Potato Juice, a Starch Industry Waste, as a Cost-Effective Medium for the Biosynthesis of Bacterial Cellulose
Abstract
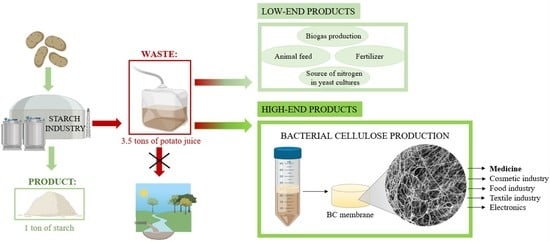
:1. Introduction
2. Results and Discussion
2.1. Characterization of PJ Medium
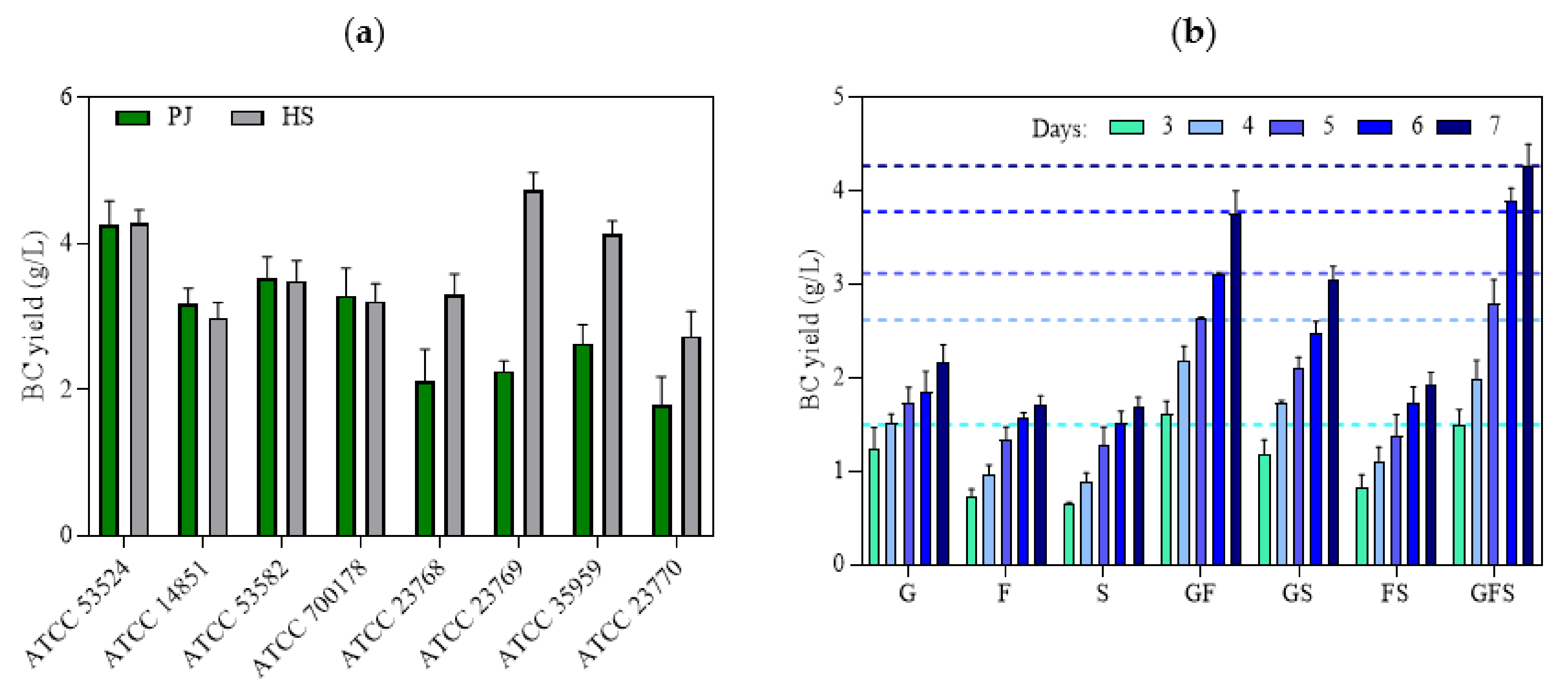
2.2. Quantification of K. xylinus Cells and BC Yield
2.3. The Changes in pH and Chemical Composition of PJ during Fermentation Process
2.4. The Role of Bacterial Strain and Presence of Sugars on BC Yield
2.5. Physicochemical Properties of BC Obtained from Potato Tuber Juice Medium
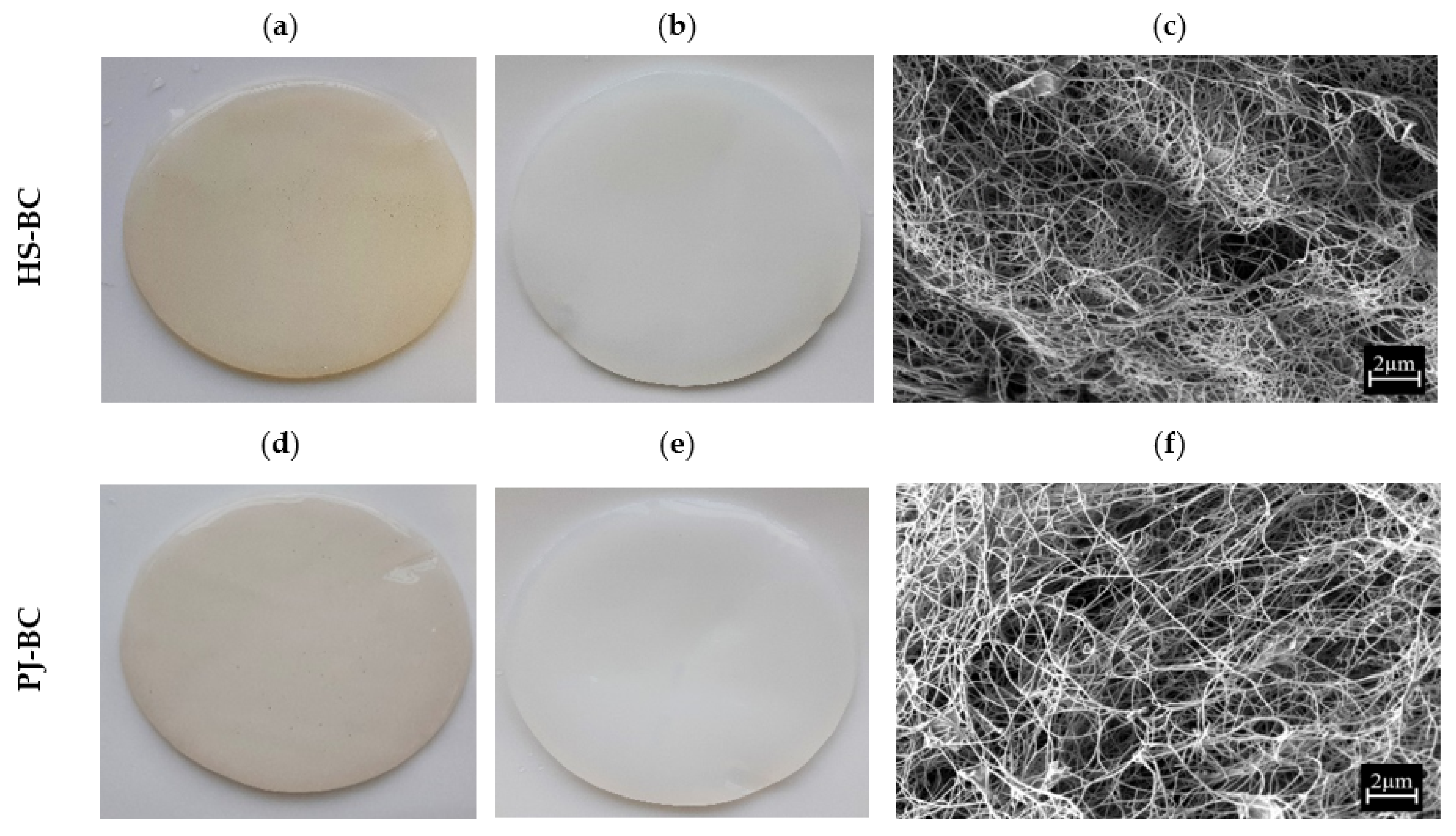
2.5.1. Macro- and Microstructure
2.5.2. Analysis of ATR-FTIR Spectra
2.5.3. Water-Related Properties, Density, and Mechanical Properties of BC
2.6. BC Cytotoxicity Screening
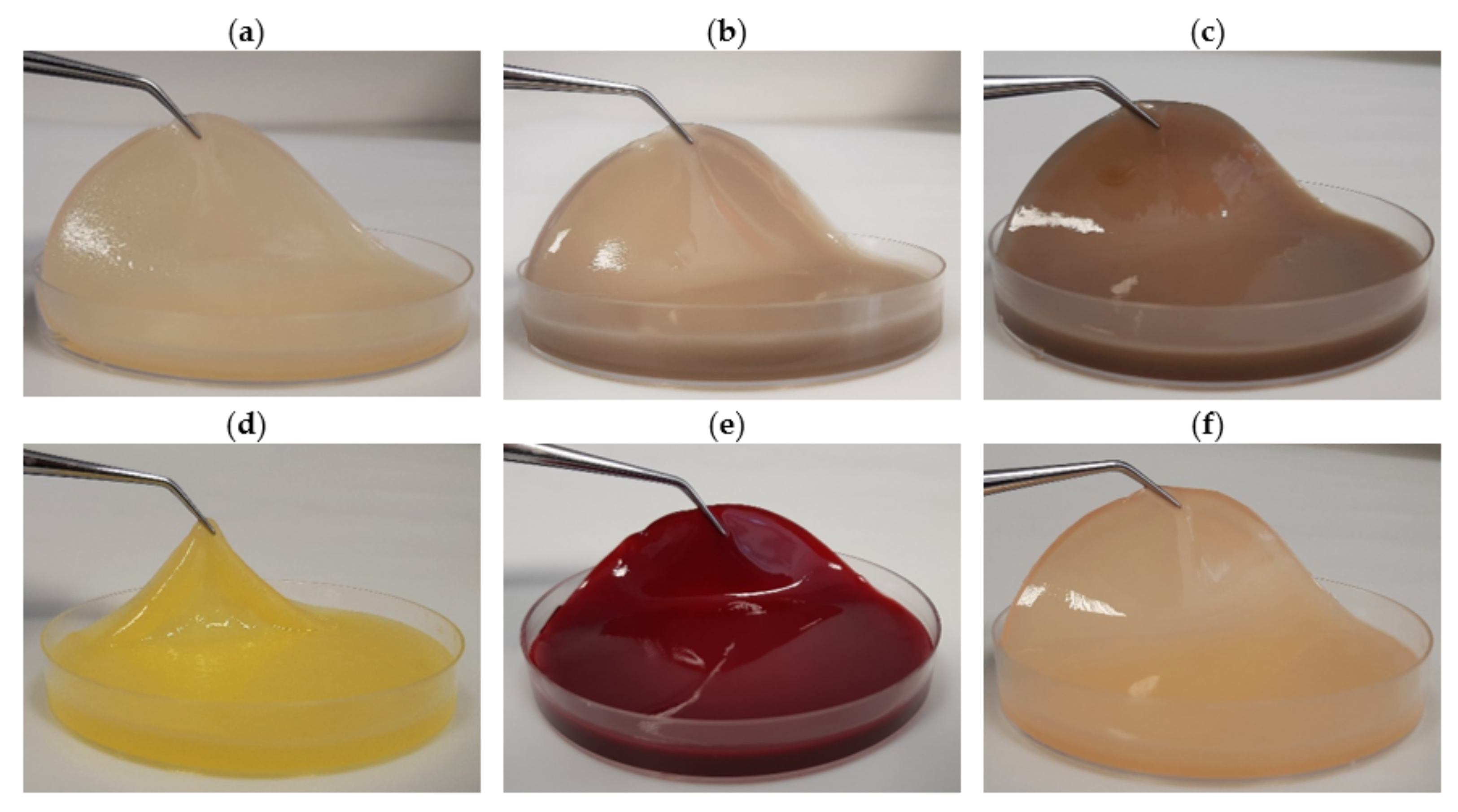
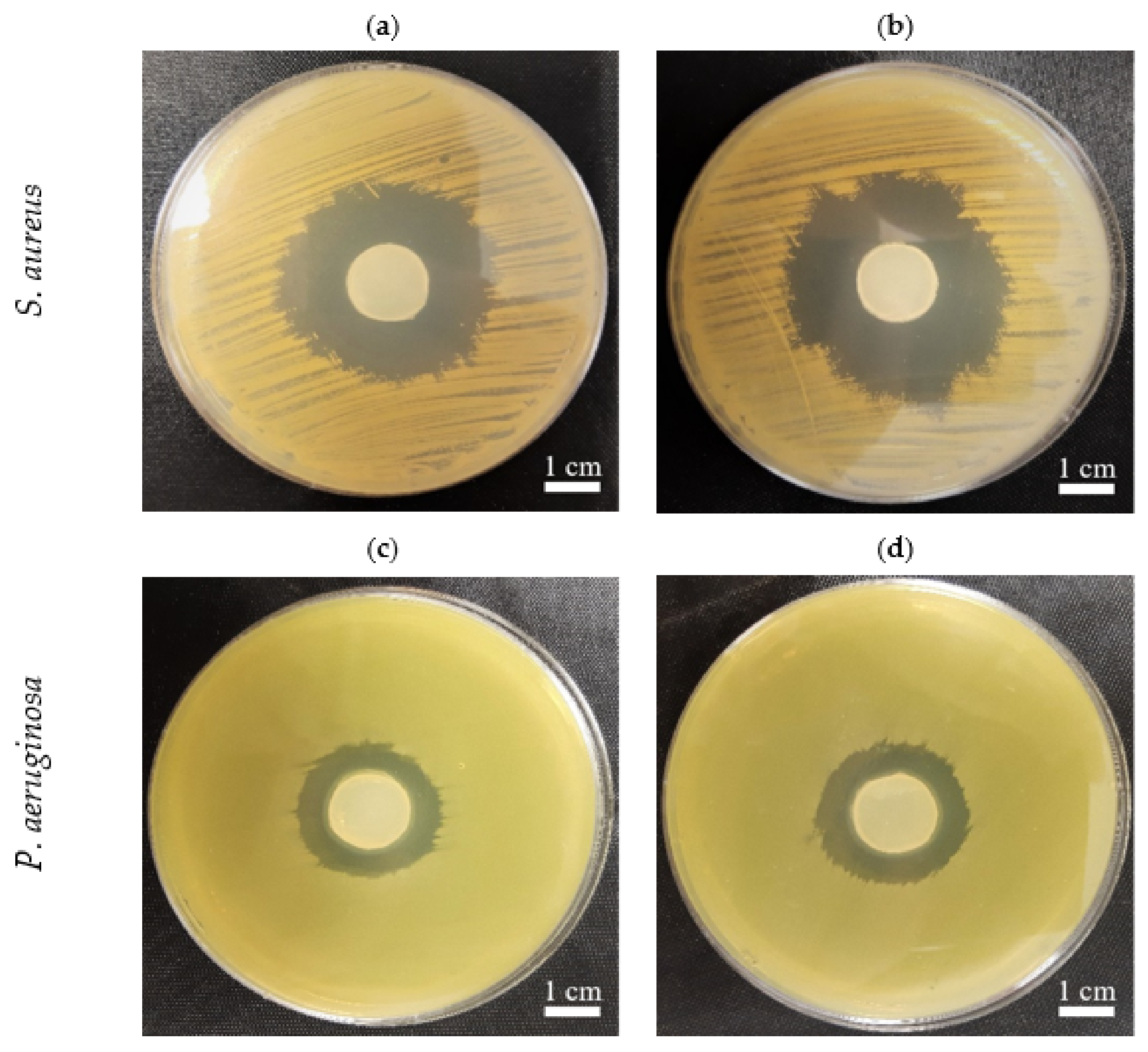
2.7. The In Vitro Activity of PJ-BC and HS-BC Soaked with Antimicrobial against Two Species of Opportunistic Pathogens
3. Materials and Methods
3.1. Preparation of Culture Medium
3.2. Determination of pH, Protein, and Carbohydrate Concentration in PJ Medium
3.3. Microorganisms and Culture Conditions
3.4. Determination of BC Yield
