L-Arginine Exerts Excellent Anti-Stress Effects on Stress-Induced Shortened Lifespan, Cognitive Decline and Depression
Abstract
1. Introduction
2. Results
2.1. Long-Term Effect of Stress
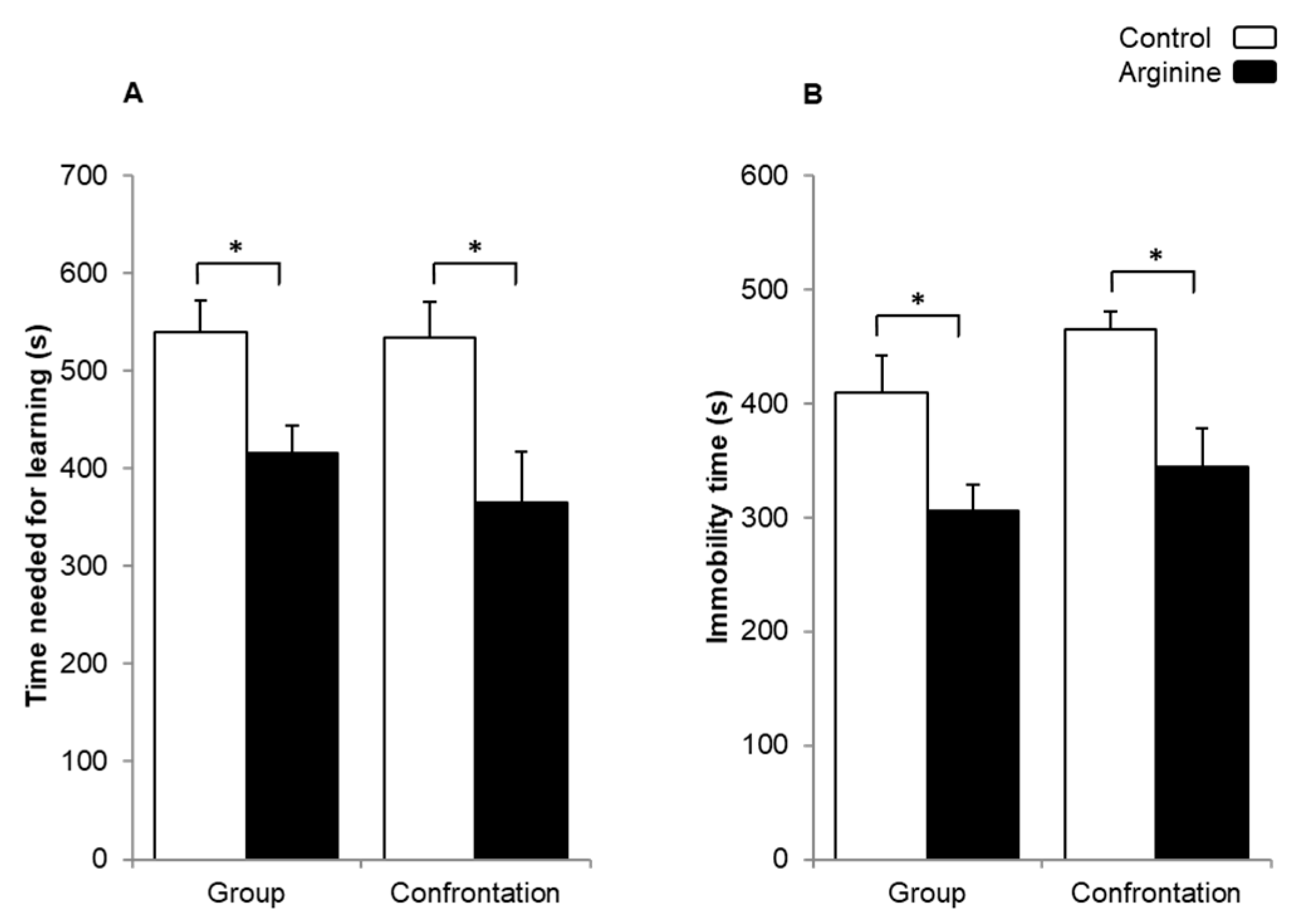
2.1.1. Improving Effect of Arg on Learning Ability and Behavioral Depression
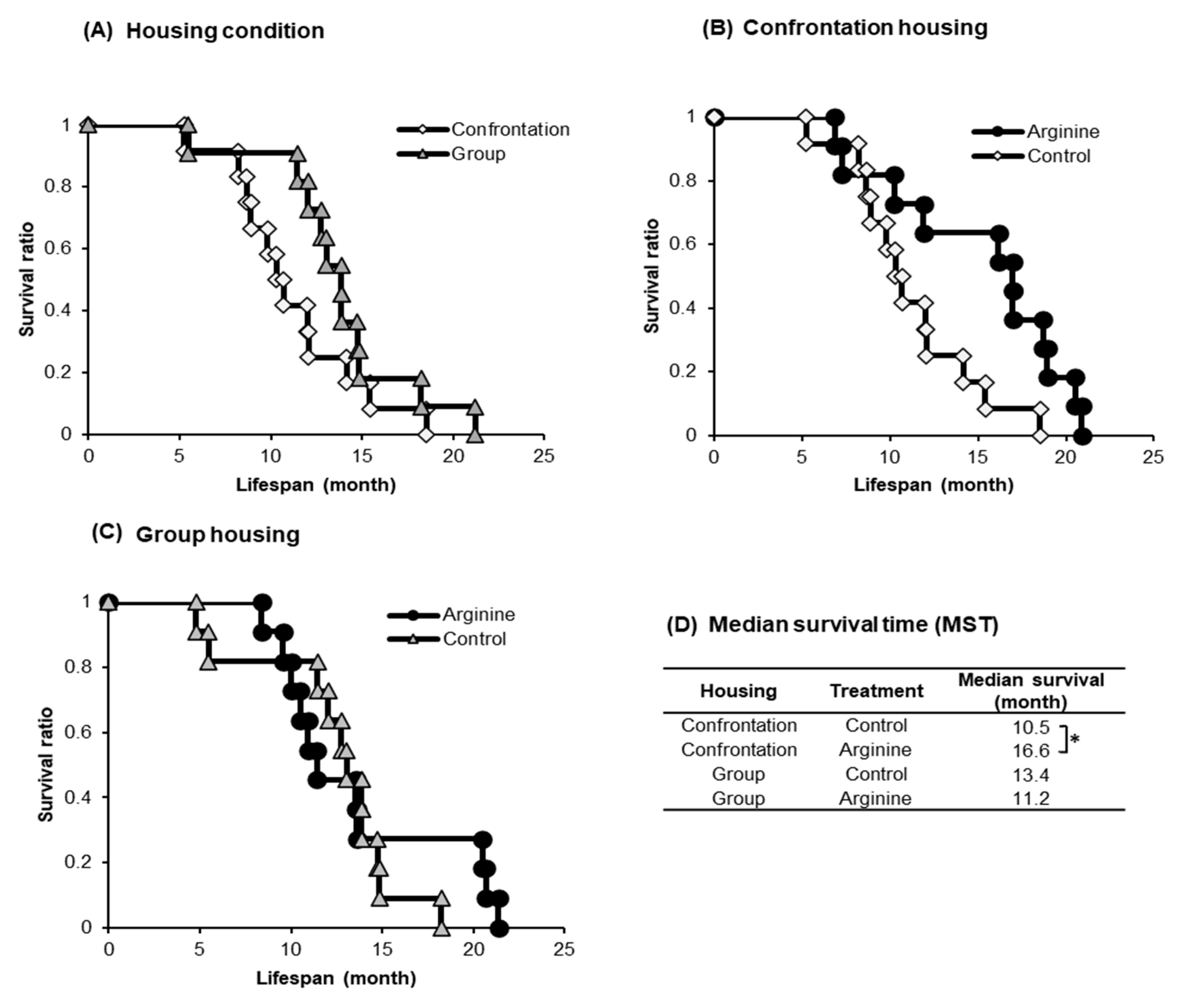
2.1.2. Improving Effect of Arg Intake on Lifespan
2.2. Initial Response to Stress
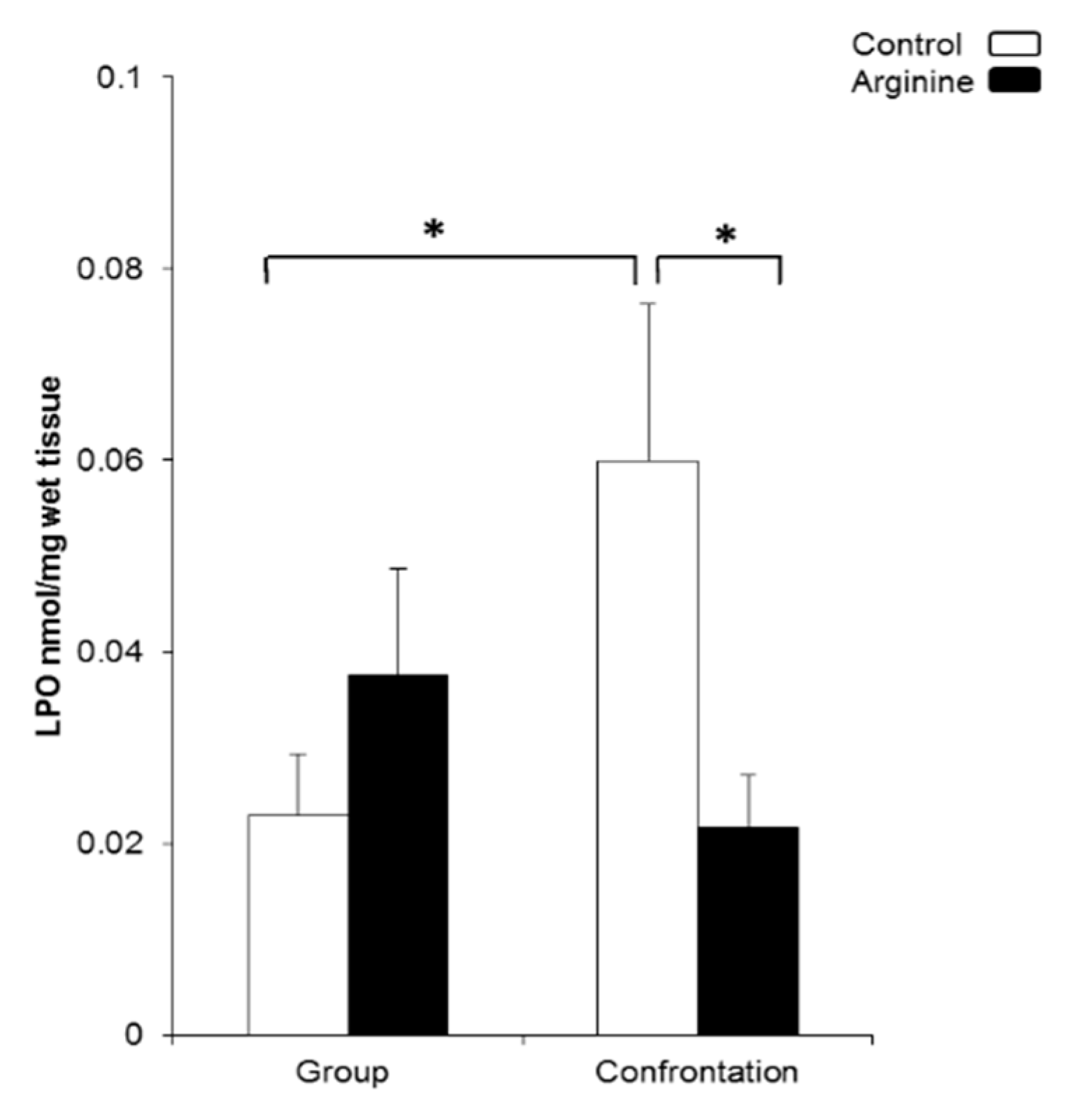
2.2.1. Oxidative Damage in the Brain
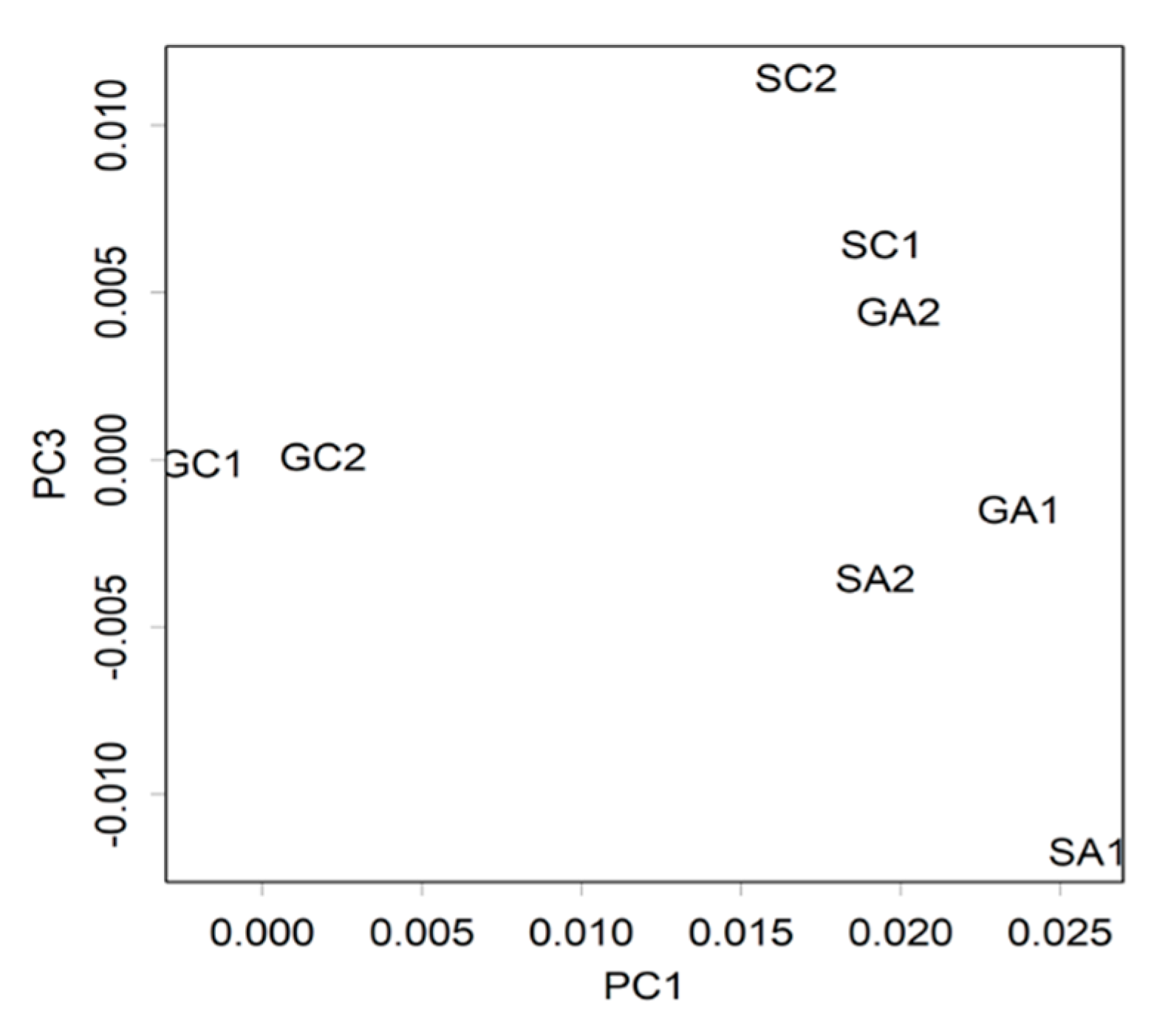
2.2.2. Effect of Arg Intake on Gene Expression in the Hippocampus of Stressed SAMP10 Mice
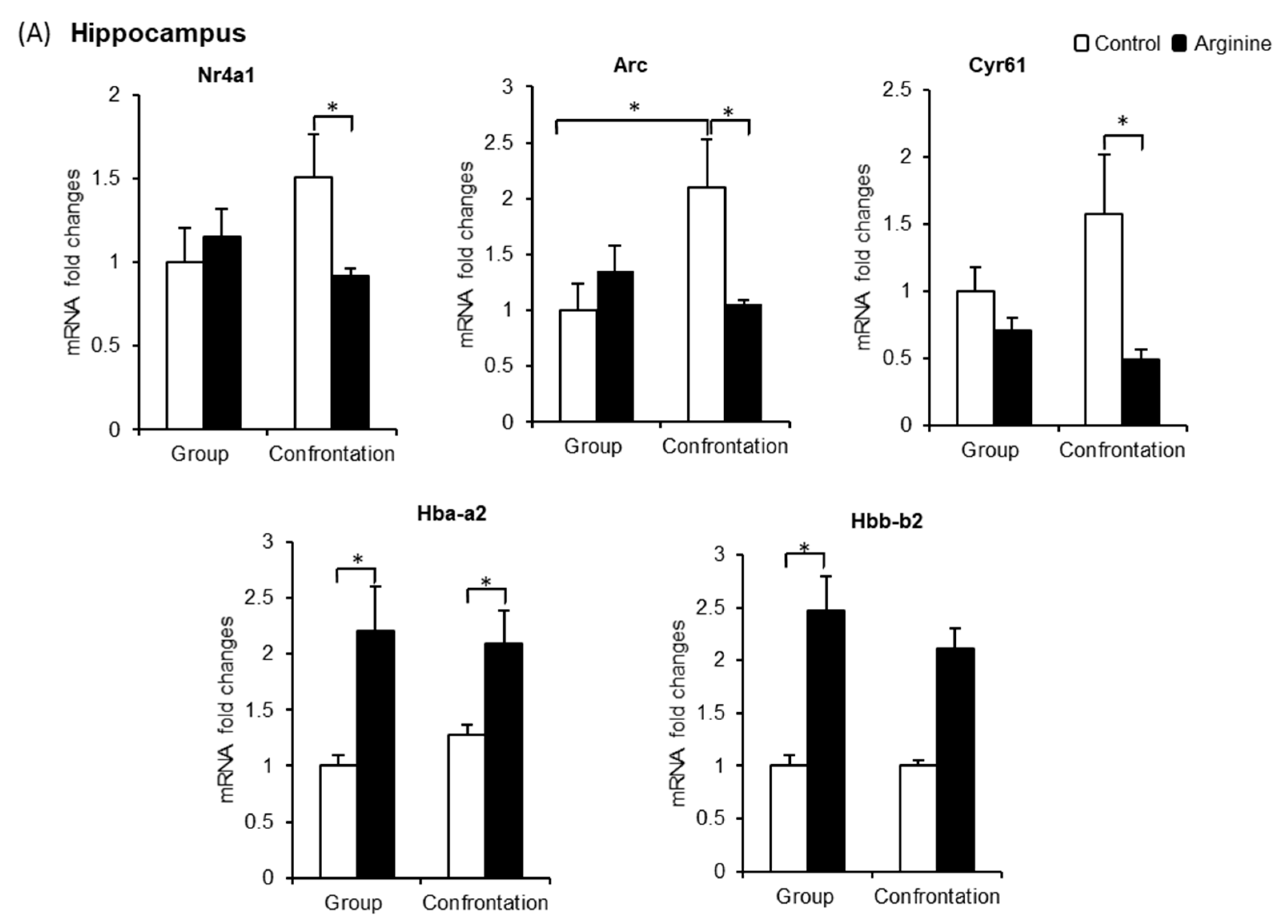
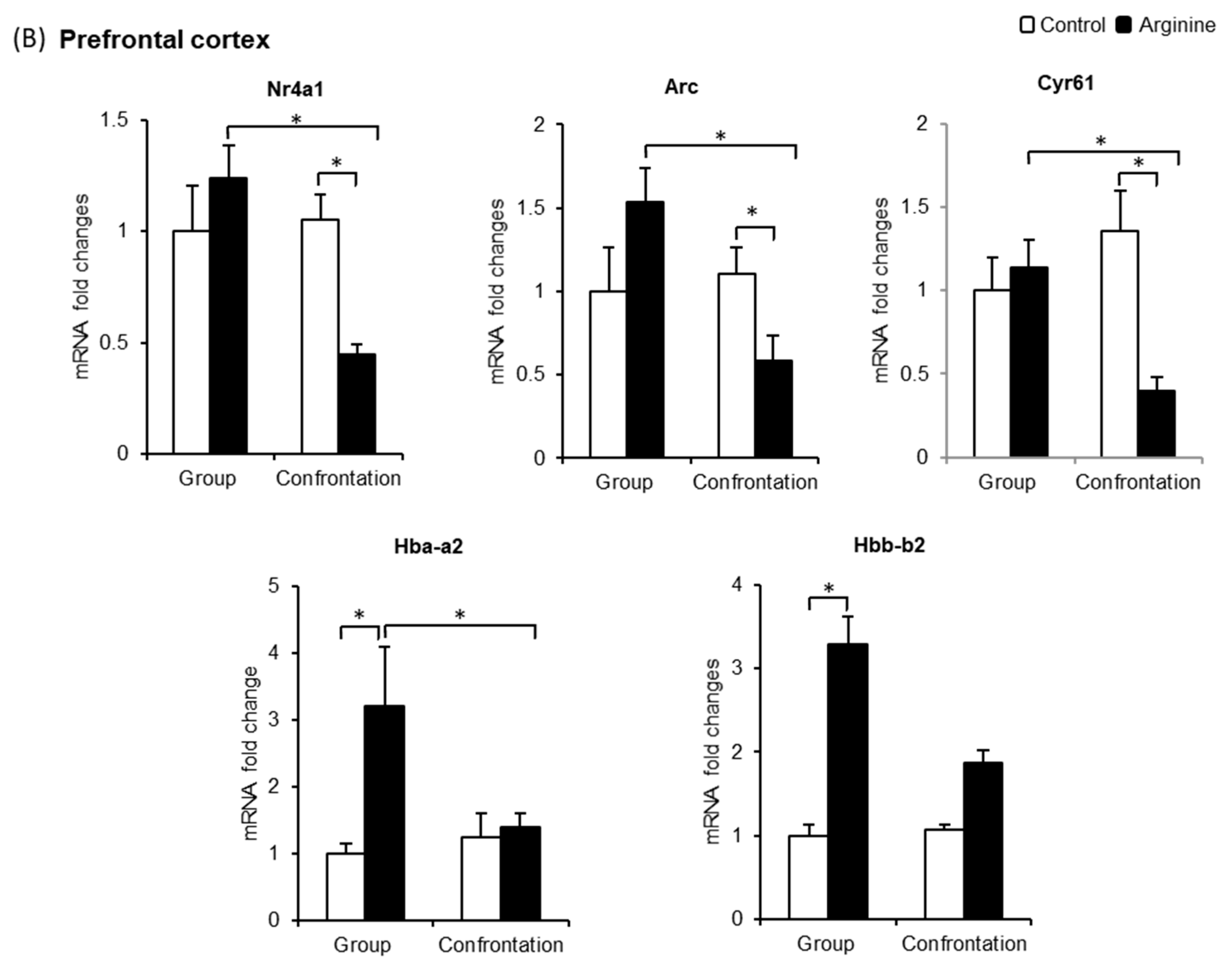
2.2.3. Effect of Arg Intake on Nr4a1, Arc, and Cyr61 Levels in the Brain
2.2.4. Effect of Arg Intake on Hba-a2 and Hbb-b2 Levels in the Brain
3. Discussion
4. Materials and Methods
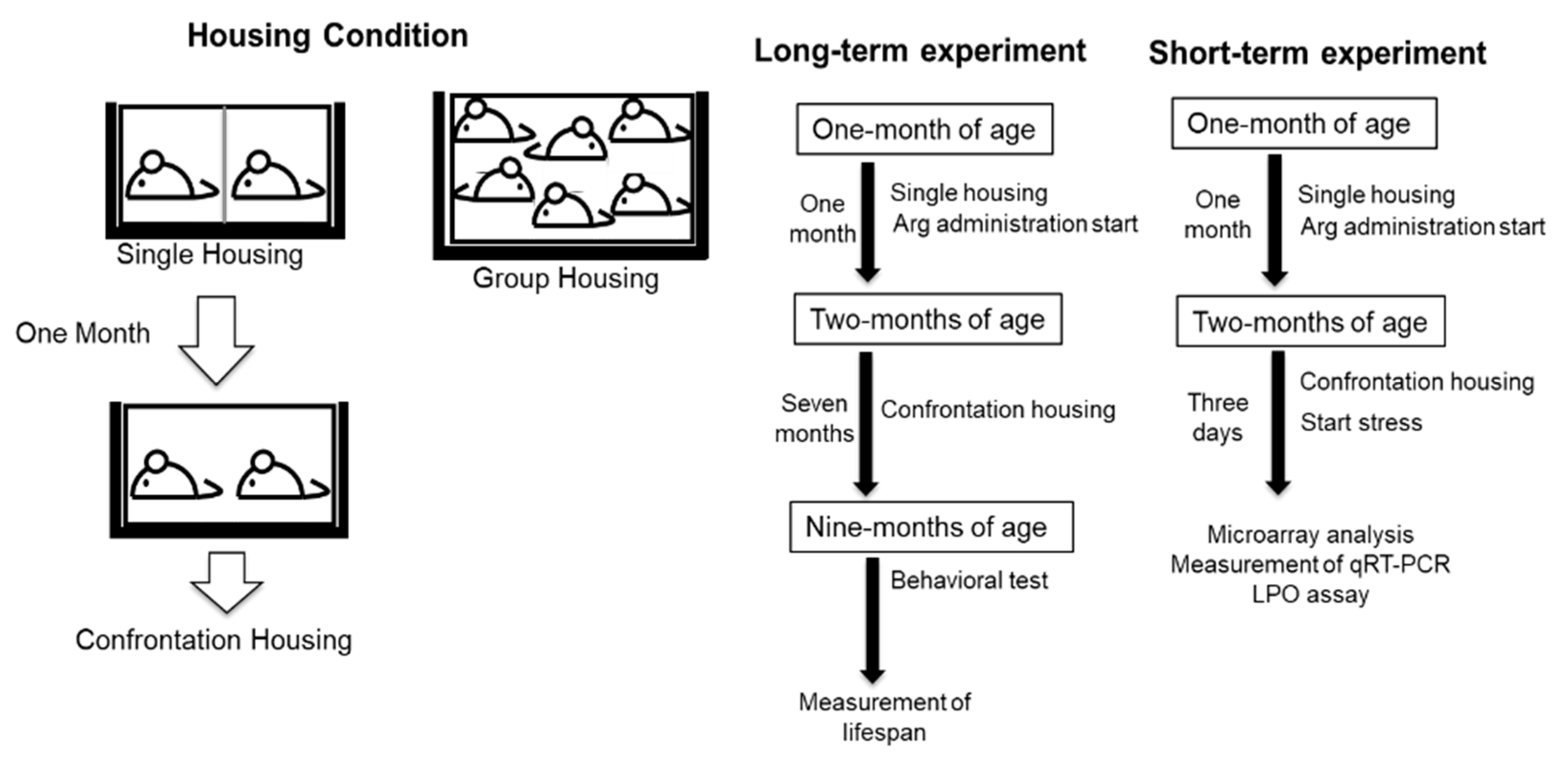
4.1. Animals, Arg Preparation, and Housing Condition
4.2. Housing Condition for Confrontation
4.3. Memory Acquisition Test
4.4. Measurement of Immobility in the Tail Suspension Test
4.5. Measurement of Oxidative Damage in the Brain
4.6. Measurement of DNA Microarray and qRT-PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Arg | Arginine |
| Arc | Activity regulated cytoskeletal-associated protein |
| Cyr61 | Cysteine rich protein 61 |
| Hba-a2 | Hemoglobin alpha, adult chain 2 |
| Hbb-b2 | Hemoglobin, beta adult minor chain |
| IEGs | Immediate-early genes |
| LPO | Lipid peroxidation |
| MST | Median survival time |
| Nr4a1 | Nuclear receptor subfamily 4, group A, member 1 |
| qRT-PCR | Quantitative real-time reverse transcription polymerase chain reaction |
| SAMP10 | Senescence-accelerated mouse prone 10 |
References
- Unno, K.; Fujitani, K.; Takamori, N.; Takabayashi, F.; Maeda, K.; Miyazaki, H.; Tanida, N.; Iguchi, K.; Shimoi, K.; Hoshino, M. Theanine intake improves the shortened lifespan, cognitive dysfunction and behavioural depression that are induced by chronic psychosocial stress in mice. Free Radic. Res. 2011, 45, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Iguchi, K.; Tanida, N.; Fujitani, K.; Takamori, N.; Yamamoto, H.; Ishii, N.; Nagano, H.; Nagashima, T.; Hara, A.; et al. Ingestion of theanine, an amino acid in tea, suppresses psychosocial stress in mice. Exp. Physiol. 