Key Properties of a Bioactive Ag-SiO2/TiO2 Coating on NiTi Shape Memory Alloy as Necessary at the Development of a New Class of Biomedical Materials
Abstract
:1. Introduction
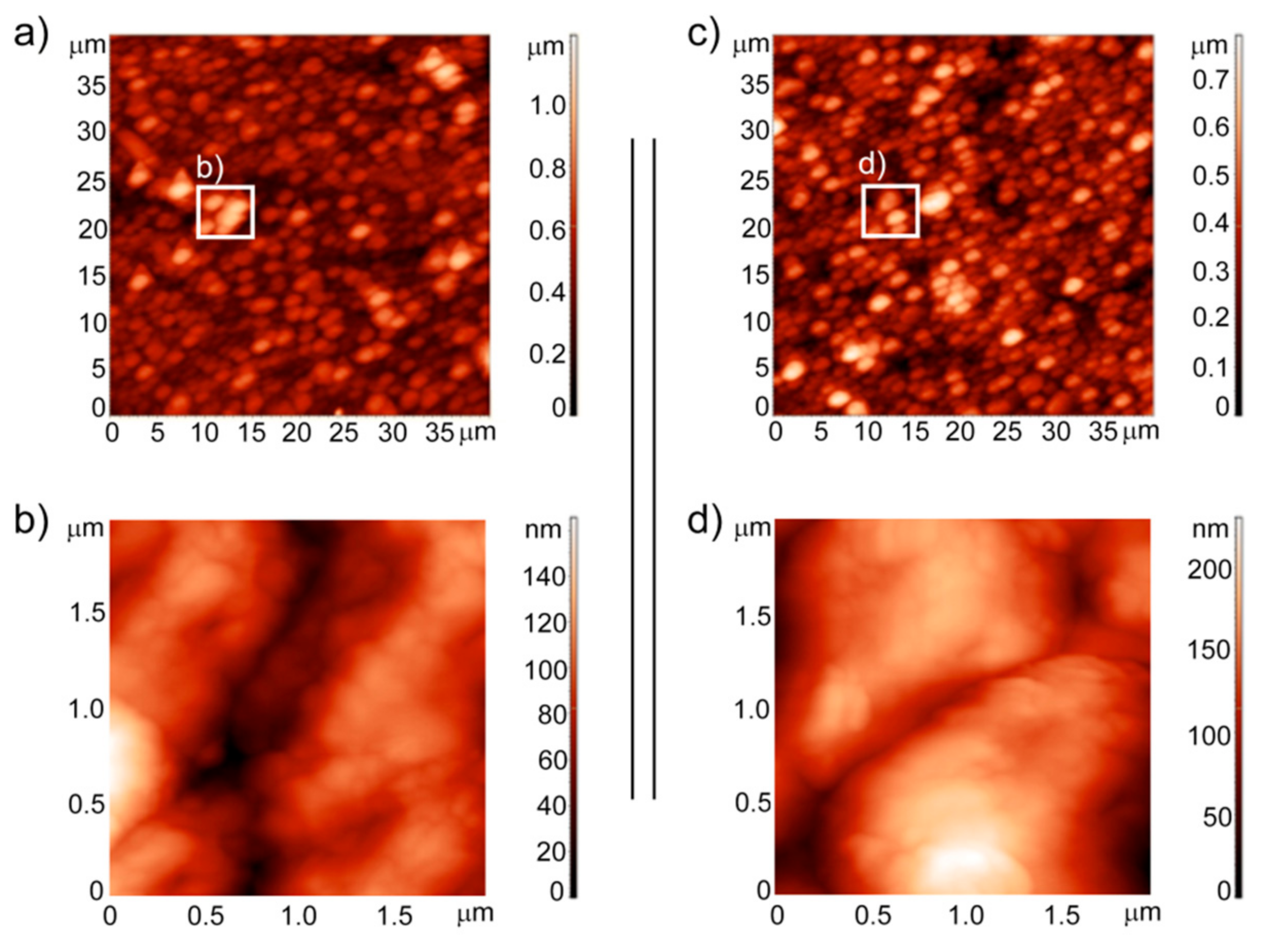
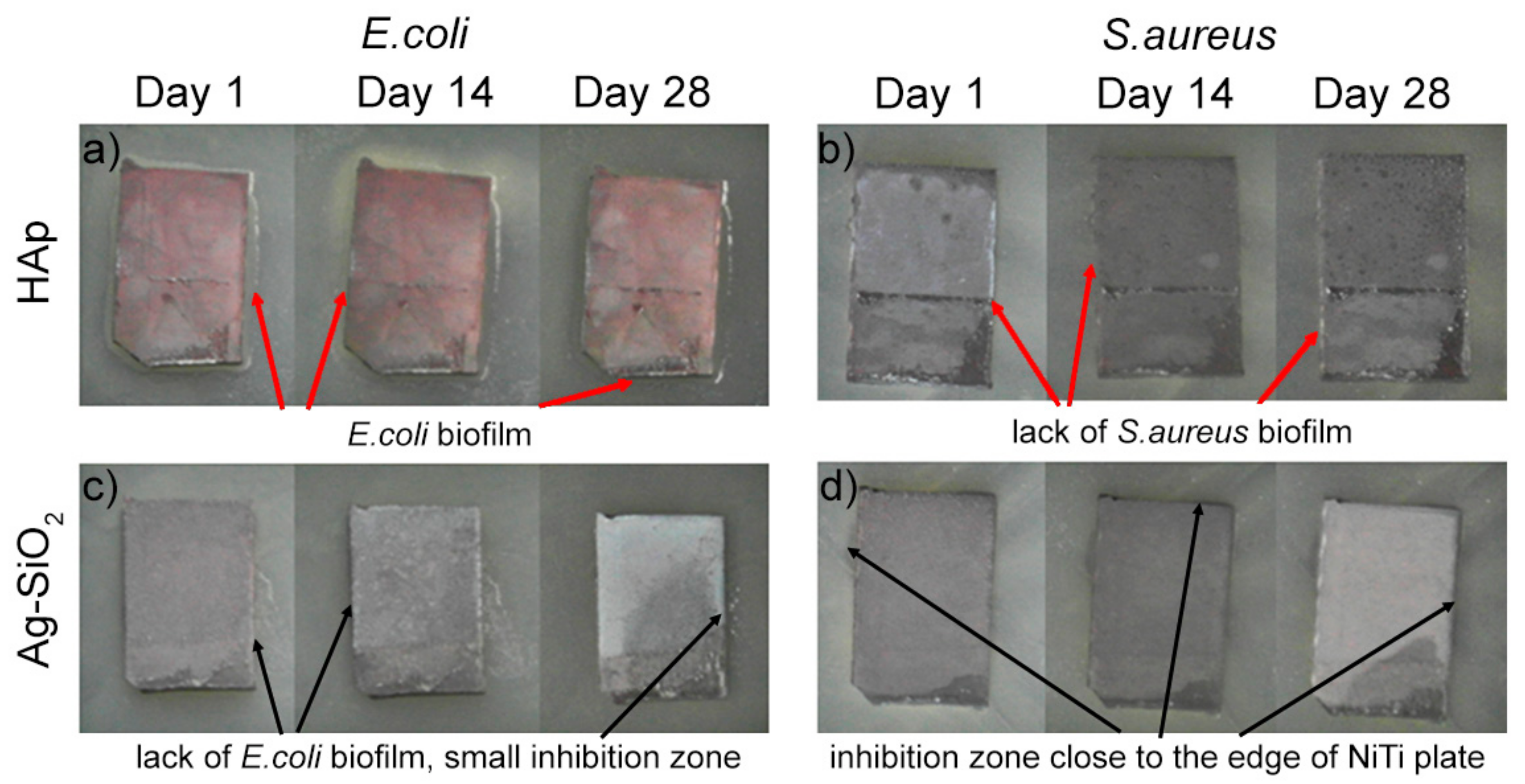
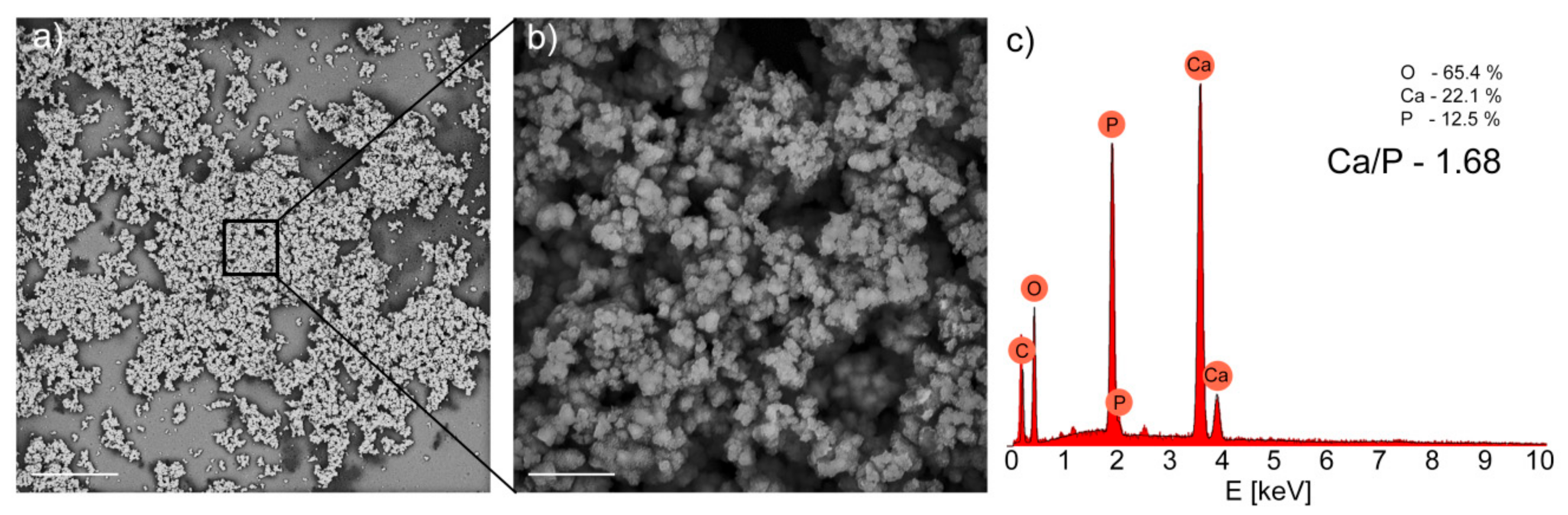
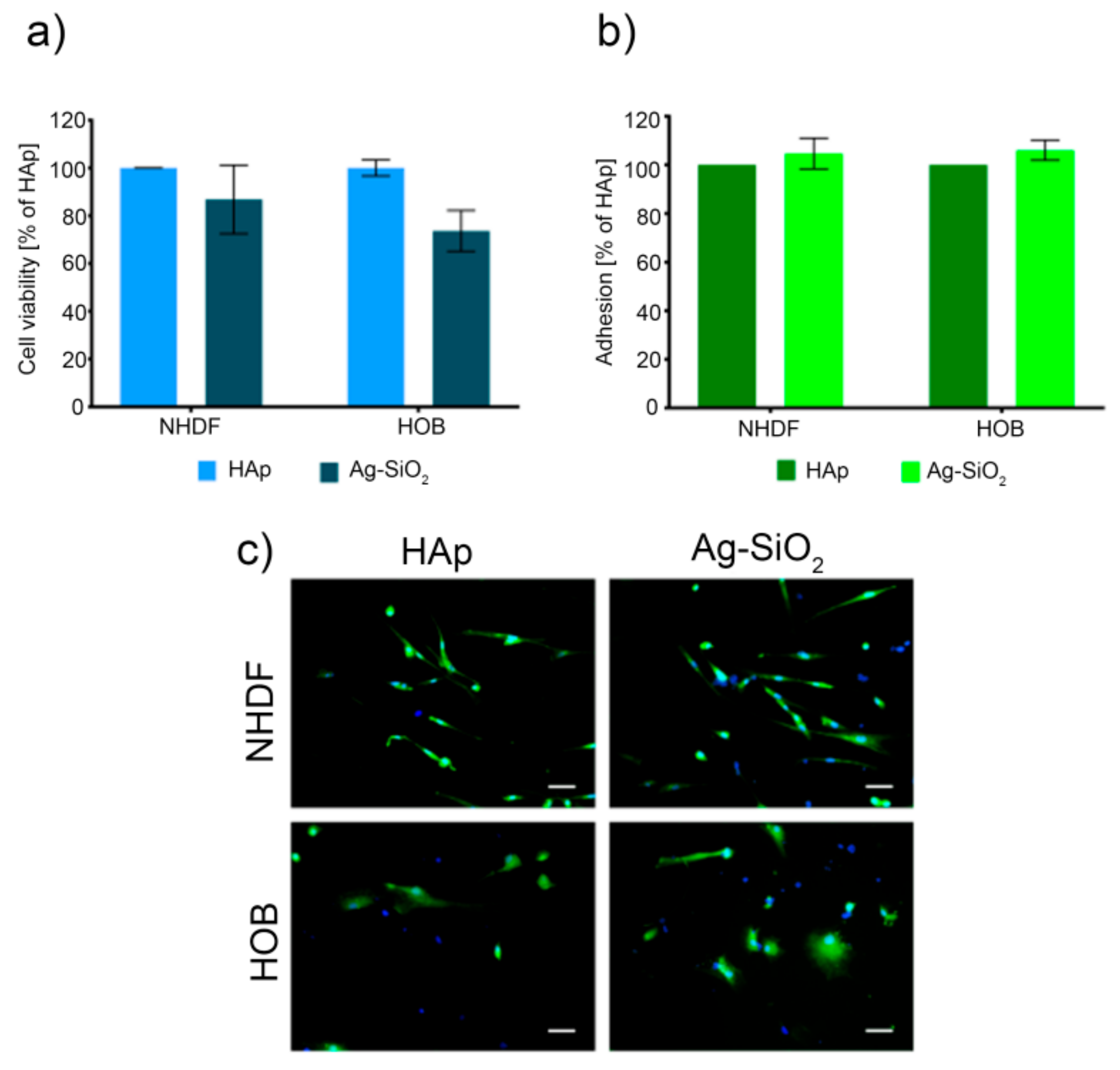
2. Results and Discussion
3. Materials and Methods
3.1. Wettability and Surface Free Energy Measurements
3.2. Tribological Tests
3.3. Analysis of the Surface Topography and Roughness Measurements
3.4. Ion Release
3.5. Immersion in Simulated Body Fluid
3.6. Zone of Inhibition Test
3.7. Cell Culture and Viability Assay
3.8. Cell Adhesion Assay and Cell Morphology
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buehler, W.J.; Gilfrich, J.V.; Wiley, R.C. Effect of Low-Temperature Phase Changes on the Mechanical Properties of Alloys near Composition TiNi. J. Appl. Phys. 1963, 34, 1475–1477. [Google Scholar] [CrossRef]
- Otsuka, K.; Ren, X. Physical metallurgy of Ti–Ni-based shape memory alloys. Prog. Mater. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef]
- Thierry, B.; Merhi, Y.; Bilodeau, L.; Trépanier, C.; Tabrizian, M. Nitinol versus stainless steel stents: Acute thrombogenicity study in an ex vivo porcine model. Biomaterials 2002, 23, 2997–3005. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.A. Shape memory alloys for medical applications. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Kallioinen, M.; Tuukkanen, J.; Junila, J.; Sandvik, P.; Serlo, W. In vivo biocompatibility evaluation of nickel-titanium shape memory metal alloy: Muscle and perineural tissue responses and encapsule membrane thickness. J. Biomed. Mater. Res. 1998, 41, 481–488. [Google Scholar] [CrossRef]
- Andani, M.T.; Saedi, S.; Turabi, A.S.; Ravari, M.R.K.; Haberland, C.; Karaca, H.; Elahinia, M. Mechanical and shape memory properties of porous Ni 50.1 Ti 49.9 alloys manufactured by selective laser melting. J. Mech. Behav. Biomed. Mater. 2017, 68, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Itin, V.; Gyunter, V.; Shabalovskaya, S.; Sachdeva, R. Mechanical properties and shape memory of porous nitinol. Mater. Charact. 1994, 32, 179–187. [Google Scholar] [CrossRef]
- Silberstein, B.M.; Gunter, V. Shape-Memory Implants in Spinal Surgery: Long-Term Results (Experimental and Clinical Studies). In Shape Memory Implants; Yahia, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 147–152. ISBN 978-3-642-59768-8. [Google Scholar]
- Bansiddhi, A.; Sargeant, T.; Stupp, S.; Dunand, D. Porous NiTi for bone implants: A review. Acta Biomater. 2008, 4, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, H.; Vogel, B.; Welle, A. Applications of shape memory alloys in medical instruments. Minim. Invasive Ther. Allied Technol. 2004, 13, 248–253. [Google Scholar] [CrossRef]