3.5. Quantification and Viability Assessment of K. xylinus Cells
3.6. Assessment of pH, Protein, Sucrose, Glucose, Fructose, and Gluconic Acid Concentration during BC Biosynthesis
3.7. Evaluation of Physical and Chemical Properties of BC Obtained from PJ Medium
3.7.1. Analysis of Microstructure Using Scanning Electron Microscopy (SEM)
3.7.2. Determination of Chemical Composition of BC Pellicles Using Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
3.7.3. Determination of Water Swelling Ratio
3.7.4. Determination of Water Holding Capacity
3.7.5. Determination of Density
3.7.6. Evaluation of Mechanical Properties
3.8. Cytotoxicity Screening
3.8.1. Extract Assay
3.8.2. Direct Contact Assay
3.8.3. Visualization of Fibroblast Viability
3.9. The In Vitro Activity of PJ-BC and HS-BC Soaked with Antimicrobial against Two Species of Opportunistic Pathogens
3.10. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M., Jr. Microbial cellulose-the natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Beluns, S.; Gaidukovs, S.; Platnieks, O.; Gaidukova, G.; Mierina, I.; Grase, L.; Starkowa, O.; Brazdausks, P.; Thakur, V.K. From wood and hemp biomass wastes to sustainable nanocellulose foams. Ind. Crops Prod. 2021, 170, 113780. [Google Scholar] [CrossRef]
