A Rationale for Hypoxic and Chemical Conditioning in Huntington’s Disease
Abstract
1. Is There Any Potential of Hypoxic and Chemical Conditioning in Huntington’s Disease?
2. Hypoxic Conditioning, Mitochondria and Neuroprotection
3. Overlapping Molecular Pathways of Hypoxic and Chemical Conditioning
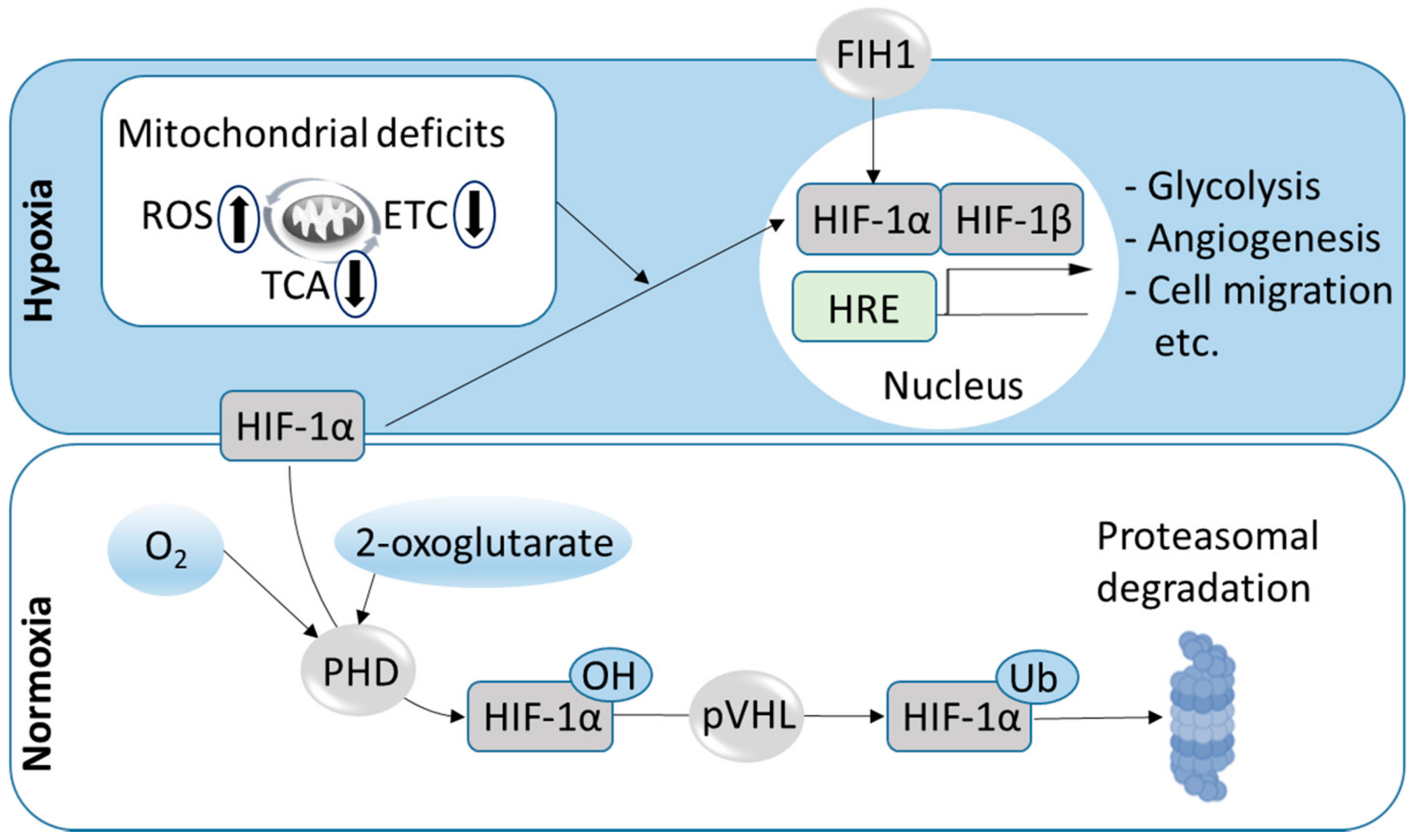
3.1. The HIF Pathway
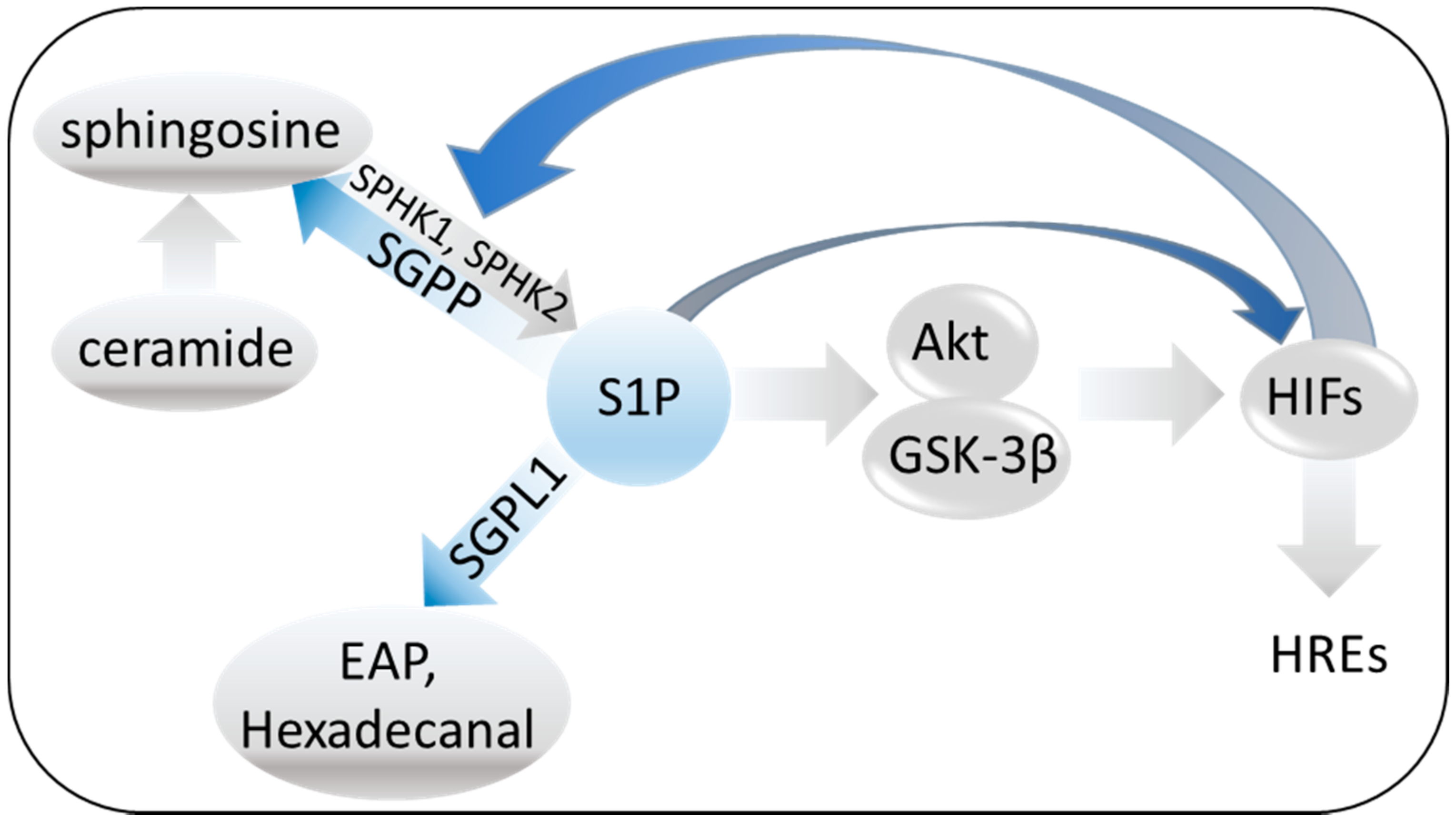
3.2. The Sphingosine System
3.3. The δ-Opioid Receptor System
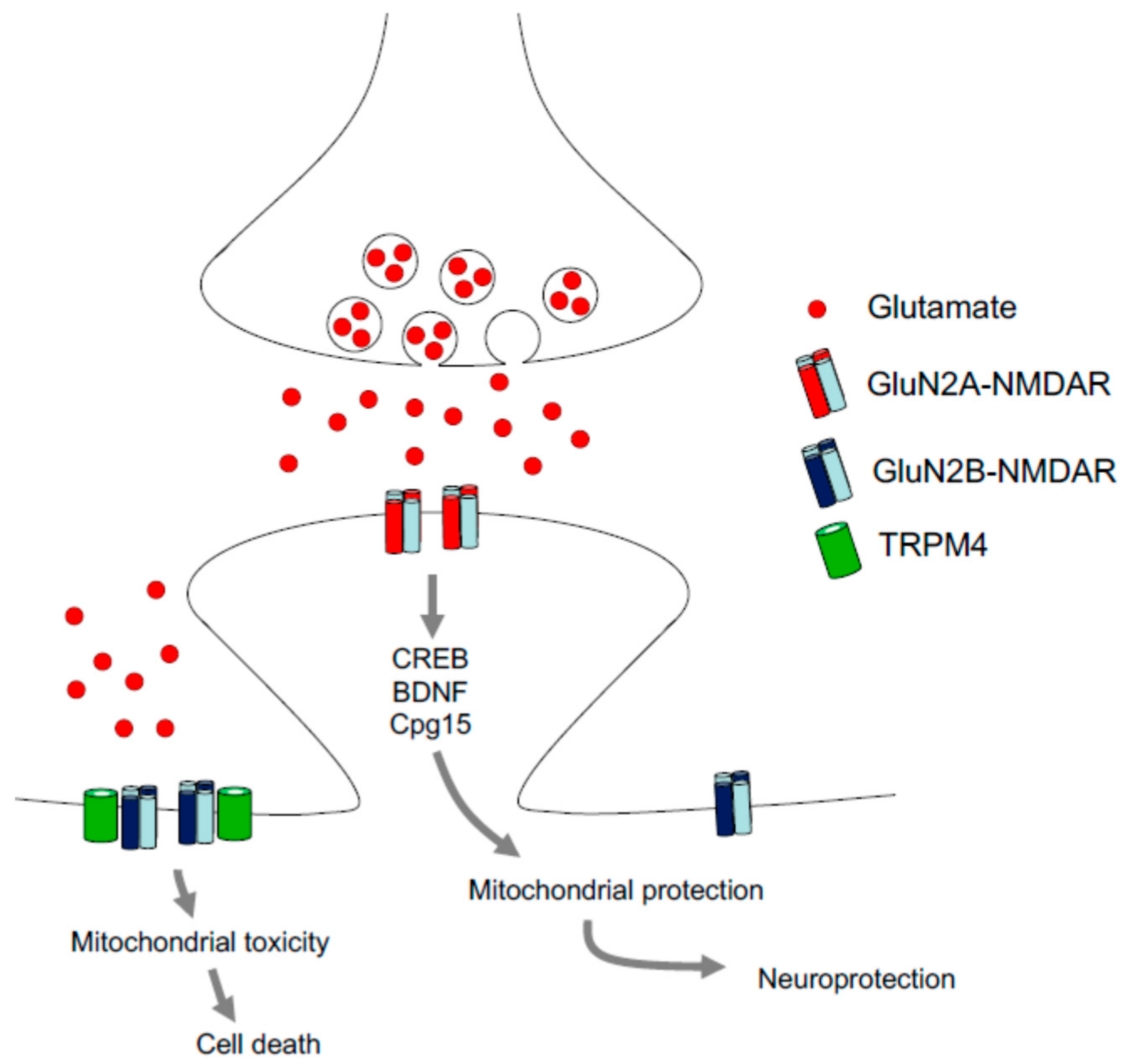
3.4. NMDA Conditioning
4. Conditioning Benefits on Mitochondrial Dysfunctions, Oxidative Stress and Neuroinflammation: The Relevance for Huntington’s Disease
4.1. Mitochondria and Oxidative Stress
4.2. Neuroinflammation
5. Approaches of Hypoxic and Chemical Conditioning in HD
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington disease: New insights into molecular pathogenesis and therapeutic opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.B.; Bates, G.P.; Steffan, J.; Saft, C.; Tabrizi, S.J. Treating the whole body in Huntington’s disease. Lancet Neurol. 2015, 14, 1135–1142. [Google Scholar] [CrossRef]
- Maglione, V.; Cannella, M.; Martino, T.; De Blasi, A.; Frati, L.; Squitieri, F. The platelet maximum number of A2A-receptor binding sites (Bmax) linearly correlates with age at onset and CAG repeat expansion in Huntington’s disease patients with predominant chorea. Neurosci. Lett. 2006, 393, 27–30. [Google Scholar] [CrossRef]
- Maglione, V.; Cannella, M.; Gradini, R.; Cislaghi, G.; Squitieri, F. Huntingtin fragmentation and increased caspase 3, 8 and 9 activities in lymphoblasts with heterozygous and homozygous Huntington’s disease mutation. Mech. Ageing Dev. 2006, 127, 213–216. [Google Scholar] [CrossRef]
- Maglione, V.; Giallonardo, P.; Cannella, M.; Martino, T.; Frati, L.; Squitieri, F. Adenosine A2A receptor dysfunction correlates with age at onset anticipation in blood platelets of subjects with Huntington’s disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 139, 101–105. [Google Scholar] [CrossRef]
- Yano, H.; Baranov, S.V.; Baranova, O.V.; Kim, J.; Pan, Y.; Yablonska, S.; Carlisle, D.L.; Ferrante, R.J.; Kim, A.H.; Friedlander, R.M. Inhibition of mitochondrial protein import by mutant huntingtin. Nat. Neurosci. 2014, 17, 822–831. [Google Scholar] [CrossRef]
- Yablonska, S.; Ganesan, V.; Ferrando, L.M.; Kim, J.; Pyzel, A.; Baranova, O.V.; Khattar, N.K.; Larkin, T.M.; Baranov, S.V.; Chen, N. Mutant huntingtin disrupts mitochondrial proteostasis by interacting with TIM23. Proc. Natl. Acad. Sci. USA 2019, 116, 16593–16602. [Google Scholar] [CrossRef]
- Duara, R.; Barker, W.W.; Chang, J.; Yoshii, F.; Loewenstein, D.A.; Pascal, S. Viability of neocortical function shown in behavioral activation state PET studies in Alzheimer disease. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1992, 12, 927–934. [Google Scholar] [CrossRef]
- Mosconi, L. Glucose metabolism in normal aging and Alzheimer’s disease: Methodological and physiological considerations for PET studies. Clin. Transl. Imaging 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Morishita, R. The roles of lipid and glucose metabolism in modulation of beta-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front. Aging Neurosci. 2015, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. The role of cerebral ischemia in Alzheimer’s disease. Neurobiol. Aging 2000, 21, 321–330. [Google Scholar] [CrossRef]