- Ayers, R.; Burkes, D.; Gottoli, G.; Yi, H.C.; Moore, J. The Application of Self-Propagating High-Temperature Synthesis of Engineered Porous Composite Biomedical Materials. Mater. Manuf. Process. 2007, 22, 481–488. [Google Scholar] [CrossRef]
- Niemi, E.; Serlo, W.; Sandvik, P.; Pernu, H.; Salo, T. Biocompatibility of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures. J. Biomed. Mater. Res. 1997, 35, 451–457. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A.; Rondelli, G.C.; Undisz, A.; Anderegg, J.W.; Burleigh, T.D.; Rettenmayr, M.E. The electrochemical characteristics of native Nitinol surfaces. Biomaterials 2009, 30, 3662–3671. [Google Scholar] [CrossRef] [PubMed]
- Es-Souni, M.; Es-Souni, M.; Fischer-Brandies, H. Assessing the biocompatibility of NiTi shape memory alloys used for medical applications. Anal. Bioanal. Chem. 2005, 381, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.; Thomas, B.; Leinenbach, C.; Eifler, D.; Minay, E.J.; Boccaccini, A.R. The electrophoretic deposition of Bioglass® particles on stainless steel and Nitinol substrates. Surf. Coatings Technol. 2006, 200, 4835–4845. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Khalil-Allafi, J.; Khalili, V. Titanium Oxide (TiO2) Coatings on NiTi Shape Memory Substrate Using Electrophoretic Deposition Process. Int. J. Eng. 2013, 26, 707–712. [Google Scholar]
- Dudek, K.; Goryczka, T. Electrophoretic deposition and characterization of thin hydroxyapatite coatings formed on the surface of NiTi shape memory alloy. Ceram. Int. 2016, 42, 19124–19132. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Peters, C.T.; Roether, J.A.; Eifler, D.; Misra, S.K.; Minay, E.J. Electrophoretic deposition of polyetheretherketone (PEEK) and PEEK/Bioglass® coatings on NiTi shape memory alloy wires. J. Mater. Sci. 2006, 41, 8152–8159. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Tantalum coated NiTi alloy by PIIID for biomedical application. Surf. Coat. Technol. 2013, 228, S2–S6. [Google Scholar] [CrossRef]
- Banerjee, P.C.; Sun, T.; Wong, J.H.W.; Wang, M. Fabrication of an Apatite/Collagen Composite Coating on the NiTi Shape Memory Alloy through Electrochemical Deposition and Coating Characterisation. Mater. Sci. Forum 2009, 618–619, 319–323. [Google Scholar] [CrossRef]