2013, 98, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Tanida, N.; Ishii, N.; Yamamoto, H.; Iguchi, K.; Hoshino, M.; Takeda, A.; Ozawa, H.; Ohkubo, T.; Juneja, L.R.; et al. Anti-stress effect of theanine on students during pharmacy practice: Positive correlation among salivary α-amylase activity, trait anxiety and subjective stress. Pharmacol. Biochem. Behav. 2013, 111, 128–135. [Google Scholar] [CrossRef]
- Unno, K.; Hara, A.; Nakagawa, A.; Iguchi, K.; Ohshio, M.; Morita, A.; Nakamura, Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine on psychosocial stress induced adrenal hypertrophy in mice. Phytomedicine 2016, 23, 1365–1374. [Google Scholar] [CrossRef]
- Unno, K.; Furushima, D.; Hamamoto, S.; Iguchi, K.; Yamada, H.; Morita, A.; Horie, H.; Nakamura, Y. Stress-reducing function of matcha green tea in animal experiments and clinical trials. Nutrients 2018, 10, 1468. [Google Scholar] [CrossRef]
- Unno, K.; Furushima, D.; Hamamoto, S.; Iguchi, K.; Yamada, H.; Morita, A.; Pervin, M.; Nakamura, Y. Stress-reducing effect of cookies containing matcha green tea: Essential ratio among theanine, arginine, caffeine and epigallocatechin gallate. Heliyon 2019, 5, e01653. [Google Scholar] [CrossRef]
- Unno, K.; Noda, S.; Kawasaki, Y.; Yamada, H.; Morita, A.; Iguchi, K.; Nakamura, Y. Reduced stress and improved sleep quality caused by green tea are associated with a reduced caffeine content. Nutrients 2017, 9, 777. [Google Scholar] [CrossRef]
- Unno, K.; Sumiyoshi, A.; Konishi, T.; Hayashi, M.; Taguchi, K.; Muguruma, Y.; Inoue, K.; Iguchi, K.; Nonaka, H.; Kawashima, R.; et al. Theanine, the main amino acid in tea, prevents stress-induced brain atrophy by modifying early stress responses. Nutrients 2020, 12, 174. [Google Scholar] [CrossRef]
- Shimada, A.; Keino, H.; Satoh, M.; Kishikawa, M.; Seriu, N.; Hosokawa, M. Age-related progressive neuronal DNA damage associated with cerebral degeneration in a mouse model of accelerated senescence. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, 415–421. [Google Scholar] [CrossRef]