- Goebel, H.H.; Heipertz, R.; Scholz, W.; Iqbal, K.; Tellez-Nagel, I. Juvenile Huntington chorea: Clinical, ultrastructural, and biochemical studies. Neurology 1978, 28, 23–31. [Google Scholar] [CrossRef]
- Gu, M.; Gash, M.T.; Mann, V.M.; Javoy-Agid, F.; Cooper, J.M.; Schapira, A.H. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann. Neurol. 1996, 39, 385–389. [Google Scholar] [CrossRef]
- Brennan, W.A., Jr.; Bird, E.D.; Aprille, J.R. Regional mitochondrial respiratory activity in Huntington’s disease brain. J. Neurochem. 1985, 44, 1948–1950. [Google Scholar] [CrossRef]
- Merril, C.; Zullo, S.; Ghanbari, H.; Herman, M.; Kleinman, J.; Bigelow, L.; Bartko, J.; Sabourin, D. Possible relationship between conditions associated with chronic hypoxia and brain mitochondrial DNA deletions. Arch. Biochem. Biophys. 1996, 326, 172–177. [Google Scholar] [CrossRef]
- Hamilton, J.; Brustovetsky, T.; Sridhar, A.; Pan, Y.; Cummins, T.R.; Meyer, J.S.; Brustovetsky, N. Energy Metabolism and Mitochondrial Superoxide Anion Production in Pre-symptomatic Striatal Neurons Derived from Human-Induced Pluripotent Stem Cells Expressing Mutant Huntingtin. Mol. Neurobiol. 2020, 57, 668–684. [Google Scholar] [CrossRef]
- Burtscher, J.; Di Pardo, A.; Maglione, V.; Schwarzer, C.; Squitieri, F. Mitochondrial Respiration Changes in R6/2 Huntington’s Disease Model Mice during Aging in a Brain Region Specific Manner. Int. J. Mol. Sci. 2020, 21, 5412. [Google Scholar] [CrossRef]
- Lou, S.; Lepak, V.C.; Eberly, L.E.; Roth, B.; Cui, W.; Zhu, X.-H.; Öz, G.; Dubinsky, J.M. Oxygen consumption deficit in Huntington disease mouse brain under metabolic stress. Hum. Mol. Genet. 2016, 25, 2813–2826. [Google Scholar] [CrossRef]
- Weindruch, R.H.; Cheung, M.K.; Anthony Verity, M.; Walford, R.L. Modification of mitochondrial respiration by aging and dietary restriction. Mech. Ageing Dev. 1980, 12, 375–392. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Jekabsons, M.B.; Chen, S.; Lin, A.; Rego, A.C.; Gonçalves, J.; Ellerby, L.M.; Nicholls, D.G. Mitochondrial dysfunction in Huntington’s disease: The bioenergetics of isolated and in situ mitochondria from transgenic mice. J. Neurochem. 2007, 101, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Youngblood, H.; Wu, C.; Zhang, Q. Mitochondria as a target for neuroprotection: Role of methylene blue and photobiomodulation. Transl. Neurodegener. 2020, 9, 1–22. [Google Scholar]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Rheims, S.; Alvarez, B.M.; Alexandre, V.; Curot, J.; Maillard, L.; Bartolomei, F.; Derambure, P.; Hirsch, E.; Michel, V.; Chassoux, F.; et al. Hypoxemia following generalized convulsive seizures. Neurology 2019, 92, e183. [Google Scholar] [CrossRef]
- Kawahara, M.; Kuroda, Y. Molecular mechanism of neurodegeneration induced by Alzheimer’s beta-amyloid protein: Channel formation and disruption of calcium homeostasis. Brain Res. Bull. 2000, 53, 389–397. [Google Scholar] [CrossRef]
