Lecithin Inclusion by α-Cyclodextrin Activates SREBP2 Signaling in the Gut and Ameliorates Postprandial Hyperglycemia
Abstract
1. Introduction
2. Results
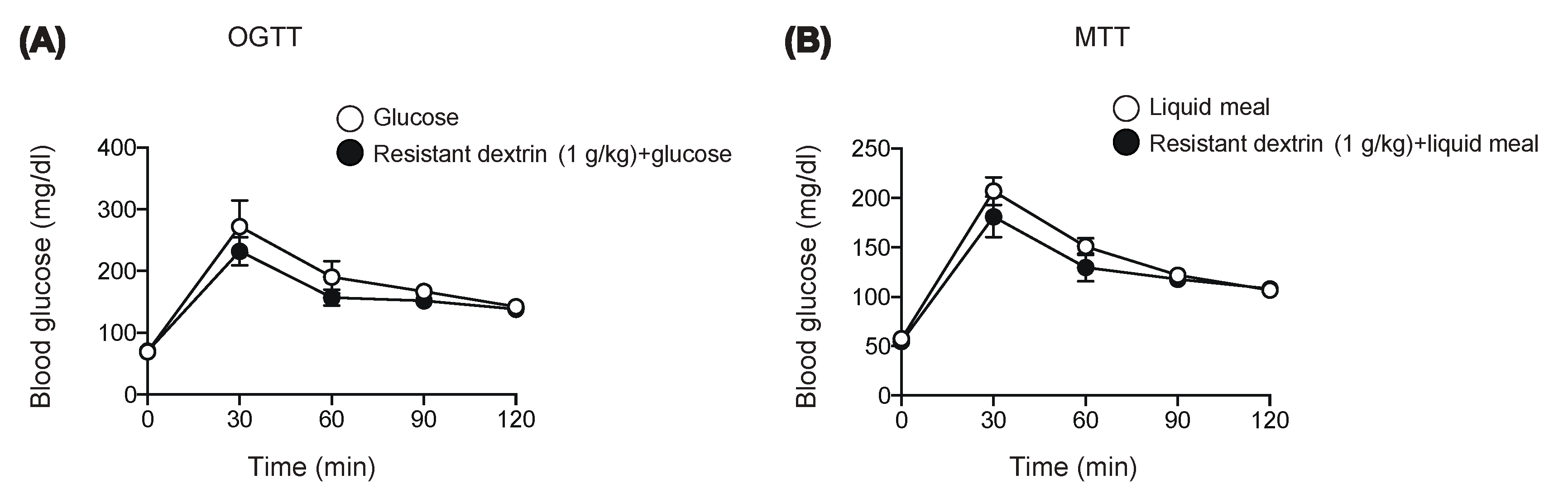
2.1. Resistant Dextrin Exhibits Modest Effects on Suppression of Hyperglycemia after Co-Administration of Glucose or Liquid Meal in Mice
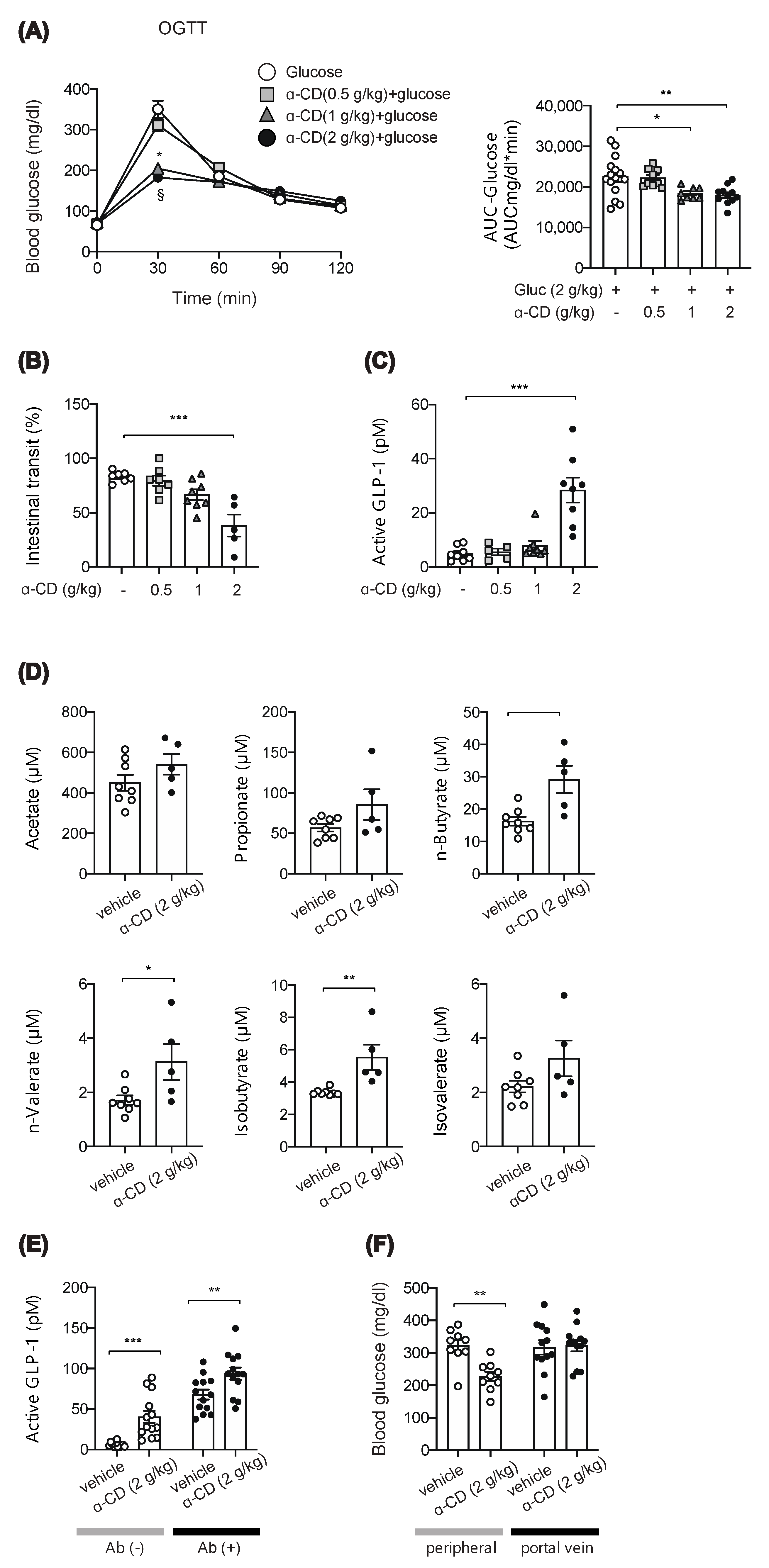
2.2. α-CD Administration with Glucose Decreases Blood Glucose via GLP-1 Dependent and Independent Pathways
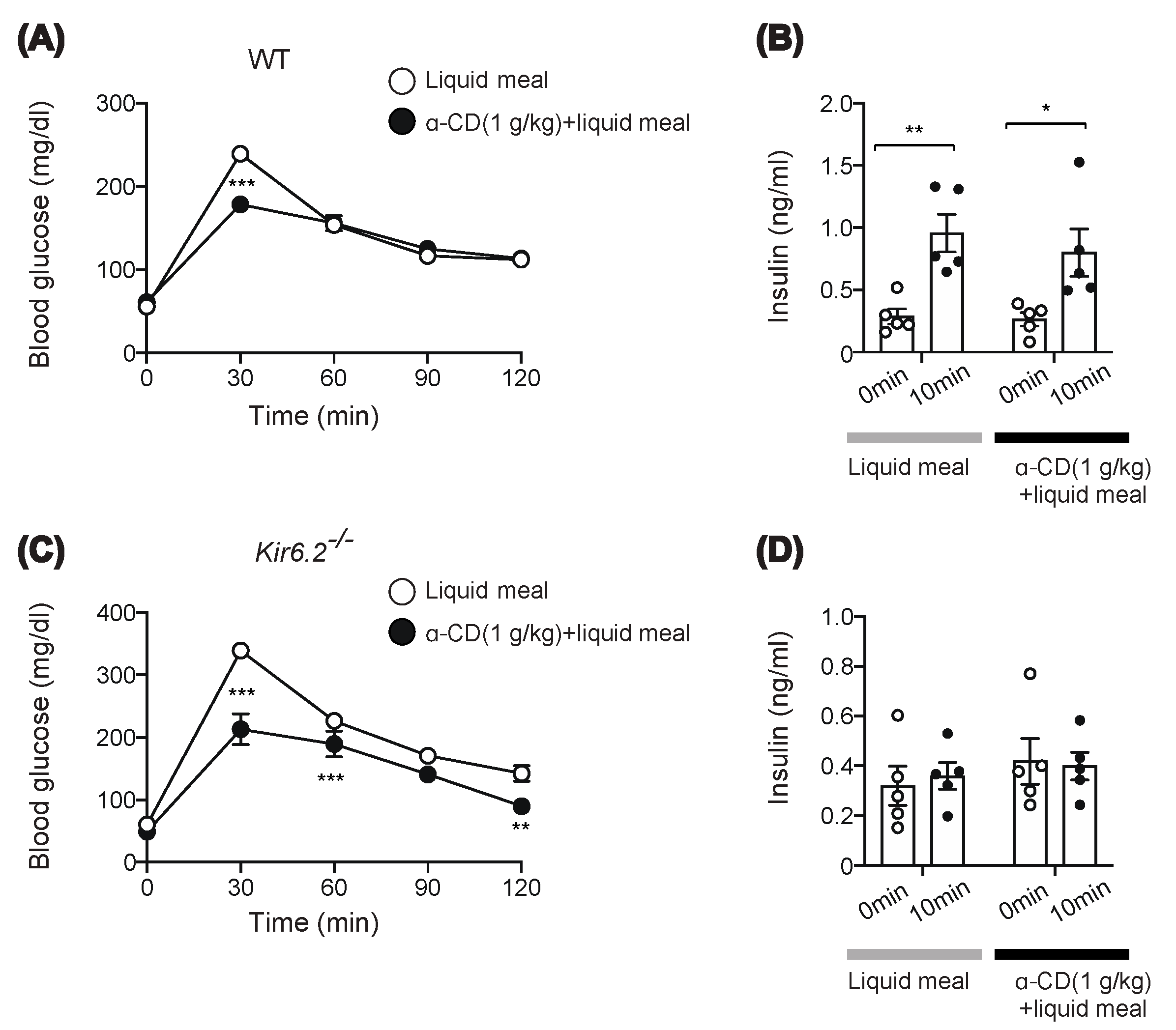
2.3. α-CD Suppresses Meal-Induced Hyperglycemia via an Insulin-Independent Pathway
2.4. Preinclusion of α-CD with Lecithin Abolishes Glucose-Lowering Effect of α-CD
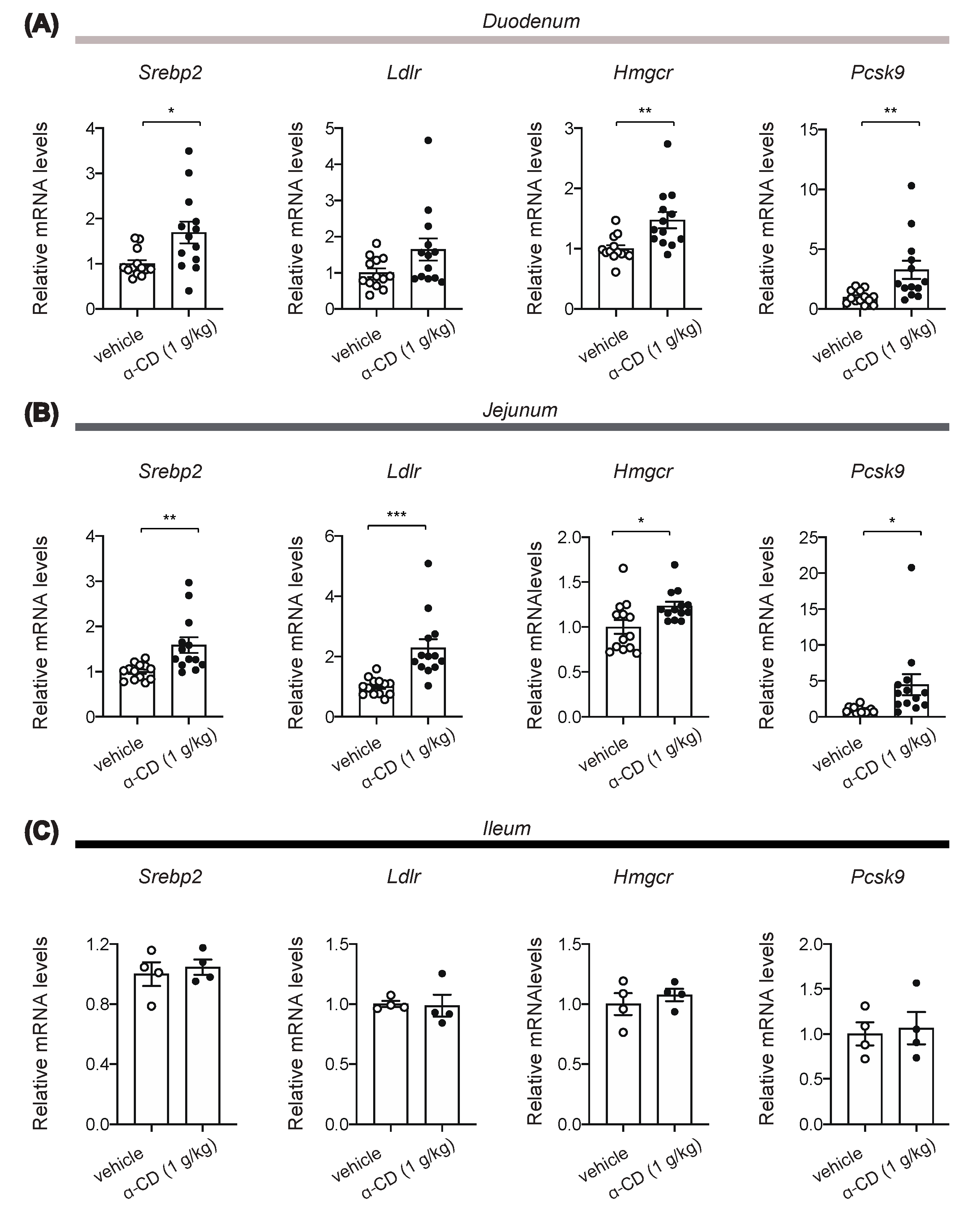
2.5. α-CD Administration Activates Intestinal SREBP2 Signaling
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Oral Glucose Tolerance Test and Meal Tolerance Test
4.2.1. Procedures for Oral Glucose or Meal Tolerance Test
4.2.2. Preparation of Resistant Dextrin, α-CD, and Lecithin and Their Use for In Vivo Experiments
4.3. Preparation of α-CD and Lecithin and Their Use for In Vitro Experiments
4.4. Measurement of Portal GLP-1, SCFAs, and Insulin
4.5. Intestinal Transit
4.6. Quantitative Real-Time PCR
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-CD | α-cyclodextrin |
| GLP-1 | glucagon-like peptide 1 |
| FOSHU | Food for Specified Health Uses |
| α-GI | α-glucosidase inhibitor |
| SCFAs | short chain fatty acids |
| MTT | meal tolerance test |
| OGTT | oral glucose tolerance test |
| CCK | cholecystokinin |
| FDA | Food and Drug Administration |
References