- Mirak, M.; Alizadeh, M.; Salahinejad, E.; Amini, R. Zn-HA-TiO2 nanocomposite coatings electrodeposited on a NiTi shape memory alloy. Surf. Interface Anal. 2014, 47, 176–183. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface 2010, 7, S581–S613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccaccini, A.R.; Zhitomirsky, I. Application of electrophoretic and electrolytic deposition techniques in ceramics processing. Curr. Opin. Solid State Mater. Sci. 2002, 6, 251–260. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Cathodic electrodeposition of ceramic and organoceramic materials. Fundamental aspects. Adv. Colloid Interface Sci. 2002, 97, 279–317. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Yousefpour, M.; Vali, I.; Saebnoori, E. Surface Activation of Ni-Ti Alloy by Using Electrochemical Process for Biomimetic Deposition of Hydroxyapatite Coating (TECHNICAL NOTE). Int. J. Eng. 2014, 27, 1627–1634. [Google Scholar] [CrossRef] [Green Version]
- Qiu, D.; Yang, L.; Yin, Y.; Wang, A. Preparation and characterization of hydroxyapatite/titania composite coating on NiTi alloy by electrochemical deposition. Surf. Coatings Technol. 2011, 205, 3280–3284. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Xu, R.; Wang, W.; Liu, X.; Yeung, K.W.; Chu, P.K. Electrochemically deposited chitosan/Ag complex coatings on biomedical NiTi alloy for antibacterial application. Surf. Coatings Technol. 2013, 232, 370–375. [Google Scholar] [CrossRef]
- Dulski, M.; Dudek, K.; Grelowski, M.; Kubacki, J.; Hertlein, J.; Wojtyniak, M.; Goryczka, T. Impact of annealing on features of BCP coating on NiTi shape memory alloy: Preparation and physicochemical characterization. Appl. Surf. Sci. 2018, 437, 28–40. [Google Scholar] [CrossRef]
- Yoneyama, T.; Miyazaki, S. Shape Memory Alloys for Biomedical Applications; Elsevier BV: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Nishida, M.; Wayman, C.M.; Honma, T. Precipitation processes in near-equiatomic TiNi shape memory alloys. Met. Mater. Trans. A 1986, 17, 1505–1515. [Google Scholar] [CrossRef]
- De Groot, K.; Wolke, J.G.C.; A Jansen, J. Calcium phosphate coatings for medical implants. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 137–147. [Google Scholar] [CrossRef]
- Jarcho, M. Biomaterial aspects of calcium phosphates. Properties and applications. Dent. Clin. N. Am. 1986, 30, 25–47. [Google Scholar] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate coatings, films and layers. Prog. Biomater. 2012, 1, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Luo, C.; Zhang, Z.; Hermawan, H.; Zhu, D.; Huang, J.; Liang, Y.; Lian, J.; Ren, L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 77, 90–105. [Google Scholar] [CrossRef]