- Antonelli Incalzi, R.; Marra, C.; Giordano, A.; Calcagni, M.L.; Cappa, A.; Basso, S.; Pagliari, G.; Fuso, L. Cognitive impairment in chronic obstructive pulmonary disease—A neuropsychological and spect study. J. Neurol. 2003, 250, 325–332. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J. Neurosci. Res. 2015, 93, 1778–1794. [Google Scholar] [CrossRef]
- Dodd, J.; Getov, S.; Jones, P.W. Cognitive function in COPD. Eur. Respir. J. 2010, 35, 913–922. [Google Scholar] [CrossRef]
- Peers, C.; Dallas, M.L.; Boycott, H.E.; Scragg, J.L.; Pearson, H.A.; Boyle, J.P. Hypoxia and neurodegeneration. Ann. N. Y. Acad. Sci. 2009, 1177, 169–177. [Google Scholar] [CrossRef]
- Noble, R.L. The development of resistance by rats and guinea pigs to amounts of trauma usually fatal. Am. J. Physiol. 1943, 138, 346–351. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Yang, X.; Wan, Y.; Jia, J. The effects of exercise preconditioning on cerebral blood flow change and endothelin-1 expression after cerebral ischemia in rats. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Manukhina, E.B.; Downey, H.F.; Shi, X.; Mallet, R.T. Intermittent hypoxia training protects cerebrovascular function in Alzheimer’s disease. Exp. Biol. Med. 2016, 241, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.C.; Santos, R.X.; Perry, G.; Zhu, X.; Moreira, P.I.; Smith, M.A. Mitochondria: The missing link between preconditioning and neuroprotection. J. Alzheimer Dis. 2010, 20 (Suppl. S2), S475–S485. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Wick, W.; Waltenberger, J.; Weller, M.; Dichgans, J.; Schulz, J.B. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J. Neurosci. 2002, 22, 6401–6407. [Google Scholar] [CrossRef]
- Lin, A.M.; Chen, C.; Ho, L. Neuroprotective effect of intermittent hypoxia on iron-induced oxidative injury in rat brain. Exp. Neurol. 2002, 176, 328–335. [Google Scholar] [CrossRef]
- Amano, S.; Obata, T.; Hazama, F.; Kashiro, N.; Shimada, M. Hypoxia prevents seizures and neuronal damages of the hippocampus induced by kainic acid in rats. Brain Res. 1990, 523, 121–126. [Google Scholar] [CrossRef]
- Pohle, W.; Rauca, C. Hypoxia protects against the neurotoxicity of kainic acid. Brain Res. 1994, 644, 297–304. [Google Scholar] [CrossRef]
- Emerson, M.; Samson, F.; Pazdernik, T. Effects of hypoxia preconditioning on expression of metallothionein-1, 2 and heme oxygenase-1 before and after kainic acid-induced seizures. Cell. Mol. Biol. Noisy Grand France 2000, 46, 619–626. [Google Scholar]
- Yang, Y.; Chen, J.; Li, L.; Gao, Y.; Chen, J.; Fei, Z.; Liu, W. Effect of Different Mild Hypoxia Manipulations on Kainic Acid-Induced Seizures in the Hippocampus of Rats. Neurochem. Res. 2013, 38, 123–132. [Google Scholar] [CrossRef]
- Mayfield, K.P.; D’Alecy, L.G. Delta-1 opioid agonist acutely increases hypoxic tolerance. J. Pharmacol. Exp. Ther. 1994, 268, 683–688. [Google Scholar] [PubMed]
- Yang, Y.; Xia, X.; Zhang, Y.; Wang, Q.; Li, L.; Luo, G.; Xia, Y. delta-Opioid receptor activation attenuates oxidative injury in the ischemic rat brain. BMC Biol. 2009, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Boeck, C.R.; Ganzella, M.; Lottermann, A.; Vendite, D. NMDA preconditioning protects against seizures and hippocampal neurotoxicity induced by quinolinic acid in mice. Epilepsia 2004, 45, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wu, J.; Yan, L.-J. Chemical conditioning as an approach to ischemic stroke tolerance: Mitochondria as the target. Int. J. Mol. Sci. 2016, 17, 351. [Google Scholar] [CrossRef] [PubMed]