- Ullah, H.; Badshah, M.; Correia, A.; Wahid, F.; Santos, H.A.; Khan, T. Functionalized bacterial cellulose microparticles for drug delivery in biomedical applications. Curr. Pharm. Des. 2019, 25, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Drozd, R.; Szymańska, M.; Przygrodzka, K.; Hoppe, J.; Leniec, G.; Kowalska, U. The Simple Method of Preparation of Highly Carboxylated Bacterial Cellulose with Ni-and Mg-Ferrite-Based Versatile Magnetic Carrier for Enzyme Immobilization. Int. J. Mol. Sci. 2021, 22, 8563. [Google Scholar] [CrossRef]
- Chawla, P.R.; Bajaj, I.B.; Survase, S.A.; Singhal, R.S. Microbial cellulose: Fermentative production and applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2009, 76, 333–335. [Google Scholar] [CrossRef]
- Lima, H.L.S.; Nascimento, E.S.; Andrade, F.K.; Brígida, A.I.S.; Borges, M.; Cassales, A.R.; Muniz, C.R.; Filho, M.D.S.M.S.; Morais, J.P.S.; Rosa, M.D.F. Bacterial Cellulose Production by Komagataeibacter hansenii ATCC 23769 Using Sisal Juice-An Agroindustry Waste. Braz. J. Chem. Eng. 2017, 34, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Med. Biol. Res. 2018, 49, 151–159. [Google Scholar] [CrossRef]
- Kongruang, S. Bacterial cellulose production by Acetobacter xylinum strains from agricultural waste products. Biotechnol. Fuels Chem. 2007, 148, 763–774. [Google Scholar]
- Li, Z.; Wang, L.; Hua, J.; Jia, S.; Zhang, J.; Liu, H. Production of nano bacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr. Polym. 2015, 120, 115–119. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 1–14. [Google Scholar]
- Cheng, Z.; Yang, R.; Liu, X.; Liu, X.; Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresour. Technol. 2017, 234, 8–14. [Google Scholar] [CrossRef]
- Goelzer, F.D.E.; Faria-Tischer, P.C.S.; Vitorino, J.C.; Sierakowski, M.R.; Tischer, C.A. Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Mat. Sci. Eng. C 2009, 29, 546–551. [Google Scholar] [CrossRef]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Bradshaw, J.E.; Bonierbale, M. Potatoes. In Root and Tuber Crops; Bradshaw, J.E., Ed.; Springer: New York, NY, USA, 2010; Volume 7, pp. 1–52. [Google Scholar]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef] [PubMed]
- The European Starch Industry. Available online: https://starch.eu/the-european-starch-industry/ (accessed on 22 May 2021).