- Costerton, J.; Montanaro, L.; Arciola, C. Biofilm in Implant Infections: Its Production and Regulation. Int. J. Artif. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Genet. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.; Furtos, G.; Rapuntean, S.; Horovitz, O.; Flore, C.; Garbo, C.; Danisteanu, A.; Rapuntean, G.; Prejmerean, C.; Tomoaia-Cotisel, M. Synthesis; characterization and antimicrobial effects of composites based on multi-substituted hydroxyapatite and silver nanoparticles. Appl. Surf. Sci. 2014, 298, 225–235. [Google Scholar] [CrossRef]
- Dudek, K.; Dulski, M.; Łosiewicz, B. Functionalization of the NiTi Shape Memory Alloy Surface by HAp/SiO2/Ag Hybrid Coatings Formed on SiO2-TiO2 Glass Interlayer. Materials 2020, 13, 1648. [Google Scholar] [CrossRef] [Green Version]
- Schreurs, W.J.; Rosenberg, H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J. Bacteriol. 1982, 152, 7–13. [Google Scholar]
- Bragg, P.D.; Rainnie, D.J. The effect of silver ions on the respiratory chain of Escherichia coli. Can. J. Microbiol. 1974, 20, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.D.; Hugo, W.B. Antimicrobial Activity and Action of Silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar] [PubMed]
- Dulski, M.; Dudek, K.; Chalon, D.; Kubacki, J.; Sułowicz, S.; Piotrowska-Seget, Z.; Mrozek-Wilczkiewicz, A.; Gawecki, R.; Nowak, A. Toward the Development of an Innovative Implant: NiTi Alloy Functionalized by Multifunctional β-TCP+Ag/SiO2 Coatings. ACS Appl. Bio Mater. 2019, 2, 987–998. [Google Scholar] [CrossRef]
- Djokić, S. (Ed.) Biomedical and Pharmaceutical Applications of Electrochemistry; Modern Aspects of Electrochemistry; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-31847-9. [Google Scholar]
- Feng, Q.L.; Cui, F.Z.; Kim, T.N.; Kim, J.W. Ag-Substituted Hydroxyapatite Coatings with Both Antimicrobial Effects and Biocompatibility. J. Mater. Sci. Lett. 1999, 18, 559–561. [Google Scholar] [CrossRef]
- Chen, W.; Oh, S.; Ong, A.; Oh, N.; Liu, Y.; Courtney, H.; Appleford, M.; Ong, J. Antibacterial and osteogenic properties of silver-containing hydroxyapatite coatings produced using a sol gel process. J. Biomed. Mater. Res. Part A 2007, 82, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Pielichowska, K.; Blazewicz, S. Bioactive Polymer/Hydroxyapatite (Nano)composites for Bone Tissue Regeneration. In Adv Polym SciIn Biopolymers: Lignin, Proteins, Bioactive Nanocomposites; Abe, A., Dusek, K., Kobayashi, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 97–207. ISBN 978-3-642-13630-6. [Google Scholar]
- Glocker, D.; Ranade, S. (Eds.) Medical Coatings and Deposition Technologies; Wiley-Scrivener: Hoboken, NJ, USA; Salem, MA, USA, 2016; ISBN 978-1-118-03194-0. [Google Scholar]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Lee, J.N.; Jiang, X.; Ryan, A.D.; Whitesides, G.M. Compatibility of Mammalian Cells on Surfaces of Poly(dimethylsiloxane). Langmuir 2004, 20, 11684–11691. [Google Scholar] [CrossRef]