- Frenkel-Denkberg, G.; Gershon, D.; Levy, A.P. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett. 1999, 462, 341–344. [Google Scholar] [CrossRef]
- Honma, Y.; Tani, M.; Takayama, M.; Yamamura, K.; Hasegawa, H. Aging abolishes the cardioprotective effect of combination heat shock and hypoxic preconditioning in reperfused rat hearts. Basic Res. Cardiol. 2002, 97, 489–495. [Google Scholar] [CrossRef]
- Bickler, P.E.; Fahlman, C.S.; Gray, J.J. Hypoxic preconditioning failure in aging hippocampal neurons: Impaired gene expression and rescue with intracellular calcium chelation. J. Neurosci. Res. 2010, 88, 3520–3529. [Google Scholar] [CrossRef]
- Benderro, G.F.; LaManna, J.C. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain Res. 2011, 1389, 50–60. [Google Scholar] [CrossRef]
- Serebrovskaya, T.V.; Manukhina, E.B.; Smith, M.L.; Downey, H.F.; Mallet, R.T. Intermittent hypoxia: Cause of or therapy for systemic hypertension? Exp. Biol. Med. 2008, 233, 627–650. [Google Scholar] [CrossRef]
- Bayer, U.; Likar, R.; Pinter, G.; Stettner, H.; Demschar, S.; Trummer, B.; Neuwersch, S.; Glazachev, O.; Burtscher, M. Intermittent hypoxic–hyperoxic training on cognitive performance in geriatric patients. Alzheimer Dement. Transl. Res. Clin. Interv. 2017, 3, 114–122. [Google Scholar] [CrossRef]
- Serebrovska, Z.O.; Serebrovska, T.V.; Kholin, V.A.; Tumanovska, L.V.; Shysh, A.M.; Pashevin, D.A.; Goncharov, S.V.; Stroy, D.; Grib, O.N.; Shatylo, V.B.; et al. Intermittent Hypoxia-Hyperoxia Training Improves Cognitive Function and Decreases Circulating Biomarkers of Alzheimer’s Disease in Patients with Mild Cognitive Impairment: A Pilot Study. Int. J. Mol. Sci. 2019, 20, 5405. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, X.; Schenck, H.; Hall, J.R.; Ross, S.E.; Kline, G.P.; Chen, S.; Mallet, R.T.; Chen, P. Intermittent Hypoxia Training for Treating Mild Cognitive Impairment: A Pilot Study. Am. J. Alzheimers Dis. Other Dement. 2020, 35, 1533317519896725. [Google Scholar] [CrossRef] [PubMed]
- Lendahl, U.; Lee, K.L.; Yang, H.; Poellinger, L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat. Rev. Genet. 2009, 10, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Brose, S.A.; Golovko, S.A.; Golovko, M.Y. Fatty Acid Biosynthesis Inhibition Increases Reduction Potential in Neuronal Cells under Hypoxia. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Almohanna, A.M.; Wray, S. Hypoxic conditioning in blood vessels and smooth muscle tissues: Effects on function, mechanisms, and unknowns. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H756–H770. [Google Scholar] [CrossRef]
- Mole, D.R.; Blancher, C.; Copley, R.R.; Pollard, P.J.; Gleadle, J.M.; Ragoussis, J.; Ratcliffe, P.J. Genome-wide association of hypoxia-inducible factor (HIF)-1α and HIF-2α DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 2009, 284, 16767–16775. [Google Scholar] [CrossRef]