- Fang, C.; Boe, K.; Angelidaki, I. Biogas production from potato-juice, a by-product from potato-starch processing, in upflow anaerobic sludge blanket (UASB) and expanded granular sludge bed (EGSB) reactors. Bioresour. Technol. 2011, 102, 5734–5741. [Google Scholar] [CrossRef]
- Grommers, H.E.; van der Krogt, D.A. Potato starch: Production, modifications and uses. Starch 2009, 11, 511–539. [Google Scholar]
- Kot, A.M.; Pobiega, K.; Piwowarek, K.; Kieliszek, M.; Błażejak, S.; Gniewosz, M.; Lipińska, E. Biotechnological methods of management and utilization of potato industry waste-a review. Potato Res. 2020, 63, 431–447. [Google Scholar] [CrossRef]
- Jayanty, S.S.; Diganta, K.; Raven, B. Effects of cooking methods on nutritional content in potato tubers. Am. J. Potato Res. 2019, 96, 183–194. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Olejnik, A.; Białas, W.; Rybicka, I.; Zielińska-Dawidziak, M.; Siger, A.; Kubiak, P.; Lewandowicz, G. The nutritional value and biological activity of concentrated protein fraction of potato juice. Nutrients 2019, 11, 1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczewski, P.; Celka, K.; Białas, W.; Lewandowicz, G. Antioxidant activity of potato juice. Acta Sci. Pol. Technol. Aliment. 2012, 11, 175–181. [Google Scholar]
- Han, G.P.; Lee, K.R.; Han, J.S.; Kozukue, N.; Kim, D.S.; Kim, J.; Bae, J.H. Quality characteristics of the potato juice-added functional white bread. Korean J. Food Sci. Technol. 2004, 36, 924–929. [Google Scholar]
- Kim, N.J.; Jang, H.L.; Yoon, K.Y. Potato juice fermented with Lactobacillus casei as a probiotic functional beverage. Food Sci. Biotechnol. 2012, 21, 1301–1307. [Google Scholar] [CrossRef]
- Jozala, A.F.; Pértile, R.A.N.; dos Santos, C.A.; de Carvalho Santos-Ebinuma, V.; Seckler, M.M.; Gama, F.M.; Pessoa, A. Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl. Microbiol. Biotechnol. 2015, 99, 1181–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperotto, G.; Stasiak, L.G.; Godoi, J.P.M.G.; Gabiatti, N.C.; De Souza, S.S. A review of culture media for bacterial cellulose production: Complex, chemically defined and minimal media modulations. Cellulose 2021, 28, 2649–2673. [Google Scholar] [CrossRef]
- Molina-Ramírez, C.; Castro, M.; Osorio, M.; Torres-Taborda, M.; Gómez, B.; Zuluaga, R.; Gómez, C.; Ganan, P.; Rojas, O.J.; Castro, C. Effect of different carbon sources on bacterial nanocellulose production and structure using the low pH resistant strain Komagataeibacter medellinensis. Materials 2017, 10, 639. [Google Scholar] [CrossRef]
- Singhsa, P.; Narain, R.; Manuspiya, H. Physical structure variations of bacterial cellulose produced by different Komagataeibacter xylinus strains and carbon sources in static and agitated conditions. Cellulose 2018, 25, 1571–1581. [Google Scholar] [CrossRef]