- Peltola, T.; Jokinen, M.J.; Veittola, S.; Rahiala, H.; Yliurpo, A. Influence of sol and stage of spinnability on in vitro bioactivity and dissolution of sol–gel-derived SiO2 fibers. Biomaterials 2001, 22, 589–598. [Google Scholar] [CrossRef]
- Akkopru, B.; Durucan, C. Preparation and microstructure of sol-gel derived silver-doped silica. J. Sol-Gel Sci. Technol. 2007, 43, 227–236. [Google Scholar] [CrossRef]
- Durucan, C.; Akkopru, B. Effect of calcination on microstructure and antibacterial activity of silver-containing silica coatings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 448–458. [Google Scholar] [CrossRef]
- Shah, A.T.; Attique, S.; Rehman, M.A.U.; Khan, A.S.; Goerke, O. Silica-based antibacterial coatings for dental implants. In Dental Implants; Elsevier BV: Amsterdam, Netherlands, 2020; pp. 145–171. [Google Scholar]
- Ferraris, S.; Perero, S.; Miola, M.; Vernè, E.; Skoglund, S.; Blomberg, E.; Wallinder, I.O. Antibacterial silver nanocluster/silica composite coatings on stainless steel. Appl. Surf. Sci. 2017, 396, 1546–1555. [Google Scholar] [CrossRef]
- Massa, M.A.; Covarrubias, C.; Bittner, M.; Fuentevilla, I.A.; Capetillo, P.; Von Marttens, A.; Carvajal, J.C. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater. Sci. Eng. C 2014, 45, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Dulski, M.; Goryczka, T.; Gerle, A. Structural changes of hydroxyapatite coating electrophoretically deposited on NiTi shape memory alloy. Ceram. Int. 2018, 44, 11292–11300. [Google Scholar] [CrossRef]
- Dudek, K.; Plawecki, M.; Dulski, M.; Kubacki, J. Multifunctional layers formation on the surface of NiTi SMA during β-tricalcium phosphate deposition. Mater. Lett. 2015, 157, 295–298. [Google Scholar] [CrossRef]
- Dudek, K.; Szaraniec, B.; Lelątko, J.; Goryczka, T. Structure of Multi-Layers Deposited on NiTi Shape Memory Alloy. Solid State Phenom. 2013, 203–204, 90–93. [Google Scholar] [CrossRef]
- Dulski, M.; Balcerzak, J.; Simka, W.; Dudek, K. Innovative Bioactive Ag-SiO2/TiO2 Coating on a NiTi Shape Memory Alloy: Structure and Mechanism of Its Formation. Materials 2021, 14, 99. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Arias, L.S.; Fernandes, R.A.; Straioto, F.G.; Barbosa, D.B.; Pessan, J.P.; Delbem, A.C.B. Role of tyrosol on Candida albicans, Candida glabrata and Streptococcus mutans biofilms developed on different surfaces. Am. J. Dent. 2017, 30, 35–39. [Google Scholar]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Rashid, H. The effect of surface roughness on ceramics used in dentistry: A review of literature. Eur. J. Dent. 2014, 8, 571–579. [Google Scholar] [CrossRef]
