Research Progress of Mitochondrial Mechanism in NLRP3 Inflammasome Activation and Exercise Regulation of NLRP3 Inflammasome
Abstract
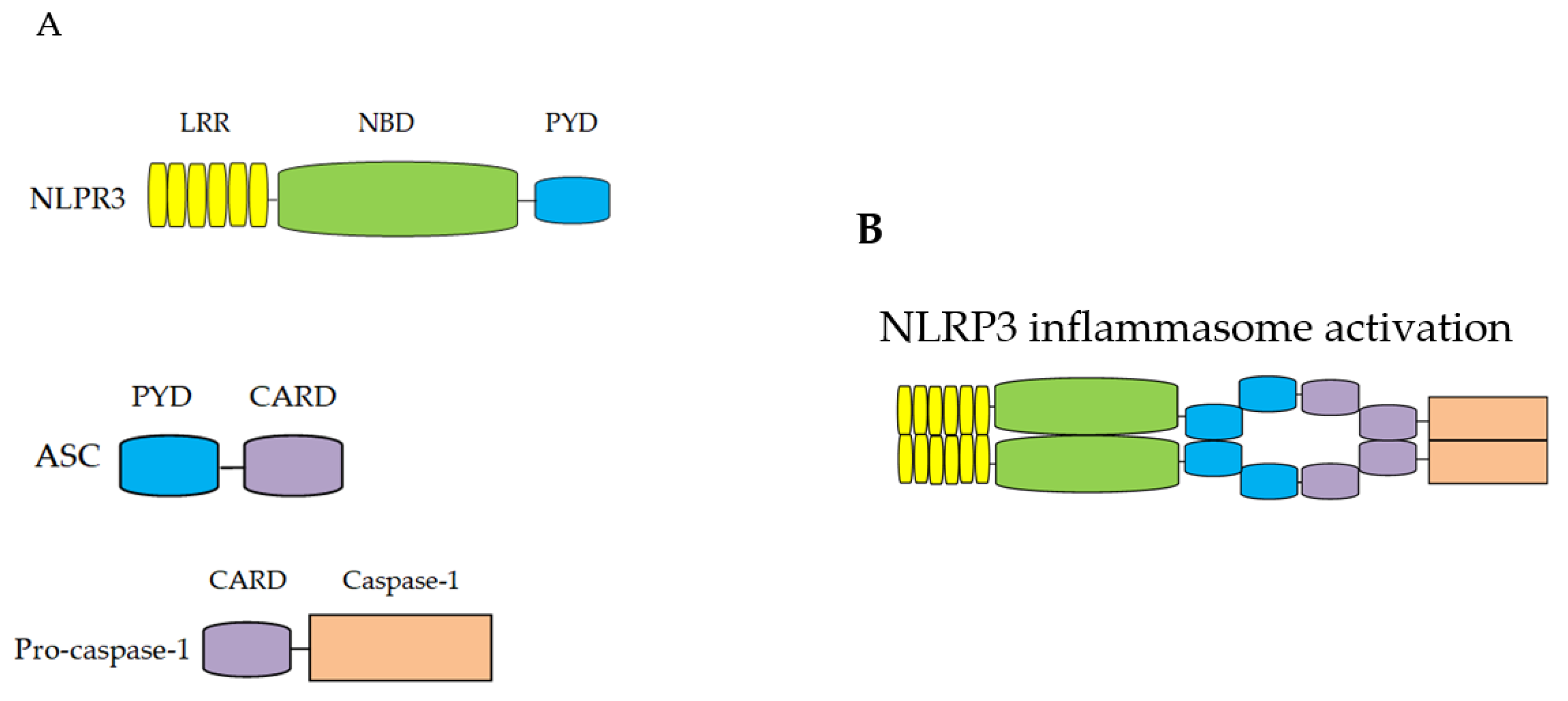
:1. NLRP3 Inflammasome
1.1. Overview of NLRP3 Inflammasome
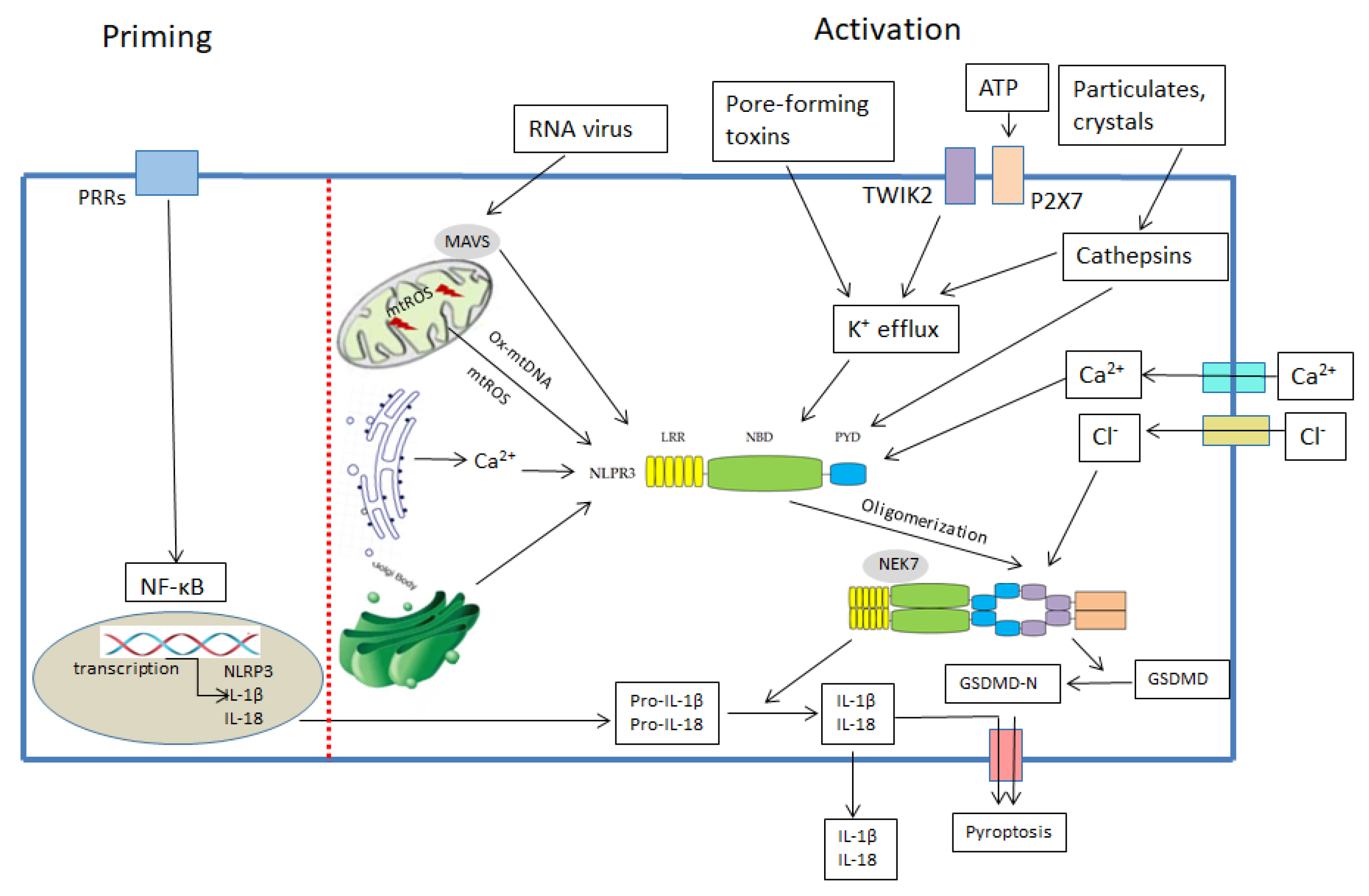
1.2. The Mechanism for NLRP3 Inflammasome Activation
1.2.1. MtDNA and NLRP3 Inflammasome
1.2.2. MtROS and NLRP3 Inflammasome
2. Effect of Exercise on NLRP3 Inflammasome Activation
2.1. Classification of Exercise
2.2. Endurance Training and NLRP3 Inflammasome
2.3. Resistance Training and NLRP3 Inflammasome
2.4. Endurance Training Combined with Resistance Training, NLRP3 Inflammasome
2.5. HIIT and NLRP3 Inflammasome
2.6. Acute Exercise and NLRP3 Inflammasome
2.7. Exercise Preconditioning and NLRP3 Inflammasome
3. Exercise, Mitochondria and NLRP3 Inflammasome
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Asher, J.L.; Molony, R.D.; Shaw, A.C.; Zeiss, C.J.; Wang, C.; Morozova-Roche, L.A.; Herzog, R.I.; Iwasaki, A.; Dixit, V.D. beta-Hydroxybutyrate Deactivates Neutrophil NLRP3 Inflammasome to Relieve Gout Flares. Cell Rep. 2017, 18, 2077–2087. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [Green Version]
- McAllister, M.J.; Chemaly, M.; Eakin, A.J.; Gibson, D.S.; McGilligan, V.E. NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarthr. Cartil. 2018, 26, 612–619. [Google Scholar] [CrossRef]
- Theivanthiran, B.; Evans, K.S.; DeVito, N.C.; Plebanek, M.; Sturdivant, M.; Wachsmuth, L.P.; Salama, A.K.; Kang, Y.; Hsu, D.; Balko, J.M.; et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J. Clin. Investig. 2020, 130, 2570–2586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamarsheh, S.; Zeiser, R. NLRP3 Inflammasome Activation in Cancer: A Double-Edged Sword. Front Immunol. 2020, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Xu, S.; Ma, Y.; Liu, G.; Jang, H.; Fang, J. Modulatory Mechanisms of the NLRP3 Inflammasomes in Diabetes. Biomolecules 2019, 9, 850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J.; et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: Implications for Parkinson disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. NAT REV Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Grzywa, R.H. The role of NOD-like receptors (NLRs) in the pathogenesis of metabolic diseases. Postepy. Biochem. 2017, 63, 205–209. [Google Scholar]
- Rovira-Llopis, S.; Apostolova, N.; Banuls, C.; Muntane, J.; Rocha, M.; Victor, V.M. Mitochondria, the NLRP3 Inflammasome, and Sirtuins in Type 2 Diabetes: New Therapeutic Targets. Antioxid. Redox Signal 2018, 29, 749–791. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Dick, M.S.; Sborgi, L.; Ruhl, S.; Hiller, S.; Broz, P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat. Commun. 2016, 7, 11929. [Google Scholar] [CrossRef] [Green Version]
- Boucher, D.; Monteleone, M.; Coll, R.C.; Chen, K.W.; Ross, C.M.; Teo, J.L.; Gomez, G.A.; Holley, C.L.; Bierschenk, D.; Stacey, K.J.; et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J. Exp. Med. 2018, 215, 827–840. [Google Scholar] [CrossRef]