- Shimada, A.; Keino, H.; Satoh, M.; Kishikawa, M.; Hosokawa, M. Age-related loss of synapses in the frontal cortex of SAMP10 mouse: A model of cerebral degeneration. Synapse 2003, 48, 198–204. [Google Scholar] [CrossRef]
- Unno, K.; Takabayashi, F.; Kishido, T.; Oku, N. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10). Exp. Gerontol. 2004, 39, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Roszik, J.; Grimm, E.A.; Ekmekcioglu, S. Impact of l-Arginine metabolism on immune response and anticancer immunotherapy. Front. Oncol. 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Debats, I.B.J.G.; Wolfs, T.G.A.M.; Gotoh, T.; Cleutjens, J.P.M.; Peutz-Kootstra, C.J.R.; van der Hulst, R.W.J. Role of Arginine in superficial wound healing in man. Nitric Oxide 2009, 21, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Harber, V.; Bell, G.J. Oral L-arginine before resistance exercise blunts growth hormone in strength trained males. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Kanazawa, S.; Ichibori, R.; Tanigawa, T.; Magome, T.; Shingaki, K.; Miyata, S.; Tohyama, M.; Hosokawa, K. L-arginine stimulates fibroblast proliferation through the GPRC6A-ERK1/2 and PI3K/Akt pathway. PLoS ONE 2014, 9, e92168. [Google Scholar] [CrossRef]
- Sanini, R.; Badole, S.I.; Zanwar, A.A. Arginine derived nitric oxide: Key to healthy skin. In Bioactive Dietary Factors and Plant. Extracts Dermatology; Watson, R.R., Zibadi, S., Eds.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2013; pp. 73–82. ISBN 978-1-62703-167-7. [Google Scholar]
- Goto, T.; Horie, H.; Ozeki, Y.; Masuda, H.; Warashina, J. Chemical composition of Japanese green teas on market. Tea Res. J. 1994, 80, 23–28. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Qiu, Y.; Yang, X.; Wang, L.; Gao, K.; Jiang, Z. L-Arginine inhibited inflammatory response and oxidative stress induced by lipopolysaccharide via Arginase-1 signaling in IPEC-J2 cells. Int. J. Mol. Sci. 2019, 20, 1800. [Google Scholar] [CrossRef]