- Lei, L.; Li, S.; Gu, Y. Cellulose synthase complexes: Composition and regulation. Front. Plant Sci. 2012, 3, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wu, G.; Chen, L.; Wang, W.; Hong, F.F.; Jönsson, L.J. Comparison of productivity and quality of bacterial nanocellulose synthesized using culture media based on seven sugars from biomass. Microb. Biotechnol. 2019, 12, 677–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aswini, K.; Gopal, N.O.; Uthandi, S. Optimized culture conditions for bacterial cellulose production by Acetobacter senegalensis MA1. BMC Biotechnol. 2020, 20, 46. [Google Scholar] [CrossRef]
- Embuscado, M.E.; Marks, J.S.; BeMiller, J.N. Bacterial cellulose. I. Factors affecting the production of cellulose by Acetobacter xylinum. Food Hydrocoll. 1994, 8, 407–418. [Google Scholar] [CrossRef]
- Drozd, R.; Szymańska, M.; Żywicka, A.; Kowalska, U.; Rakoczy, R.; Kordas, M.; Konopacki, M.; Junka, A.F.; Fijałkowski, K. Exposure to non-continuous rotating magnetic field induces metabolic strain-specific response of Komagataeibacter xylinus. Biochem. Eng. J. 2020, 166, 107855. [Google Scholar] [CrossRef]
- Ramana, K.V.; Tomar, A.; Singh, L. Effect of various carbon and nitrogen sources on cellulose synthesis by Acetobacter xylinum. World J. Microbiol. Biotechnol. 2000, 16, 245–248. [Google Scholar] [CrossRef]
- La China, S.; Bezzecchi, A.; Moya, F.; Petroni, G.; Di Gregorio, S.; Gullo, M. Genome sequencing and phylogenetic analysis of K1G4: A new Komagataeibacter strain producing bacterial cellulose from different carbon sources. Biotechnol. Lett. 2020, 42, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, M.; Robotti, F.; La China, S.; Anguluri, K.; Haghighi, H.; Bottan, S.; Ferrari, A.; Gullo, M. Assessing effectiveness of Komagataeibacter strains for producing surface-microstructured cellulose via guided assembly-based biolithography. Sci. Rep. 2021, 11, 19311. [Google Scholar] [CrossRef] [PubMed]
- Kechkar, M.; Sayed, W.; Cabrol, A.; Aziza, M.; Ahmed Zaid, T.; Amrane, A.; Djelal, H. Isolation and identification of yeast strains from sugarcane molasses, dates and figs for ethanol production under conditions simulating algal hydrolysate. Braz. J. Chem. Eng. 2019, 36, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, P.; Izquierdo, P.M.; Seseña, S.; Palop, M.L. Intraspecific genetic diversity of lactic acid bacteria from malolactic fermentation of Cencibel wines as derived from combined analysis of RAPD-PCR and PFGE patterns. Food Microbiol. 2008, 25, 942–948. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef] [Green Version]
- Hungund, B.; Prabhu, S.; Shetty, C.; Acharya, S.; Prabhu, V.; Gupta, S.G. Production of bacterial cellulose from Gluconacetobacter persimmonis GH-2 using dual and cheaper carbon sources. J. Microb. Biochem. Technol. 2013, 5, 31–33. [Google Scholar] [CrossRef] [Green Version]
- Hungund, B.S.; Gupta, S.G. Improved production of bacterial cellulose from Gluconacetobacter persimmonis GH-2. J. Microb. Biochem. Technol. 2010, 2, 127–133. [Google Scholar] [CrossRef]
- Wang, S.S.; Han, Y.H.; Chen, J.L.; Zhang, D.C.; Shi, X.X.; Ye, Y.X.; Chen, D.-L.; Li, M. Insights into bacterial cellulose biosynthesis from different carbon sources and the associated biochemical transformation pathways in Komagataeibacter sp. W1. Polymers 2018, 10, 963. [Google Scholar] [CrossRef] [Green Version]