- Zheng, Y.; Ley, S.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2017, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Sato-Mito, N.; Nagata, J.; Umegaki, K. Health Claim Evidence Requirements in Japan. J. Nutr. 2008, 138, 1192S–1198S. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on the Definition of Dietary Fiber; The Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes Proposed Definition of Dietary Fiber; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Hobden, M.R.; Guérin-Deremaux, L.; Rowland, I.; Gibson, G.R.; Kennedy, O.B. Potential anti-obesogenic properties of non-digestible carbohydrates: Specific focus on resistant dextrin. Proc. Nutr. Soc. 2015, 74, 258–267. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Kishimoto, Y.; Matsuoka, A. Effects of indigestible dextrin on glucose tolerance in rats. J. Endocrinol. 1995, 144, 533–538. [Google Scholar] [CrossRef]
- Bock, P.M.; Telo, G.H.; Ramalho, R.; Sbaraini, M.; Leivas, G.; Martins, A.F.; Schaan, B.D. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: A systematic review and meta-analysis. Diabetologia 2020, 64, 26–41. [Google Scholar] [CrossRef]
- Lee, E.Y.; Kaneko, S.; Jutabha, P.; Zhang, X.; Seino, S.; Jomori, T.; Anzai, N.; Miki, T. Distinct action of the α-glucosidase inhibitor miglitol on SGLT3, enteroendocrine cells, and GLP1 secretion. J. Endocrinol. 2015, 224, 205–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, E.Y.; Zhang, X.; Miyamoto, J.; Kimura, I.; Taknaka, T.; Furusawa, K.; Jomori, T.; Fujimoto, K.; Uematsu, S.; Miki, T. Gut carbohydrate inhibits GIP secretion via a microbiota/SCFA/FFAR3 pathway. J. Endocrinol. 