- McKee, C.M.; Coll, R.C. NLRP3 inflammasome priming: A riddle wrapped in a mystery inside an enigma. J. Leukoc. Biol. 2020, 108, 937–952. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Planillo, R.; Kuffa, P.; Martinez-Colon, G.; Smith, B.L.; Rajendiran, T.M.; Nunez, G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, H.; Bording-Jorgensen, M.; Chan, R.; Wine, E. Nigericin Promotes NLRP3-Independent Bacterial Killing in Macrophages. Front. Immunol. 2019, 10, 2296. [Google Scholar] [CrossRef] [Green Version]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1beta secretion in response to ATP. Nat. Commun. 2016, 7, 10555. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Li, C.; Chen, M.; He, X.; Ouyang, D. A mini-review onion fluxes that regulate NLRP3 inflammasome activation. Acta. Biochim. Biophys. Sin. 2021, 53, 131–139. [Google Scholar] [CrossRef]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Lang, X.; Xu, C.; Wang, X.; Gong, T.; Yang, Y.; Cui, J.; Bai, L.; Wang, J.; Jiang, W.; et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 2017, 8, 202. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 2018, 564, 71–76. [Google Scholar] [CrossRef]
- Katsnelson, M.A.; Lozada-Soto, K.M.; Russo, H.M.; Miller, B.A.; Dubyak, G.R. NLRP3 inflammasome signaling is activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: Roles for K+ efflux and Ca2+ influx. Am. J. Physiol. Cell Physiol. 2016, 311, C83–C100. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.M.; O’Neill, L. Metabolic regulation of NLRP3. Immunol. Rev. 2018, 281, 88–98. [Google Scholar] [CrossRef]
- Hillen, H.S.; Morozov, Y.I.; Sarfallah, A.; Temiakov, D.; Cramer, P. Structural Basis of Mitochondrial Transcription Initiation. Cell 2017, 171, 1072–1081.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.; Lee, S.J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [Google Scholar] [CrossRef]
- Yan, C.; Duanmu, X.; Zeng, L.; Liu, B.; Song, Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells 2019, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Gaumer, S.; Guenal, I.; Brun, S.; Theodore, L.; Mignotte, B. Bcl-2 and Bax mammalian regulators of apoptosis are functional in Drosophila. Cell Death Differ. 2000, 7, 804–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renault, T.T.; Floros, K.V.; Chipuk, J.E. BAK/BAX activation and cytochrome c release assays using isolated mitochondria. Methods 2013, 61, 146–155. [Google Scholar] [CrossRef] [Green Version]
- McArthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; San, C.H.; et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 2018, 359, 6378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, J.S.; Quarato, G.; Cloix, C.; Lopez, J.; O’Prey, J.; Pearson, M.; Chapman, J.; Sesaki, H.; Carlin, L.M.; Passos, J.F.; et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 2018, 37, e99238. [Google Scholar] [CrossRef]
- Kim, J.; Gupta, R.; Blanco, L.P.; Yang, S.; Shteinfer-Kuzmine, A.; Wang, K.; Zhu, J.; Yoon, H.E.; Wang, X.; Kerkhofs, M.; et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 2019, 366, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Cruz, C.M.; Rinna, A.; Forman, H.J.; Ventura, A.L.; Persechini, P.M.; Ojcius, D.