- Tabaii, M.J.; Emtiazi, G. Comparison of bacterial cellulose production among different strains and fermented media. Appl. Food Biotechnol. 2016, 3, 35–41. [Google Scholar]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Algar, I.; Fernandes, S.C.; Mondragon, G.; Castro, C.; Garcia-Astrain, C.; Gabilondo, N.; Retegi, A.; Eceiza, A. Pineapple agroindustrial residues for the production of high value bacterial cellulose with different morphologies. J. Appl. Polym. Sci. 2015, 132, 1–17. [Google Scholar] [CrossRef]
- Huang, H.C.; Chen, L.C.; Lin, S.B.; Hsu, C.P.; Chen, H.H. In situ modification of bacterial cellulose network structure by adding interfering substances during fermentation. Bioresour. Technol. 2010, 101, 6084–6091. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, E.; Hilczer, B.; Pawlaczyk, C. Dielectric and acoustic response of biocellulose. Ferroelectrics 2004, 304, 39–42. [Google Scholar] [CrossRef]
- Lin, W.C.; Lien, C.C.; Yeh, H.J.; Yu, C.M.; Hsu, S.H. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef]
- Zhijiang, C.; Guang, Y. Bacterial cellulose/collagen composite: Characterization and first evaluation of cytocompatibility. J. Appl. Polym. Sci. 2011, 120, 2938–2944. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Junka, A.; Fijałkowski, K.; Ząbek, A.; Mikołajewicz, K.; Chodaczek, G.; Szymczyk, P.; Smutnicka, D.; Żywicka, A.; Sedghizadeh, P.P.; Dziadas, M.; et al. Correlation between type of alkali rinsing, cytotoxicity of bio-nanocellulose and presence of metabolites within cellulose membranes. Carbohydr. Polym. 2017, 157, 371–379. [Google Scholar] [CrossRef]
- Wiegand, C.; Moritz, S.; Hessler, N.; Kralisch, D.; Wesarg, F.; Müller, F.A.; Fischer, D.; Hipler, U.C. Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone-iodine. J. Mater. Sci. Mater. Med. 2015, 26, 245. [Google Scholar] [CrossRef]
- De Mattos, I.B.; Nischwitz, S.P.; Tuca, A.C.; Groeber-Becker, F.; Funk, M.; Birngruber, T.; Mautner, S.I.; Kamolz, L.P.; Holzer, J.C.J. Delivery of antiseptic solutions by a bacterial cellulose wound dressing: Uptake, release and antibacterial efficacy of octenidine and povidone-iodine. Burns 2020, 46, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Dydak, K.; Junka, A.; Dydak, A.; Brożyna, M.; Paleczny, J.; Fijałkowski, K.; Kubielas, G.; Aniołek, O.; Bartoszewicz, M. In vitro efficacy of bacterial cellulose dressings chemisorbed with antiseptics against biofilm formed by pathogens isolated from chronic wounds. Int. J. Mol. Sci. 2021, 22, 3996. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Ampawong, S.; Harnsilpong, T.; Angspatt, A.; Aramwit, P. Inflammatory reaction, clinical efficacy, and safety of bacterial cellulose wound dressing containing silk sericin and polyhexamethylene biguanide for wound treatment. Arch. Derm. Res. 2018, 310, 795–805. [Google Scholar] [CrossRef]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest advances on bacterial cellulose-based antibacterial materials as wound dressings. Front Bioeng. Biotechnol. 2020, 8, 1334. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sulistyarti, H.; Fardiyah, Q.; Febriyanti, S. A simple and safe spectrophotometric method for iodide determination. Makara J. Sci. 2015, 19, 43–48. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kondo, T. Quantitative analysis for the cellulose Iα crystalline phase in developing wood cell walls. Int. J. Biol. Macromol. 1999, 24, 37–41. [Google Scholar] [CrossRef]