2018, 239, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.D.; Thorp, A.A.; Murphy, K.J.; Howe, P.R. Dose-dependent inhibition of the post-prandial glycaemic response to a standard carbohydrate meal following incorporation of alpha-cyclodextrin. Ann. Nutr. Metab. 2006, 50, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Matsumoto, Y.; Sekimizu, K. Inhibitory effects of alpha-cyclodextrin and its derivative against su-crose-induced hyperglycemia in an in vivo evaluation system. Drug Discov. Ther. 2018, 12, 122–125. [Google Scholar] [CrossRef]
- Koukiekolo, R. Mechanism of porcine pancreatic alpha-amylase. Inhibition of amylose and maltopentaose hydrolysis by α-, β- and γ-cyclodextrins. Eur. J. Biochem. 2001, 268, 841–848. [Google Scholar] [CrossRef]
- Oudjeriouat, N.; Moreau, Y.; Santimone, M.; Svensson, B.; Marchis-Mouren, G.; Desseaux, V. On the mechanism of α-amylase. JBIC J. Biol. Inorg. Chem. 2003, 270, 3871–3879. [Google Scholar] [CrossRef]
- Bär, A.; Diamantis, I.; Venetz, W.P. Alpha-Cyclodextrin Attenuates the Glycemic and Insulinemic Impact of White Bread in Healthy Male Volunteers. Foods 2020, 9, 62. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Nihei, N.; Okamoto, H.; Furune, T.; Ikuta, N.; Sasaki, K.; Rimbach, G.; Yoshikawa, Y.; Terao, K. Dietary α-cyclodextrin modifies gut microbiota and reduces fat accumulation in high-fat-diet-fed obese mice. BioFactors 2018, 44, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2011, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Minami, K.; Shinozaki, H.; Matsumura, K.; Saraya, A.; Ikeda, H.; Yamada, Y.; Holst, J.J.; Seino, S. Distinct Effects of Glucose-Dependent Insulinotropic Polypeptide and Glucagon-Like Peptide-1 on Insulin Secretion and Gut Motility. Diabetes 2005, 54, 1056–1063. [Google Scholar] [CrossRef]