- Tan, J.; Liu, S.; Guo, Y.; Applegate, T.J.; Eicher, S.D. Dietary L-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 2014, 111, 1394–1404. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Tong, B.C.; Barbul, A. Cellular and physiological effects of arginine. Mini Rev. Med. Chem. 2004, 4, 823–832. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.M. Regulation of neuronal proliferation and differentiation by nitric oxide. Mol. Neurobiol. 2003, 27, 107–120. [Google Scholar] [CrossRef]
- Paul, V.; Ekambaram, P. Involvement of nitric oxide in learning & memory processes. Indian J. Med. Res. 2011, 133, 471–478. [Google Scholar] [PubMed]
- Gulati, K.; Ray, A. Differential neuromodulatory role of NO in anxiety and seizures: An experimental study. Nitric Oxide 2014, 43, 55–61. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Wang, Y.; Liu, L.; Zhang, X.; Li, B.; Cuia, R. The effects of psychological stress on depression. Curr. Neuropharmacol. 2015, 13, 494–504. [Google Scholar] [CrossRef]
- Byers, A.L.; Yaffe, K. Depression and risk of developing dementia. Nat. Rev. Neurol. 2011, 7, 323–331. [Google Scholar] [CrossRef]
- Razzoli, M.; Nyuyki-Dufe, K.; Gurney, A.; Erickson, C.; McCallum, J.; Spielman, N.; Marzullo, M.; Patricelli, J.; Kurata, M.; Pope, E.A.; et al. Social stress shortens lifespan in mice. Aging Cell 2018, 17, e12778. [Google Scholar] [CrossRef]
- Han, B.; Wang, J.H.; Geng, Y.; Shen, L.; Wang, H.L.; Wang, Y.Y.; Wang, M.W. Chronic stress contributes to cognitive dysfunction and hippocampal metabolic abnormalities in APP/PS1 Mice. Cell Physiol. Biochem. 2017, 41, 1766–1776. [Google Scholar] [CrossRef]
- Marin, M.F.; Lord, C.; Andrews, J.; Juster, R.P.; Sindi, S.; Arsenault-Lapierre, G.; Fiocco, A.J.; Lupien, S.J. Chronic stress, cognitive functioning and mental health. Neurobiol. Learn. Mem. 2011, 96, 583–595. [Google Scholar] [CrossRef]
- Olver, J.S.; Pinney, M.; Maruff, P.; Norman, T.R. Impairments of spatial working memory and attention following acute psychosocial stress. Stress Health 2015, 31, 115–123. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Dedic, N.; Pöhlmann, M.L.; Häusl, A.; Karst, H.; Engelhardt, C.; Westerholz, S.; Wagner, K.V.; Labermaier, C.; Hoeijmakers, L.; et al. Glutamatergic, but Not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol. Psychiatry 2017, 22, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M. Stress, Glucocorticoids, and Damage