- Dobrzyński, M.; Pajaczkowska, M.; Nowicka, J.; Jaworski, A.; Kosior, P.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Bogucki, Z.A.; Filipiak, J.; et al. Study of Surface Structure Changes for Selected Ceramics Used in the CAD/CAM System on the Degree of Microbial Colonization, In Vitro Tests. BioMed Res. Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Giljean, S.; Bigerelle, M.; Anselme, K. Roughness statistical influence on cell adhesion using profilometry and multiscale analysis. Scanning 2012, 36, 2–10. [Google Scholar] [CrossRef]
- Richards, R. The effect of surface roughness on fibroblast adhesion in vitro. Injury 1996, 27, S/C38–S/C43. [Google Scholar] [CrossRef]
- Biazar, E. The relationship between cellular adhesion and surface roughness in polystyrene modified by microwave plasma radiation. Int. J. Nanomed. 2011, 6, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zreiqat, H.; Standard, O.C.; Gengenbach, T.; Steele, J.G.; Howlett, C.R. The Role of Surface Characteristics in the Initial Adhesion of Human Bone-Derived Cells on Ceramics. Cells Mater. 1996, 6, 45–56. [Google Scholar]
- Hollister, S. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [Green Version]
- Sansone, V. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Mol. Ecol. Evol. Approaches Appl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [Green Version]
- Lansdown, A.B. Silver in Health Care: Antimicrobial Effects and Safety in Use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. 2), S96–S101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Zheng, Y. A Study of ZrN/Zr Coatings Deposited on NiTi Alloy by PIIID Technique. IEEE Trans. Plasma Sci. 2006, 34, 1105–1108. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.A.; Fadl-Allah, S.A.; El-Bagoury, N.; El-Rab, S.M.G. Improvement of corrosion resistance and antibacterial effect of NiTi orthopedic materials by chitosan and gold nanoparticles. Appl. Surf. Sci. 2014, 292, 390–399. [Google Scholar] [CrossRef]
- Schrader, M.E. Young-Dupre Revisited. Langmuir 1995, 11, 3585–3589. [Google Scholar] [CrossRef]
- Busscher, H.; De Jong, H.; Arends, J. Surface free energies of hydroxyapatite, fluorapatite and calcium fluoride. Mater. Chem. Phys. 1987, 17, 553–558. [Google Scholar] [CrossRef]
- Batory, D.; Gorzędowski, J.; Kołodziejczyk, Ł.; Szymański, W. Modyfikacja powłok DLC metodą implantacji jonów srebra. Eng. Biomater. 2011, 14, 5–12. [Google Scholar]
- Razali, M.F.; Mahmud, A.S.; Mokhtar, N.; Abdullah, J. Influence of sliding friction on leveling force of superelastic NiTi arch wire: A computational analysis. In Proceedings of the 2nd International Conference on Applied Science and Technology, (ICAST’17) 2017, Kedah, Malaysia, 3–5 April 2017; p. 020122. [Google Scholar]