- Ciecholewska-Juśko, D.; Żywicka, A.; Junka, A.; Drozd, R.; Sobolewski, P.; Migdał, P.; Kowalska, U.; Toporkiewicz, M.; Fijałkowski, K. Superabsorbent crosslinked bacterial cellulose biomaterials for chronic wound dressings. Carbohydr. Polym. 2021, 253, 117247. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Sittampalam, G., Coussens, N., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004; pp. 1–23. [Google Scholar]



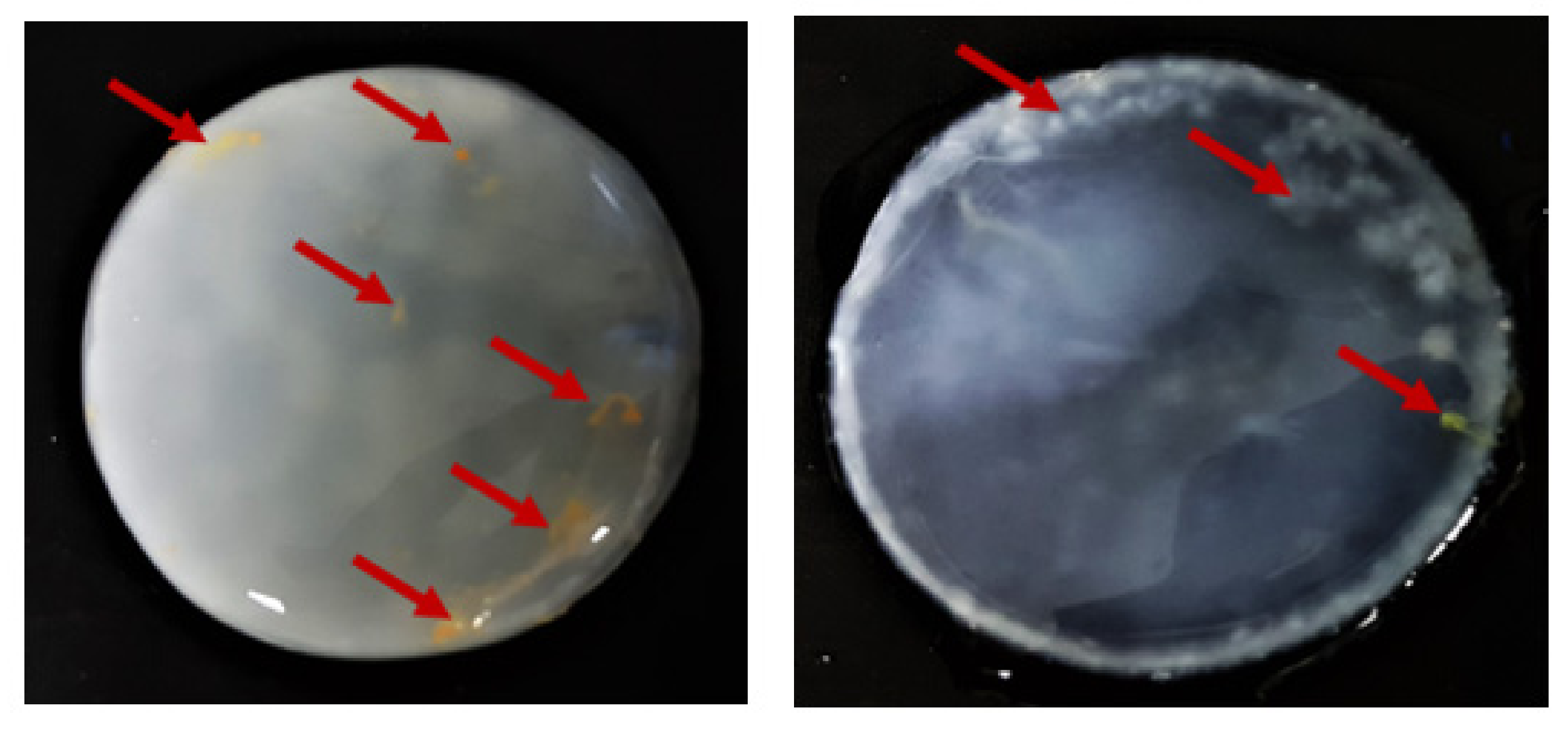


| Dry BC Yield (g/L) | |
|---|---|
| PJ:water dilution ratio 1:1 * | 4.26 ± 0.32 |
| PJ:water dilution ratio 1:2 | 1.97 ± 0.11 |
| PJ:water dilution ratio 2:1 | 2.27 ± 0.18 |
| HS with 1% of ethanol | 4.28 ± 0.18 |
| HS without ethanol | 2.04 ± 0.37 |
| PJ with 1% of ethanol * | 4.26 ± 0.32 |
| PJ without ethanol | 1.97 ± 0.53 |
| non-centrifuged PJ medium containing starch solids (with 0.67 g/L starch content) | 1.689 ± 0.143 |
| PJ medium prepared with decantation and centrifugation (with <0.1 g/L starch content) * | 4.26 ± 0.32 |
| SR | WHC | EB | TS | |
|---|---|---|---|---|
| HS-BC | 299 ± 33 | 3.70 ± 1.24 | 20.0 ± 3.67 | 2.00 ± 0.26 |
| PJ-BC | 298 ± 33 | 4.07 ± 1.45 | 19.80 ± 3.28 | 2.05 ± 0.23 |
| Iα fraction | I.C.11370/2900 | I.C.21430/900 | ρ | |
| HS-BC | 0.44 ± 0.013 | 1.62 ± 0.21 | 1.04 ± 0.06 | 1.55 ± 0.02 |
| PJ-BC | 0.45 ± 0.018 | 1.57 ± 0.27 | 1.10 ± 0.11 | 1.49 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciecholewska-Juśko, D.; Broda, M.; Żywicka, A.; Styburski, D.; Sobolewski, P.; Gorący, K.; Migdał, P.; Junka, A.; Fijałkowski, K. Potato Juice, a Starch Industry Waste, as a Cost-Effective Medium for the Biosynthesis of Bacterial Cellulose. Int. J. Mol. Sci. 2021, 22, 10807. https://doi.org/10.3390/ijms221910807
Ciecholewska-Juśko D, Broda M, Żywicka A, Styburski D, Sobolewski P, Gorący K, Migdał P, Junka A, Fijałkowski K. Potato Juice, a Starch Industry Waste, as a Cost-Effective Medium for the Biosynthesis of Bacterial Cellulose. International Journal of Molecular Sciences. 2021; 22(19):10807. https://doi.org/10.3390/ijms221910807
Chicago/Turabian StyleCiecholewska-Juśko, Daria, Michał Broda, Anna Żywicka, Daniel Styburski, Peter Sobolewski, Krzysztof Gorący, Paweł Migdał, Adam Junka, and Karol Fijałkowski. 2021. "Potato Juice, a Starch Industry Waste, as a Cost-Effective Medium for the Biosynthesis of Bacterial Cellulose" International Journal of Molecular Sciences 22, no. 19: 10807. https://doi.org/10.3390/ijms221910807
APA StyleCiecholewska-Juśko, D., Broda, M., Żywicka, A., Styburski, D., Sobolewski, P., Gorący, K., Migdał, P., Junka, A., & Fijałkowski, K. (2021). Potato Juice, a Starch Industry Waste, as a Cost-Effective Medium for the Biosynthesis of Bacterial Cellulose. International Journal of Molecular Sciences, 22(19), 10807. https://doi.org/10.3390/ijms221910807