to the Nervous System: The Current State of Confusion. Stress 1996, 1, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Munhoz, C.D.; Manley, N.C.; Yen, S.; Sapolsky, R.M. Glucocorticoids increase excitotoxic injury and inflammation in the hippocampus of adult male rats. Neuroendocrinology 2014, 100, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Tatomir, A.; Micu, C.; Crivii, C. The impact of stress and glucocorticoids on memory. Clujul Med. 2014, 87, 3–6. [Google Scholar] [CrossRef]
- Helbling, J.C.; Minni, A.M.; Pallet, V.; Moisan, M.P. Stress and glucocorticoid regulation of NR4A genes in mice. J. Neurosci. Res. 2014, 92, 825–834. [Google Scholar] [CrossRef]
- Dai, Y.; Jin, W.; Cheng, L.; Yu, C.; Chen, C.; Ni, H. Nur77 is a promoting factor in traumatic brain injury-induced nerve cell apoptosis. Biomed. Pharmacother. 2018, 108, 774–782. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, W.; Sun, Q.; Zhang, X.; Zhou, X.; Hu, Y.; Shi, J. Nuclear receptor nur77 promotes cerebral cell apoptosis and induces early brain injury after experimental subarachnoid hemorrhage in rats. J. Neurosci. Res. 2014, 92, 1110–1121. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, J.; Yang, L.K.; Feng, Y.; Lin, W.; Wang, Y.H. Glutamate-induced rapid induction of Arc/Arg3.1 requires NMDA receptor-mediated phosphorylation of ERK and CREB. Neurosci. Lett. 2017, 661, 23–28. [Google Scholar] [CrossRef]
- Deng, J.; Yang, X.; Zhang, B. MS4a6D exacerbates immunological pathology in experimental viral fulminant hepatitis. Int. J. Sci. 2019, 8, 98–104. [Google Scholar] [CrossRef]
- Matarin, M.; Salih, D.A.; Yasvoina, M.; Cummings, D.M.; Guelfi, S.; Liu, W.; Solim, M.A.N.; Moens, T.G.; Paublete, R.M.; Ali, S.S.; et al. A genome-wide gene-expression analysis and database in transgenic mice during development of amyloid or tau pathology. Cell. Rep. 2015, 10, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; DeStafano, A.L.; Bis, J.C.; Beecham, G.W.; Grenier-Boley, B.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, S.; Matthes, F.; Posey, K.; Kickstein, E.; Weber, S.; Hettich, M.M.; Pfurtscheller, S.; Ehninger, D.; Schneider, R.; Krauß, S. Resveratrol induces dephosphorylation of tau by interfering with the MID1-PP2A complex. Sci. Rep. 2017, 7, 13753. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Min, Y.K.; Baik, J.H.; Lau, L.F.; Chaqour, B.; Chung, K.C. Expression of angiogenic factor Cyr61 during neuronal cell death via the activation of c-Jun N-terminal kinase and serum response factor. J. Biol. Chem. 2003, 278, 13847–13854. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; Meurers, B.H.; Zhu, C.; Medvedeva, V.P.; Chesselet, M.F. Neurons express hemoglobin alpha- and beta-chains in rat and human brains. J. Comp. Neurol. 2009, 515, 538–547. [Google Scholar] [CrossRef]
- Shephard, F.; Greville-Heygate, O.; Marsh, O.; Anderson, S.; Chakrabarti, L. A mitochondrial location for haemoglobins—Dynamic distribution in ageing and Parkinson’s disease. Mitochondrion 2014, 14, 64–72. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Nakaya, J. Effect of oral administration of l-arginine on senile dementia. Am. J. Med. 2000, 108, 439. [Google Scholar] [CrossRef]
- Xu, A.; Liu, J.; Liu, P.; Jia, M.; Wang, H.; Tao, L. Mitochondrial translocation of Nur77 induced by ROS contributed to cardiomyocyte apoptosis in metabolic syndrome. Biochem. Biophys. Res. Commun. 2014, 446, 1184–1189. [Google Scholar] [CrossRef]
- Qin, Z.; Robichaud, P.; He, T.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Oxidant exposure induces cysteine-rich protein 61 (CCN1) via c-Jun/AP-1 to reduce collagen expression in human dermal fibroblasts. PLoS ONE 2014, 9, e115402. [Google Scholar] [CrossRef]
- Kondoh, T.; Kameishi, M.; Mallick, H.N.; Ono, T.; Torii, K. Lysine and Arginine reduce the effects of cerebral ischemic insults and inhibit glutamate-induced neuronal activity in rats. Front. Integr. Neurosci. 2010, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Gómez, A.; Carmona, M.; Huesa, G.; Porta, S.; Riera-Codina, M.; Biagioli, M.; Gustincich, S.; Aso, E. Neuronal hemoglobin is reduced in Alzheimer’s disease, argyrophilic grain disease, Parkinson’s disease, and dementia with Lewy bodies. J. Alzheimers Dis. 2011, 23, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Vanni, S.; Zattoni, M.; Moda, F.; Giaccone, G.; Tagliavini, F.; Haïk, S.; Deslys, J.P.; Zanusso, G.; Ironside, J.W.; Carmona, M.; et al. Hemoglobin mRNA Changes in the Frontal Cortex of Patients with Neurodegenerative Diseases. Front. Neurosci. 