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol. Chem. 2007, 282, 2871–2879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.; Brickey, W.J.; Ting, J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Jabaut, J.; Ather, J.L.; Taracanova, A.; Poynter, M.E.; Ckless, K. Mitochondria-targeted drugs enhance Nlrp3 inflammasome-dependent IL-1beta secretion in association with alterations in cellular redox and energy status. Free Radic. Biol. Med. 2013, 60, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.S.; He, Q.; Janczy, J.R.; Elliott, E.I.; Zhong, Z.; Olivier, A.K.; Sadler, J.J.; Knepper-Adrian, V.; Han, R.; Qiao, L.; et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 2013, 39, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichinohe, T.; Yamazaki, T.; Koshiba, T.; Yanagi, Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc. Natl. Acad. Sci. USA 2013, 110, 17963–17968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Mardare, C.; Kruger, K.; Liebisch, G.; Seimetz, M.; Couturier, A.; Ringseis, R.; Wilhelm, J.; Weissmann, N.; Eder, K.; Mooren, F.C. Endurance and Resistance Training Affect High Fat Diet-Induced Increase of Ceramides, Inflammasome Expression, and Systemic Inflammation in Mice. J Diabetes. Res. 2016, 2016, 4536470. [Google Scholar] [CrossRef] [Green Version]
- ZhuGe, D.L.; Javaid, H.; Sahar, N.E.; Zhao, Y.Z.; Huh, J.Y. Fibroblast growth factor 2 exacerbates inflammation in adipocytes through NLRP3 inflammasome activation. Arch. Pharm. Res. 2020, 43, 1311–1324. [Google Scholar] [CrossRef]
- Yang, W.; Liu, L.; Wei, Y.; Fang, C.; Liu, S.; Zhou, F.; Li, Y.; Zhao, G.; Guo, Z.; Luo, Y.; et al. Exercise suppresses NLRP3 inflammasome activation in mice with diet-induced NASH: A plausible role of adropin. Lab. Investig. 2021, 101, 369–380. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, L.; Ji, B.; Li, L.; Qi, Z.; Ding, S. Endurance training but not high-intensity interval training reduces liver carcinogenesis in mice with hepatocellular carcinogen diethylnitrosamine. Exp. Gerontol. 2020, 133, 110853. [Google Scholar] [CrossRef]
- Ma, M.; Chen, W.; Hua, Y.; Jia, H.; Song, Y.; Wang, Y. Aerobic exercise ameliorates cardiac hypertrophy by regulating mitochondrial quality control and endoplasmic reticulum stress through M2 AChR. J. Cell. Physiol. 2021, 236, 6581–6596. [Google Scholar] [CrossRef]
- Hong, J.; Park, E.; Lee, J.; Lee, Y.; Rooney, B.V.; Park, Y. Exercise training mitigates ER stress and UCP2 deficiency-associated coronary vascular dysfunction in atherosclerosis. Sci. Rep. 2021, 11, 15449. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Wang, K.; Liang, X.; Wang, W.; Hu, X.; Huang, Z.; Wang, Y. Aerobic Exercise Ameliorates Myocardial Inflammation, Fibrosis and Apoptosis in High-Fat-Diet Rats by Inhibiting P2X7 Purinergic Receptors. Front. Physiol. 2019, 10, 1286. [Google Scholar] [CrossRef]
- Cai, M.; Wang, H.; Li, J.J.; Zhang, Y.L.; Xin, L.; Li, F.; Lou, S.J. The signaling mechanisms of hippocampal endoplasmic reticulum stress affecting neuronal plasticity-related protein levels in high fat diet-induced obese rats and the regulation of aerobic exercise. Brain. Behav. Immun. 2016, 57, 347–359. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Sheng, H.; Ni, X.; Lu, J. Exercise amelioration of depression-like behavior in OVX mice is associated with suppression of NLRP3 inflammasome activation in hippocampus. Behav. Brain. Res. 2016, 307, 18–24. [Google Scholar] [CrossRef]