- Ruas, J.L.; Poellinger, L.; Pereira, T. Role of CBP in regulating HIF-1-mediated activation of transcription. J. Cell Sci. 2005, 118, 301–311. [Google Scholar] [CrossRef]
- Holmquist-Mengelbier, L.; Fredlund, E.; Löfstedt, T.; Noguera, R.; Navarro, S.; Nilsson, H.; Pietras, A.; Vallon-Christersson, J.; Borg, Å.; Gradin, K. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell 2006, 10, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, J.; Chang, Y.; ShiDu Yan, S.; Shi, H. Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Curr. Med. Chem. 2011, 18, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.C.; Moreira, P.I. Hypoxia-inducible factor 1: A new hope to counteract neurodegeneration? J. Neurochem. 2010, 112, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.C.; Carvalho, C.; Cardoso, S.; X Santos, R.; I Plácido, A.; Candeias, E.; I Duarte, A.; I Moreira, P. Defective HIF signaling pathway and brain response to hypoxia in neurodegenerative diseases: Not an “iffy” question! Curr. Pharm. Des. 2013, 19, 6809–6822. [Google Scholar] [CrossRef] [PubMed]
- Merelli, A.; Rodríguez, J.C.G.; Folch, J.; Regueiro, M.R.; Camins, A.; Lazarowski, A. Understanding the role of hypoxia inducible factor during neurodegeneration for new therapeutics opportunities. Curr. Neuropharmacol. 2018, 16, 1484–1498. [Google Scholar] [CrossRef] [PubMed]
- Speer, R.E.; Karuppagounder, S.S.; Basso, M.; Sleiman, S.F.; Kumar, A.; Brand, D.; Smirnova, N.; Gazaryan, I.; Khim, S.J.; Ratan, R.R. Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by “antioxidant” metal chelators: From ferroptosis to stroke. Free Radic. Biol. Med. 2013, 62, 26–36. [Google Scholar] [CrossRef]
- Ashok, B.S.; Ajith, T.A.; Sivanesan, S. Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin. Exp. Pharmacol. Physiol. 2017, 44, 327–334. [Google Scholar] [CrossRef]
- Youdim, M.B.; Kupershmidt, L.; Amit, T.; Weinreb, O. Promises of novel multi-target neuroprotective and neurorestorative drugs for Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20, S132–S136. [Google Scholar] [CrossRef]
- Li, X.; Cui, X.-X.; Chen, Y.-J.; Wu, T.-T.; Xu, H.; Yin, H.; Wu, Y.-C. Therapeutic potential of a prolyl hydroxylase inhibitor FG-4592 for Parkinson’s diseases in vitro and in vivo: Regulation of redox biology and mitochondrial function. Front. Aging Neurosci. 2018, 10, 121. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, Y.; Simmering, J.E.; Schultz, J.L.; Li, Y.; Fernandez-Carasa, I.; Consiglio, A.; Raya, A.; Polgreen, P.M.; Narayanan, N.S. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J. Clin. Investig. 2019, 129, 4539–4549. [Google Scholar] [CrossRef]
- Kandil, E.A.; Sayed, R.H.; Ahmed, L.A.; Abd El Fattah, M.A.; El-Sayeh, B.M. Hypoxia-inducible factor 1 alpha and nuclear-related receptor 1 as targets for neuroprotection by albendazole in a rat rotenone model of Parkinson’s disease. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Currò, M.; Giunta, M.L.; Longo, D.; Rizzo, V.; Caccamo, D.; Ientile, R. Up-regulation of HIF-1α is associated with neuroprotective effects of agmatine against rotenone-induced toxicity in differentiated SH-SY5Y cells. Amino Acids 2020, 52, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-F.; Zhang, Y.-H.; Wang, S.; Pang, Z.-Q.; Fan, Y.