2018, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T. Principal component analysis for designed experiments. BMC Bioinform. 2015, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T. Three-parameter lognormal distribution ubiquitously found in cDNA microarray data and its application to parametric data treatment. BMC Bioinform. 2004, 5, 5. [Google Scholar] [CrossRef]
- Shandilya, M.C.V.; Gautam, A. The temporal effect of hippocampal Arc in the working memory paradigm during novelty exploration. Brain. Res. Bull. 2020, 158, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kurumaji, A.; Ito, T.; Ishii, S.; Nishikawa, T. Effects of FG7142 and immobilization stress on the gene expression in the neocortex of mice. Neurosci. Res. 2008, 62, 155–159. [Google Scholar] [CrossRef]
- Choi, Y.G.; Yeo, S.; Hong, Y.M.; Kim, S.H.; Lim, S. Changes of gene expression profiles in the cervical spinal cord by acupuncture in an MPTP-intoxicated mouse model: Microarray analysis. Gene 2011, 481, 7–16. [Google Scholar] [CrossRef]
- Mansergh, F.C.; Wride, M.A.; Walker, V.E.; Adams, S.; Hunter, S.M.; Evans, M.J. Gene expression changes during cataract progression in Sparc null mice: Differential regulation of mouse globins in the lens. Mol. Vis. 2004, 10, 490–511. [Google Scholar]
| Symbol | Full Name | ΔZ | p | |
|---|---|---|---|---|
| Downregulated | Nr4a1 | Nuclear receptor subfamily 4, group A, member 1 | −0.2726 | 3.51 × 10−10 |
| Arc | Activity regulated cytoskeleton-associated protein | −0.2345 | 2.51 × 10−7 | |
| Olfr1384 | Olfactory receptor 1384 | −0.2446 | 0.00702 | |
| Cryba1 | Crystallin, beta A1 | −0.2270 | 0.00280 | |
| Ms4a6d | Membrane-spanning 4-domains, subfamily A, member 6D | −0.2371 | 0.00674 | |
| Mid1 | Midline 1 | −0.2399 | 2.93 × 10−9 | |
| Cyr61 | Cysteine rich protein 61 | −0.2591 | 8.73 × 10−10 | |
| Prss2 | Protease, serine 2 | −0.2197 | 0.00620 | |
| H2-Q6 | Histocompatibility 2, Q region locus 6 | −0.2550 | 0.00548 | |
| Mir1983 | MicroRNA 1983 | −0.1860 | 0.00375 | |
| Upregulated | Mela | Melanoma antigen | 0.3509 | 1.42 × 10−5 |