- Liang, F.; Huang, T.; Li, B.; Zhao, Y.; Zhang, X.; Xu, B. High-intensity interval training and moderate-intensity continuous training alleviate beta-amyloid deposition by inhibiting NLRP3 inflammasome activation in APPswe/PS1dE9 mice. Neuroreport 2020, 31, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, X.; Wang, Z.; Wang, Y.; Luo, L.; Cheng, J.; Chen, S.F.; Liu, H.; Wan, Q.; Wang, Q. Exercise ameliorates post-stroke depression by inhibiting PTEN elevation-mediated upregulation of TLR4/NF-kappaB/NLRP3 signaling in mice. Brain. Res. 2020, 1736, 146777. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.M.; Camargo, A.; Wolin, I.; Kaster, M.P.; Rodrigues, A. Physical exercise prevents amyloid beta1–40-induced disturbances in NLRP3 inflammasome pathway in the hippocampus of mice. Metab. Brain. Dis. 2021, 36, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Liu, B.; Li, F.; Hu, J.; Wang, Q.; Li, M.; Lou, S. Mechanisms of Aerobic Exercise Upregulating the Expression of Hippocampal Synaptic Plasticity-Associated Proteins in Diabetic Rats. Neural. Plast. 2019, 2019, 7920540. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lv, Z.; Gao, J.; Liu, M.; Wang, Y.; Tang, C.; Xiang, J. Treadmill exercise alleviates neuronal damage by suppressing NLRP3 inflammasome and microglial activation in the MPTP mouse model of Parkinson’s disease. Brain. Res. Bull. 2021, 174, 349–358. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Liu, Y.; Li, J.; Liu, B.; Li, M.; Lou, S. Aerobic Exercise Improves Synaptic-Related Proteins of Diabetic Rats by Inhibiting FOXO1/NF-kappaB/NLRP3 Inflammatory Signaling Pathway and Ameliorating PI3K/Akt Insulin Signaling Pathway. J. Mol. Neurosci. 2019, 69, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Khakroo, A.I.; Rahmani-Nia, F.; Lombardi, G. The Effects of Acute and Chronic Aerobic Activity on the Signaling Pathway of the Inflammasome NLRP3 Complex in Young Men. Medicina 2019, 55, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Wang, H.; Wang, Y.; Li, H.; Ji, L. Metabolic factors-triggered inflammatory response drives antidepressant effects of exercise in CUMS rats. Psychiatry. Res. 2015, 228, 257–264. [Google Scholar] [CrossRef]
- Bai, Y.; Feng, Y.; Jiang, B.; Yang, Y.; Pei, Z.; Yang, Q.; Cui, Y. The Role of Exercise in Reducing Hyperlipidemia-Induced Neuronal Damage in Apolipoprotein E-Deficient Mice. Biomed. Res. Int. 2021, 2021, 5512518. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, Y.; LaVoy, E.C.; Umetani, M.; Hong, J.; Park, Y. Physical activity protects NLRP3 inflammasome-associated coronary vascular dysfunction in obese mice. Physiol. Rep. 2018, 6, e13738. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Bi, X.; Hu, C.; Ding, F.; Ding, W. Therapeutic Approaches in Mitochondrial Dysfunction, Inflammation, and Autophagy in Uremic Cachexia: Role of Aerobic Exercise. Mediat. Inflamm. 2019, 2019, 2789014. [Google Scholar] [CrossRef]
