/10.3390/ijms221910752
Good SC, Dewison KM, Radford SE, van Oosten-Hawle P. Global Proteotoxicity Caused by Human β2 Microglobulin Variants Impairs the Unfolded Protein Response in C. elegans. International Journal of Molecular Sciences. 2021; 22(19):10752. https://doi.org/10.3390/ijms221910752
Chicago/Turabian StyleGood, Sarah C., Katherine M. Dewison, Sheena E. Radford, and Patricija van Oosten-Hawle. 2021. "Global Proteotoxicity Caused by Human β2 Microglobulin Variants Impairs the Unfolded Protein Response in C. elegans" International Journal of Molecular Sciences 22, no. 19: 10752. https://doi.org/10.3390/ijms221910752
APA StyleGood, S. C., Dewison, K. M., Radford, S. E., & van Oosten-Hawle, P. (2021). Global Proteotoxicity Caused by Human β2 Microglobulin Variants Impairs the Unfolded Protein Response in C. elegans. International Journal of Molecular Sciences, 22(19), 10752. https://doi.org/10.3390/ijms221910752