| Olfr2 | Olfactory receptor 2 | 0.3302 | 0.00402 | |
| Slitrk6 | SLIT and NTRK-like family, member 6 | 0.1712 | 0.00023 | |
| Hbb-b2 | Hemoglobin, beta adult minor chain | 0.3780 | 3.55 × 10−8 | |
| Itih2 | Inter-alpha trypsin inhibitor, heavy chain 2 | 0.2867 | 7.42 × 10−6 | |
| Olfr535 | Olfactory receptor 535 | 0.3544 | 0.00262 | |
| Zic1 | Zinc finger protein of the cerebellum 1 | 0.0703 | 4.38 × 10−9 | |
| LOC666331 | Uncharacterized LOC666331 | 0.2908 | 0.00262 | |
| Hba-a2 | Hemoglobin alpha, adult chain 2 | 0.3284 | 6.38 × 10−17 | |
| Tcf712 | Transcription factor 7 like 2, T cell specific, HMG box | 0.1181 | 1.27 × 10−11 |
| Gene | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) | Ref. |
|---|---|---|---|
| Nr4a1 | CTGCCTTCCTGGAACTCTTCA | CGGGTTTAGATCGGTATGCC | [37] |
| Arc | ACGATCTGGCTTCCTCATTCTGCT | AGGTTCCCTCAGCATCTCTGCTTT | [57] |
| Cyr61 | CCCCCGGCTGGTGAAAGTC | ATGGGCGTGCAGAGGGTTGAAAAG | [58] |
| Hba-a2 | GAAGCCCTGGAAAGGATGTT | GCCGTGGCTTACATCAAAGT | [59] |
| Hbb-b2 | CACCTGACTGATGCTGAGAAGT | CCCTTGAGGTTGTCCAGGTTT | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pervin, M.; Unno, K.; Konishi, T.; Nakamura, Y. L-Arginine Exerts Excellent Anti-Stress Effects on Stress-Induced Shortened Lifespan, Cognitive Decline and Depression. Int. J. Mol. Sci. 2021, 22, 508. https://doi.org/10.3390/ijms22020508
Pervin M, Unno K, Konishi T, Nakamura Y. L-Arginine Exerts Excellent Anti-Stress Effects on Stress-Induced Shortened Lifespan, Cognitive Decline and Depression. International Journal of Molecular Sciences. 2021; 22(2):508. https://doi.org/10.3390/ijms22020508
Chicago/Turabian StylePervin, Monira, Keiko Unno, Tomokazu Konishi, and Yoriyuki Nakamura. 2021. "L-Arginine Exerts Excellent Anti-Stress Effects on Stress-Induced Shortened Lifespan, Cognitive Decline and Depression" International Journal of Molecular Sciences 22, no. 2: 508. https://doi.org/10.3390/ijms22020508
APA StylePervin, M., Unno, K., Konishi, T., & Nakamura, Y. (2021). L-Arginine Exerts Excellent Anti-Stress Effects on Stress-Induced Shortened Lifespan, Cognitive Decline and Depression. International Journal of Molecular Sciences, 22(2), 508. https://doi.org/10.3390/ijms22020508