- Mejias-Pena, Y.; Estebanez, B.; Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; Almar, M.; de Paz, J.A.; Gonzalez-Gallego, J.; Cuevas, M.J. Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging 2017, 9, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, H.; Byrkjeland, R.; Njerve, I.U.; Akra, S.; Solheim, S.; Arnesen, H.; Seljeflot, I.; Opstad, T.B. Effects of exercise training on inflammasome-related mediators and their associations to glucometabolic variables in patients with combined coronary artery disease and type 2 diabetes mellitus: Sub-study of a randomized control trial. Diab. Vasc. Dis. Res. 2019, 16, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Quiroga, R.; Nistal, E.; Estebanez, B.; Porras, D.; Juarez-Fernandez, M.; Martinez-Florez, S.; Garcia-Mediavilla, M.V.; de Paz, J.A.; Gonzalez-Gallego, J.; Sanchez-Campos, S.; et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef]
- Comassi, M.; Santini, E.; Rossi, C.; Vitolo, E.; Seghieri, M.; Tocchini, L.; Franzoni, F.; Solini, A. The level of physical training modulates cytokine levels through P2X7 receptor in healthy subjects. Eur. J. Clin. Investig. 2018, 48, e12880. [Google Scholar] [CrossRef]
- Li, Y.; Xu, P.; Wang, Y.; Zhang, J.; Yang, M.; Chang, Y.; Zheng, P.; Huang, H.; Cao, X. Different Intensity Exercise Preconditions Affect Cardiac Function of Exhausted Rats through Regulating TXNIP/TRX/NF-kBp65/NLRP3 Inflammatory Pathways. Evid. Based Complement Alternat. Med. 2020, 2020, 5809298. [Google Scholar] [PubMed]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef]
- Rothschild, J.A.; Bishop, D.J. Effects of Dietary Supplements on Adaptations to Endurance Training. Sports Med. 2020, 50, 25–53. [Google Scholar] [CrossRef] [Green Version]
- Lixandrao, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceicao, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Ashton, R.E.; Tew, G.A.; Aning, J.J.; Gilbert, S.E.; Lewis, L.; Saxton, J.M. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: Systematic review with meta-analysis. Br. J. Sports Med. 2020, 54, 341–348. [Google Scholar]
- Maillard, F.; Pereira, B.; Boisseau, N. Effect of High-Intensity Interval Training on Total, Abdominal and Visceral Fat Mass: A Meta-Analysis. Sports Med. 2018, 48, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Miguet, M.; Fearnbach, N.S.; Metz, L.; Khammassi, M.; Julian, V.; Cardenoux, C.; Pereira, B.; Boirie, Y.; Duclos, M.; Thivel, D. Effect of HIIT versus MICT on body composition and energy intake in dietary restrained and unrestrained adolescents with obesity. Appl. Physiol. Nutr. Metab. 2020, 45, 437–445. [Google Scholar] [CrossRef]
- Ross, L.M.; Porter, R.R.; Durstine, J.L. High-intensity interval training (HIIT) for patients with chronic diseases. J. Sport Health Sci. 2016, 5, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.J.; Smith, K.J. Effects of Exercise on Vascular Function, Structure, and Health in Humans. Cold Spring Harb. Perspect. Med. 2018, 8, a029819. [Google Scholar] [CrossRef] [Green Version]