-G.; Li, J.-Y.; Wang, Z.-Y.; Guo, C. Lactoferrin ameliorates dopaminergic neurodegeneration and motor deficits in MPTP-treated mice. Redox Biol. 2019, 21, 101090. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Hao, L.-J.; Yang, Z.-H.; Chai, R.; Zhang, S.; Gu, Y.; Gao, H.-L.; Zhong, M.-L.; Wang, T.; Li, J.-Y. Deferoxamine-mediated up-regulation of HIF-1α prevents dopaminergic neuronal death via the activation of MAPK family proteins in MPTP-treated mice. Exp. Neurol. 2016, 280, 13–23. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [PubMed]
- Goni, F.M.; Sot, J.; Alonso, A. Biophysical properties of sphingosine, ceramides and other simple sphingolipids. Biochem. Soc. Trans. 2014, 42, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef]
- Mencarelli, C.; Martinez-Martinez, P. Ceramide function in the brain: When a slight tilt is enough. Cell Mol. Life Sci. 2013, 70, 181–203. [Google Scholar] [CrossRef]
- Brann, A.B.; Scott, R.; Neuberger, Y.; Abulafia, D.; Boldin, S.; Fainzilber, M.; Futerman, A.H. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J. Neurosci. 1999, 19, 8199–8206. [Google Scholar] [CrossRef]
- Schwarz, A.; Futerman, A.H. Distinct roles for ceramide and glucosylceramide at different stages of neuronal growth. J. Neurosci. 1997, 17, 2929–2938. [Google Scholar] [CrossRef]
- Czubowicz, K.; Strosznajder, R. Ceramide in the molecular mechanisms of neuronal cell death. The role of sphingosine-1-phosphate. Mol. Neurobiol. 2014, 50, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, J.; Oya, Y.; Ezoe, T.; Vanier, M.T.; Nakayasu, H.; Fujita, N.; Suzuki, K. Ceramide accumulation is associated with increased apoptotic cell death in cultured fibroblasts of sphingolipid activator protein-deficient mouse but not in fibroblasts of patients with Farber disease. J. Inherit. Metab. Dis. 1999, 22, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Hogan, E.L.; Pahan, K. Ceramide and neurodegeneration: Susceptibility of neurons and oligodendrocytes to cell damage and death. J. Neurol. Sci. 2009, 278, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.D.; Obeid, L.M. Ceramide and apoptosis: Exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med. Chem. 2012, 12, 340–363. [Google Scholar] [CrossRef] [PubMed]
- Van Echten-Deckert, G.; Hagen-Euteneuer, N.; Karaca, I.; Walter, J. Sphingosine-1-phosphate: Boon and bane for the brain. Cell Physiol. Biochem. 2014, 34, 148–157. [Google Scholar] [CrossRef]
- Mendelson, K.; Evans, T.; Hla, T. Sphingosine 1-phosphate signalling. Development 2014, 141, 5–9. [Google Scholar] [CrossRef]
- Le Stunff, H.; Peterson, C.; Liu, H.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate and lipid phosphohydrolases. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2002, 1582, 8–17. [Google Scholar] [CrossRef]
- Morozov, V.I.; Sakuta, G.A.; Kalinski, M.I. Sphingosine-1-phosphate: Distribution, metabolism and role in the regulation of cellular functions. Ukr Biokhim Zh (1999) 2013, 85, 5–21. [Google Scholar] [CrossRef]
- Di Pardo, A.; Amico, E.; Basit, A.; Armirotti, A.; Joshi, P.; Neely, D.M.; Vuono, R.; Castaldo, S.; Digilio, A.F.; Scalabri, F.; et al. Defective Sphingosine-1-phosphate metabolism is a druggable target in Huntington’s disease. Sci. Rep. 2017, 7, 5280. [Google Scholar] [CrossRef]