- Rossi, E.; Paroni, M.; Landini, P. Biofilm and motility in response to environmental and host-related signals in Gram negative opportunistic pathogens. J. Appl. Microbiol. 2018, 125, 1587–1602. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.C.; De Sousa, L.L.; Ricci, V.P.; Rigo, E.C.D.S.; Ramos, A.S.; Campos, M.; Mariano, N.A. Titanium Biomimetically Coated With Hydroxyapatite, Silver Nitrate and Polycaprolactone, for Use In Biomaterials (Biomedicine). Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Zhao, L.; Ashraf, A.M. Influence of Ag/HA Nanocomposite Coating on Biofilm Formation of Joint Prosthesis and Its Mechanism. West Indian Med. J. 2016, 64, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Bharati, S.; Sinha, M.K.; Basu, D. Hydroxyapatite coating by biomimetic method on titanium alloy using concentrated SBF. Bull. Mater. Sci. 2005, 28, 617–621. [Google Scholar] [CrossRef]
- Khalili, V.; Khalil-Allafi, J.; Frenzel, J.; Eggeler, G. Bioactivity and electrochemical behavior of hydroxyapatite-silicon-multi walled carbon nano-tubes composite coatings synthesized by EPD on NiTi alloys in simulated body fluid. Mater. Sci. Eng. C 2017, 71, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Messer, R.L.; Lucas, L. Evaluations of metabolic activities as biocompatibility tools: A study of individual ions’ effects on fibroblasts. Dent. Mater. 1999, 15, 1–6. [Google Scholar] [CrossRef]
- Taira, M.; Toguchi, M.S.; Hamada, Y.; Takahashi, J.; Itou, R.; Toyosawa, S.; Ijyuin, N.; Okazaki, M. Studies on cytotoxic effect of nickel ions on three cultured fibroblasts. J. Mater. Sci. Mater. Electron. 2001, 12, 373–376. [Google Scholar] [CrossRef]
- Zhang, D.; Wong, C.S.; Wen, C.; Li, Y. Cellular responses of osteoblast-like cells to 17 elemental metals. J. Biomed. Mater. Res. Part A 2017, 105, 148–158. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hieda, Y.; Kogai, Y. Effect of hydroxyapatite surface morphology on cell adhesion. Mater. Sci. Eng. C 2016, 69, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hori, N.; Ando, H.; Namba, S.; Toyama, T.; Nishimiya, N.; Yamashita, K. Surface free energy predominates in cell adhesion to hydroxyapatite through wettability. Mater. Sci. Eng. C 2016, 62, 283–292. [Google Scholar] [CrossRef]
- Ueno, H.; Inoue, M.; Okonogi, A.; Kotera, H.; Suzuki, T. Correlation between Cells-on-Chips materials and cell adhesion/proliferation focused on material’s surface free energy. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 565, 188–194. [Google Scholar] [CrossRef]