- Abdelbasset, W.K.; Alqahtani, B.A.; Elshehawy, A.A.; Tantawy, S.A.; Elnegamy, T.E.; Kamel, D.M. Examining the impacts of 12 weeks of low to moderate-intensity aerobic exercise on depression status in patients with systolic congestive heart failure-A randomized controlled study. Clinics 2019, 74, e1017. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, D.; Da, S.C.; Hill-Haas, S.; Wong, D.P.; Natali, A.J.; De Lima, J.R.; Bara, F.M.; Marins, J.J.; Garcia, E.S.; Karim, C. Heart rate monitoring in soccer: Interest and limits during competitive match play and training, practical application. J Strength. Cond. Res. 2012, 26, 2890–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, B.M.; Rossi, F.E.; Oyama, L.M.; Rosa-Neto, J.C.; Lira, F.S. Exercise intensity and physical fitness modulate lipoproteins profile during acute aerobic exercise session. Sci. Rep. 2020, 10, 4160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achten, J.; Gleeson, M.; Jeukendrup, A.E. Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 2002, 34, 92–97. [Google Scholar] [CrossRef]
- Ribeiro-Alvares, J.B.; Marques, V.B.; Vaz, M.A.; Baroni, B.M. Four Weeks of Nordic Hamstring Exercise Reduce Muscle Injury Risk Factors in Young Adults. J Strength. Cond. Res. 2018, 32, 1254–1262. [Google Scholar] [CrossRef]
- Mak, M.K.; Wong-Yu, I.S.; Shen, X.; Chung, C.L. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat. Rev. Neurol. 2017, 13, 689–703. [Google Scholar] [CrossRef]
- Kwon, S.M.; Park, H.G.; Jun, J.K.; Lee, W.L. Exercise, but not quercetin, ameliorates inflammation, mitochondrial biogenesis, and lipid metabolism in skeletal muscle after strenuous exercise by high-fat diet mice. J. Exerc. Nutr. Biochem. 2014, 18, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Lezi, E.; Burns, J.M.; Swerdlow, R.H. Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol. Aging 2014, 35, 2574–2583. [Google Scholar]
- Chen, W.K.; Tsai, Y.L.; Shibu, M.A.; Shen, C.Y.; Chang-Lee, S.N.; Chen, R.J.; Yao, C.H.; Ban, B.; Kuo, W.W.; Huang, C.Y. Exercise training augments Sirt1-signaling and attenuates cardiac inflammation in D-galactose induced-aging rats. Aging 2018, 10, 4166–4174. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.; Lim, W.; Kim, T.; Kang, C. Strenuous exercise induces mitochondrial damage in skeletal muscle of old mice. Biochem. Biophys. Res. Commun. 2015, 461, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, R.; Grant, A.R.; Zhang, J.; Gordon, P.M.; Wei, Y.; Chen, P. Immune adaptation to chronic intense exercise training: New microarray evidence. BMC Genom. 2017, 18, 29. [Google Scholar] [CrossRef] [Green Version]
- Gan, Z.; Fu, T.; Kelly, D.P.; Vega, R.B. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell. Res. 2018, 28, 969–980. [Google Scholar] [CrossRef]
- Memme, J.M.; Erlich, A.T.; Phukan, G.; Hood, D.A. Exercise and mitochondrial health. J. Physiol. 2021, 599, 803–817. [Google Scholar] [CrossRef]
- Salo, D.C.; Donovan, C.M.; Davies, K.J. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic. Biol. Med. 1991, 11, 239–246. [Google Scholar] [CrossRef]
- de Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simoes, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox. Biol. 2020, 35, 101471. [Google Scholar] [CrossRef] [PubMed]

| Object | Exercise Type/Intensity/Time | Changes of NLRP3 Inflammasome after Exercise | Reference |
|---|---|---|---|
| Human | Calorie restriction and exercise, 1 year | The mRNA expressions of NLRP3 and IL-1β in subcutaneous fat of T2DM patients were decreased | [48] |
| Endurance training | |||
| Mouse | Treadmill training, 80% VO2max, 30 min/d, 5 d/w, 10 weeks. | The mRNA expressions of IL-1β and IL-18 were decreased in adipose tissue of HFD mice | [49] |