- Furune, T.; Ikuta, N.; Ishida, Y.; Okamoto, H.; Nakata, D.; Terao, K.; Sakamoto, N. A study on the inhibitory mechanism for cholesterol absorption by α-cyclodextrin administration. Beilstein J. Org. Chem. 2014, 10, 2827–2835. [Google Scholar] [CrossRef]
- Brown, M.S.; Radhakrishnan, A.; Goldstein, J.L. Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu. Rev. Biochem. 2018, 87, 783–807. [Google Scholar] [CrossRef]
- Jeon, T.-I.; Zhu, B.; Larson, J.L.; Osborne, T.F. SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J. Clin. Investig. 2008, 118, 3693–3700. [Google Scholar] [CrossRef]
- Cheung, G.W.; Kokorovic, A.; Lam, C.K.; Chari, M.; Lam, T.K. Intestinal Cholecystokinin Controls Glucose Production through a Neuronal Network. Cell Metab. 2009, 10, 99–109. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Gu, Z.; Chen, J.; Wu, J. Alpha-cyclodextrin: Enzymatic production and food applications. Trends Food Sci. Technol. 2014, 35, 151–160. [Google Scholar] [CrossRef]
- Bessell, E.; Fuller, N.R.; Markovic, T.P.; Lau, N.S.; Burk, J.; Hendy, C.; Picone, T.; Li, A.; Caterson, I.D. Effects of α-Cyclodextrin on Cholesterol Control and Hydrolyzed Ginseng Extract on Glycemic Control in People with Prediabetes: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2023491. [Google Scholar] [CrossRef]
- Gentilcore, D.; Vanis, L.; Teng, J.C.; Wishart, J.M.; Buckley, J.D.; Rayner, C.K.; Horowitz, M.; Jones, K.L. The oligosaccharide α-cyclodextrin has modest effects to slow gastric emptying and modify the glycaemic response to sucrose in healthy older adults. Br. J. Nutr. 2011, 106, 583–587. [Google Scholar] [CrossRef]