- Strub, G.M.; Paillard, M.; Liang, J.; Gomez, L.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Price, M.M.; Chen, Q.; Simpson, D.C.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011, 25, 600–612. [Google Scholar] [CrossRef]
- Gomez, L.; Paillard, M.; Price, M.; Chen, Q.; Teixeira, G.; Spiegel, S.; Lesnefsky, E.J. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Basic Res. Cardiol. 2011, 106, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A. New endogenous regulators of class I histone deacetylases. Sci. Signal. 2010, 3, pe1. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Sankala, H.; Hait, N.C.; Le Stunff, H.; Liu, H.; Toman, R.; Collier, C.; Zhang, M.; Satin, L.S.; Merrill, A.H., Jr.; et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005, 280, 37118–37129. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Saba, J.D. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv. Enzym. Regul. 2010, 50, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Strub, G.M.; Maceyka, M.; Hait, N.C.; Milstien, S.; Spiegel, S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 2010, 688, 141–155. [Google Scholar]
- Martin, R.; Sospedra, M. Sphingosine-1 phosphate and central nervous system. Curr. Top. Microbiol. Immunol. 2014, 378, 149–170. [Google Scholar] [CrossRef]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Karliner, J.S.; Honbo, N.; Summers, K.; Gray, M.O.; Goetzl, E.J. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J. Mol. Cell. Cardiol. 2001, 33, 1713–1717. [Google Scholar] [CrossRef]
- Schwalm, S.; Döll, F.; Römer, I.; Bubnova, S.; Pfeilschifter, J.; Huwiler, A. Sphingosine kinase-1 is a hypoxia-regulated gene that stimulates migration of human endothelial cells. Biochem. Biophys. Res. Commun. 2008, 368, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Dehne, N.; Brüne, B. HIF-1 in the inflammatory microenvironment. Exp. Cell Res. 2009, 315, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Ader, I.; Malavaud, B.; Cuvillier, O. When the sphingosine kinase 1/sphingosine 1-phosphate pathway meets hypoxia signaling: New targets for cancer therapy. Cancer Res. 2009, 69, 3723–3726. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.D.; Robitaille, G.v.A.; Gratton, J.-P.; Richard, D.E. Sphingosine-1-phosphate: A novel nonhypoxic activator of hypoxia-inducible factor-1 in vascular cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 902–908. [Google Scholar] [CrossRef]
- Heo, K.; Park, K.-A.; Kim, Y.-H.; Kim, S.-H.; Oh, Y.-S.; Kim, I.-H.; Ryu, S.-H.; Suh, P.-G. Sphingosine 1-phosphate induces vesicular endothelial growth factor expression in endothelial cells. BMB Rep. 2009, 42, 685–690. [Google Scholar] [CrossRef]
- Tao, R.; Zhang, J.; Vessey, D.A.; Honbo, N.; Karliner, J.S. Deletion of the Sphingosine Kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc. Res. 2007, 74, 56–63. [Google Scholar] [CrossRef]
- Chawla, S.; Rahar, B.; Saxena, S. S1P prophylaxis mitigates acute hypobaric hypoxia-induced molecular, biochemical, and metabolic disturbances: A preclinical report. IUBMB Life 2016, 68, 365–375. [Google Scholar] [CrossRef]