| Mouse | Treadmill training, 70% VO2max, 20–50 min/d, 5 d/w, 8 weeks | The protein expressions of NLRP3 in epididymis and subcutaneous fat of HFD nice were decreased | [50] |
| Mouse | Treadmill training, 12 m/min, 1 h/d, 5 d/w, 12 and 8 weeks | Serum IL-1β, the hepatic mRNA and protein expressions of NLRP3, ASC, caspase-1 and IL-1β were decreased in NASH mice | [51] |
| Mouse | Treadmill training, 13 m/min, 40 min/d, 5 d/w, 18 weeks | The hepatic mRNA expression of IL-1β was decreased, whereas NLRP3 mRNA expression was increased in mice with DEN damage | [52] |
| Mouse | Treadmill training, 12–15 m/min, 1 h/d, 5 d/w, 8 weeks | The protein expressions of NLRP3, caspase-1 and IL-1β were decreased in myocardium of mice with myocardial hypertrophy. | [53] |
| Mouse | Treadmill training, 15 m/min, 1 h/d, 5 d/w, 15–16 weeks | The overactivation of NLRP3 inflammasome signaling was inhibited in myocardium of ApoE−/− mice | [54] |
| Rat | Treadmill training, from 5 m/min to 10 m/min, 1 h/d, 7 d/w, 12 weeks | Serum IL-1β, the mRNA and protein expressions of NLRP3 and caspase-1 in myocardium of rats were decreased | [55] |
| Mouse | Treadmill training, 18 m/min, 40 min/d, 5 d/w, 8 weeks | The protein expressions of NLRP3 and IL-1β were decreased in hippocampus of HFD mice | [56] |
| Mouse | Treadmill training, 15 m/min, 1 h/d, 4 weeks | The mRNA expression of NLRP3 and protein expressions of IL-1β, IL-18, caspase-1 and NLRP3 were decreased in hippocampus of ovariectomized mice | [57] |
| Mouse | treadmill training, 60% Smax, 5 d/w, 12 weeks | The protein expressions of NLRP3, ASC, IL-1β and caspase-1 were decreased in hippocampus of APP/PS1 mice | [58] |
| Mouse | Treadmill training, 80% SLT, 7 d/w, 4 weeks | The protein expression of NLRP3 was decreased in hippocampus of mice with depression | [59] |
| Mouse | Treadmill training, from 6 m/min to 12 m/min, 40 min/d, 5 d/w, 4 weeks | The protein expression of NLRP3 and the ratio of caspase-1/pro-caspase-1 were decreased in hippocampus of mice | [60] |
| Rat | Treadmill training, from 15 m/min, 30 min/d to 20 m/min, 90 min/d, 6 d/w, 4 weeks | The protein expressions of NLRP3 and IL-1β were decreased in hippocampus of T2DM rats | [61] |
| Mouse | Treadmill training, 15 m/min, 1 h/d, 5 d/w, 6 weeks | The overactivation of NLRP3 signaling was decreased in substantia nigra of PD mice | [62] |
| Rat | Treadmill training, from 15 m/min, 30 min/d to 20 m/min, 90 min/d, 5 d/w, 4 weeks | The protein expression of NLRP3 was decreased in prefrontal cortex of T2DM rats | [63] |
| Healthy young man | Running, Moderate-intensity: High-intensity: 3 d/w, 3 months | The protein expression of NLRP3 and serum IL-1β, IL-18 were decreased after moderate-intensity exercise, while increased after high-intensity exercise | [64] |
| Rat | Swimming, 1 h/d, 5 d/w, 4 weeks | The protein expression of NLRP3 was decreased in prefrontal cortex of rat with depression | [65] |
| Mouse | Swimming, 40 min/d, 5 d/w, 12 weeks | The overactivation of NLRP3 signaling induced by HFD was decreased in neuronal tissue of ApoE-/- mice | [66] |
| Mouse | Voluntary wheel running, 13 weeks | The protein expression of IL-1β in myocardium of HFD mice was decreased | [67] |