- Haimhoffer, A.; Rusznyák, A.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F.; et al. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef]
- Buchan, A.; Polak, J.M.; Solcia, E.; Capella, C.; Hudson, D.; Pearse, A.G. Electron immunohistochemical evidence for the human intestinal I cell as the source of CCK. Gut 1978, 19, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.; Bloom, S.; Rayford, P.; Pearse, A.; Buchan, A.; Thompson, J. Identification of cholecystokinin-secreting cells. Lancet 1975, 306, 1016–1018. [Google Scholar] [CrossRef]
- Jarosz, P.A.; Fletcher, E.; Elserafy, E.; Artiss, J.D.; Jen, K.-L.C. The Effect of α-Cyclodextrin on postprandial lipid and glycemic responses to a fat-containing meal. Metabolism 2013, 62, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Nagashima, K.; Tashiro, F.; Kotake, K.; Yoshitomi, H.; Tamamoto, A.; Gonoi, T.; Iwanaga, T.; Miyazaki, J.-I.; Seino, S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 10402–10406. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Sakurai, K.; Zhang, X.; Toda, C.; Tanaka, T.; Jiang, M.; Shirasawa, T.; Tachibana, K.; Yokote, K.; Vidal-Puig, A.; et al. Unsuppressed lipolysis in adipocytes is linked with enhanced gluconeogenesis and altered bile acid physiology in InsrP1195L/+ mice fed high-fat-diet. Sci. Rep. 2015, 5, 17565. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Yukawa, K.; Ogasawara, H.; Suzawa, K.; Maekawa, T.; Yamamoto, Y.; Ohta, T.; Lee, E.; Miki, T. GPR52 accelerates fatty acid biosynthesis in a ligand-dependent manner in hepatocytes and in response to excessive fat intake in mice. iScience 2021, 24, 102260. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Miedzybrodzka, E.L.; Zhang, X.; Hatano, R.; Miyamoto, J.; Kimura, I.; Fujimoto, K.; Uematsu, S.; Rodriguez-Cuenca, S.; Vidal-Puig, A.; et al. Diet-Induced Obese Mice and Leptin-Deficient Lep(ob/ob) Mice Exhibit Increased Circulating GIP Levels Produced by Different Mechanisms. Int. J. Mol. Sci. 2019, 20, 4448. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.; Zhang, X.; Noda, T.; Miyamoto, J.; Kimura, I.; Tanaka, T.; Sakurai, K.; Hatano, R.; Miki, T. Lecithin Inclusion by α-Cyclodextrin Activates SREBP2 Signaling in the Gut and Ameliorates Postprandial Hyperglycemia. Int. J. Mol. Sci. 2021, 22, 10796. https://doi.org/10.3390/ijms221910796
Lee E, Zhang X, Noda T, Miyamoto J, Kimura I, Tanaka T, Sakurai K, Hatano R, Miki T. Lecithin Inclusion by α-Cyclodextrin Activates SREBP2 Signaling in the Gut and Ameliorates Postprandial Hyperglycemia. International Journal of Molecular Sciences. 2021; 22(19):10796. https://doi.org/10.3390/ijms221910796
Chicago/Turabian StyleLee, Eunyoung, Xilin Zhang, Tomoe Noda, Junki Miyamoto, Ikuo Kimura, Tomoaki Tanaka, Kenichi Sakurai, Ryo Hatano, and Takashi Miki. 2021. "Lecithin Inclusion by α-Cyclodextrin Activates SREBP2 Signaling in the Gut and Ameliorates Postprandial Hyperglycemia" International Journal of Molecular Sciences 22, no. 19: 10796. https://doi.org/10.3390/ijms221910796
APA StyleLee, E., Zhang, X., Noda, T., Miyamoto, J., Kimura, I., Tanaka, T., Sakurai, K., Hatano, R., & Miki, T. (2021). Lecithin Inclusion by α-Cyclodextrin Activates SREBP2 Signaling in the Gut and Ameliorates Postprandial Hyperglycemia. International Journal of Molecular Sciences, 22(19), 10796. https://doi.org/10.3390/ijms221910796