- Annamalai, M.; Gopinadhan, K.; Han, S.A.; Saha, S.; Park, H.J.; Cho, E.B.; Kumar, B.; Patra, A.; Kim, S.-W.; Venkatesan, T. Surface energy and wettability of van der Waals structures. Nanoscale 2016, 8, 5764–5770. [Google Scholar] [CrossRef] [Green Version]
| f | Adhesive Force [mN] | WCA [°] | Surface Free Energy | ||||
|---|---|---|---|---|---|---|---|
| SFE [mN/m] | D [mN/m] | P [mN/m] | |||||
| HAp | pristine | 0.415 ± 0.025 | 2.00 | 65.0 ± 1.4 | 62.2 | 44.5 | 17.7 |
| after sintering | 0.397 ± 0.007 | 1.14 | 70.6 ± 2.3 | 46.8 | 38.5 | 8.3 | |
| Ag-SiO2 | pristine | 0.424 ± 0.003 | 1.99 | 65.7 ± 2.1 | 61.0 | 47.7 | 13.3 |
| after sintering | 0.247 ± 0.012 | 1.04 | 74.8 ± 1.6 | 45.4 | 40.1 | 5.3 | |
| Duration of the Immersion [Days] | Ion Concentration [mg/L] | |
|---|---|---|
| Ni | Ti | |
| 1 | 0.10 ± 0.05 | <0.01 |
| 5 | 0.48 ± 0.20 | |
| 8 | 0.66 ± 0.20 | |
| 16 | 1.24 ± 0.77 | |
| 23 | 1.29 ± 0.80 | |
| Duration of the Immersion [Days] | Ion Concentration [mg/L] | |||||
|---|---|---|---|---|---|---|
| Ni | Ca | P | Si | Ag | Ti | |
| 1 | 0.05 ± 0.04 | 90.25 ± 0.49 | <0.05 | 0.03 ± 0.01 | <0.01 | <0.01 |
| 5 | 0.13 ± 0.05 | 104.34 ± 0.03 | 0.08 ± 0.01 | |||
| 8 | 0.15 ± 0.04 | 107.28 ± 0.16 | 0.11 ± 0.01 | |||
| 16 | 0.19 ± 0.03 | 109.40 ± 0.50 | 0.19 ± 0.02 | |||
| 23 | 0.18 ± 0.03 | 109.64 ± 0.52 | 0.20 ± 0.01 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dulski, M.; Gawecki, R.; Sułowicz, S.; Cichomski, M.; Kazek-Kęsik, A.; Wala, M.; Leśniak-Ziółkowska, K.; Simka, W.; Mrozek-Wilczkiewicz, A.; Gawęda, M.; et al. Key Properties of a Bioactive Ag-SiO2/TiO2 Coating on NiTi Shape Memory Alloy as Necessary at the Development of a New Class of Biomedical Materials. Int. J. Mol. Sci. 2021, 22, 507. https://doi.org/10.3390/ijms22020507
Dulski M, Gawecki R, Sułowicz S, Cichomski M, Kazek-Kęsik A, Wala M, Leśniak-Ziółkowska K, Simka W, Mrozek-Wilczkiewicz A, Gawęda M, et al. Key Properties of a Bioactive Ag-SiO2/TiO2 Coating on NiTi Shape Memory Alloy as Necessary at the Development of a New Class of Biomedical Materials. International Journal of Molecular Sciences. 2021; 22(2):507. https://doi.org/10.3390/ijms22020507
Chicago/Turabian StyleDulski, Mateusz, Robert Gawecki, Sławomir Sułowicz, Michal Cichomski, Alicja Kazek-Kęsik, Marta Wala, Katarzyna Leśniak-Ziółkowska, Wojciech Simka, Anna Mrozek-Wilczkiewicz, Magdalena Gawęda, and et al. 2021. "Key Properties of a Bioactive Ag-SiO2/TiO2 Coating on NiTi Shape Memory Alloy as Necessary at the Development of a New Class of Biomedical Materials" International Journal of Molecular Sciences 22, no. 2: 507. https://doi.org/10.3390/ijms22020507