| Mouse | Voluntary wheel running, 6.5 m/min, 1 h/d, 5 d/w, 8 weeks | The protein expressions of NLRP3, ASC, Caspase-1, IL-18 and IL-1β in skeletal muscle of mice with chronic kidney disease were decreased | [68] |
| Resistance training | |||
| Mouse | Ladder climbing, 3 times/d, 1 min for each interval, 5 d/w, 10 weeks | The mRNA expression of NLRP3 in adipose tissue of HFD mice was decreased | [49] |
| Elderly subjects | Cycle ergometer, 8 weeks | The protein expression of NLRP3 and the ratio of caspase-1/procaspase-1 in PBMCs of elderly subjects were decreased | [69] |
| Endurance training combined with resistance training | |||
| Adults | Endurance training combined with resistance training, 1 year | The activity of NLRP3 inflammasome in serum leucocyte and adipose tissue of adults with T2DM and CAD were unaffected | [70] |
| Obese Children | Endurance training combined with resistance training, 12 weeks | The protein expressions of NLRP3 and caspase-1 in PBMCs of obese children were decreased | [71] |
| High-intensity interval training | |||
| Mouse | Treadmill training, 85% Smax, 1.5 min; 45% Smax, 2 min, 5 d/w, 12 weeks | The protein expressions of NLRP3, IL-1β and caspase-1 in hippocampus of APP/PS1 mice were decreased | [58] |
| Mouse | Treadmill training, 60–70% Smax, 5 d/w, 4 weeks | The protein expression of NLRP3 in hippocampus of mice with post-stroke depression was decreased | [59] |
| Mouse | Treadmill training, 25 m/min, 5 d/w, 18 weeks | The hepatic mRNA expression of IL-1β was unaffected, while NLRP3 mRNA expression was increased in mouse with DEN damage | [52] |
| Acute exercise | |||
| Healthy young men | Running, moderate and high- intensity acute exercise | NLRP3 inflammasome activity in PBMCs of health young men was unaffected after moderate-intensity acute exercise, while increased after high-intensity acute exercise | [64] |
| Trained/untrained men | A single bout of maximal exercise | The expressions of NLRP3, caspase-1 and IL-1β were increased after acute exercise in untrained men, while decreased or unaffected in trained individuals. | [72] |
| Exercise preconditioning | |||
| Rat | Treadmill training, low, moderate and high-intensity, 8 weeks | The overactivation of NLRP3 inflammasome induced by high-intensity acute exercise was blunted by low, moderate and high-intensity exercise preconditioning, and moderate-intensity exercise exhibits the best effect. | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Ding, S.; Wang, R. Research Progress of Mitochondrial Mechanism in NLRP3 Inflammasome Activation and Exercise Regulation of NLRP3 Inflammasome. Int. J. Mol. Sci. 2021, 22, 10866. https://doi.org/10.3390/ijms221910866
Zhang T, Ding S, Wang R. Research Progress of Mitochondrial Mechanism in NLRP3 Inflammasome Activation and Exercise Regulation of NLRP3 Inflammasome. International Journal of Molecular Sciences. 2021; 22(19):10866. https://doi.org/10.3390/ijms221910866
Chicago/Turabian StyleZhang, Tan, Shuzhe Ding, and Ru Wang. 2021. "Research Progress of Mitochondrial Mechanism in NLRP3 Inflammasome Activation and Exercise Regulation of NLRP3 Inflammasome" International Journal of Molecular Sciences 22, no. 19: 10866. https://doi.org/10.3390/ijms221910866