- Chawla, S.; Rahar, B.; Tulswani, R.; Saxena, S. Preventive preclinical efficacy of intravenously administered sphingosine-1-phosphate (S1P) in strengthening hypoxia adaptive responses to acute and sub-chronic hypobaric hypoxia. Eur. J. Pharm. 2020, 870, 172877. [Google Scholar] [CrossRef]
- Wacker, B.K.; Perfater, J.L.; Gidday, J.M. Hypoxic preconditioning induces stroke tolerance in mice via a cascading HIF, sphingosine kinase, and CCL 2 signaling pathway. J. Neurochem. 2012, 123, 954–962. [Google Scholar] [CrossRef]
- Ke, M.; Tang, Q.; Pan, Z.; Yin, Y.; Zhang, L.; Wen, K. Sphingosine-1-phosphate attenuates hypoxia/reoxygenation-induced cardiomyocyte injury via a mitochondrial pathway. Biochem. Biophys. Res. Commun. 2019, 510, 142–148. [Google Scholar] [CrossRef]
- Anelli, V.; Gault, C.R.; Cheng, A.B.; Obeid, L.M. Sphingosine Kinase 1 Is Up-regulated during Hypoxia in U87MG Glioma Cells ROLE OF HYPOXIA-INDUCIBLE FACTORS 1 AND 2. J. Biol. Chem. 2008, 283, 3365–3375. [Google Scholar] [CrossRef] [PubMed]
- Bouquerel, P.; Gstalder, C.; Müller, D.; Laurent, J.; Brizuela, L.; Sabbadini, R.; Malavaud, B.; Pyronnet, S.; Martineau, Y.; Ader, I. Essential role for SphK1/S1P signaling to regulate hypoxia-inducible factor 2α expression and activity in cancer. Oncogenesis 2016, 5, e209. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Maiti, A.; Xu, P.; Qi, Q.; Kawaguchi, T.; Okano, M.; Takabe, K.; Yan, L.; Luo, C. Regulation of hypoxia-inducible factor functions in the nucleus by sphingosine-1-phosphate. FASEB J. 2020, 34, 4293–4310. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhang, Y.; D’Alessandro, A.; Nemkov, T.; Song, A.; Wu, H.; Liu, H.; Adebiyi, M.; Huang, A.; Wen, Y.E.; et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun. 2016, 7, 12086. [Google Scholar] [CrossRef]
- Jęśko, H.; Stępień, A.; Lukiw, W.J.; Strosznajder, R.P. The Cross-Talk Between Sphingolipids and Insulin-Like Growth Factor Signaling: Significance for Aging and Neurodegeneration. Mol. Neurobiol. 2019, 56, 3501–3521. [Google Scholar] [CrossRef]
- Czubowicz, K.; Jęśko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer’s Disease and Other Neurodegenerative Disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef]
- Karunakaran, I.; van Echten-Deckert, G. Sphingosine 1-phosphate–A double edged sword in the brain. Biochim. Biophys. Acta BBA Biomembr. 2017, 1859, 1573–1582. [Google Scholar] [CrossRef]
- Manchon, J.F.M.; Uzor, N.-E.; Dabaghian, Y.; Furr-Stimming, E.E.; Finkbeiner, S.; Tsvetkov, A.S. Cytoplasmic sphingosine-1-phosphate pathway modulates neuronal autophagy. Sci. Rep. 2015, 5, 15213. [Google Scholar] [CrossRef]
- Pirhaji, L.; Milani, P.; Leidl, M.; Curran, T.; Avila-Pacheco, J.; Clish, C.B.; White, F.M.; Saghatelian, A.; Fraenkel, E. Revealing disease-associated pathways by network integration of untargeted metabolomics. Nat. Methods 2016, 13, 770–776. [Google Scholar] [CrossRef]
- Pirhaji, L.; Milani, P.; Dalin, S.; Wassie, B.T.; Dunn, D.E.; Fenster, R.J.; Avila-Pacheco, J.; Greengard, P.; Clish, C.B.; Heiman, M.; et al. Identifying therapeutic targets by combining transcriptional data with ordinal clinical measurements. Nat. Commun. 2017, 8, 623. [Google Scholar] [CrossRef]
- Di Pardo, A.; Pepe, G.; Capocci, L.; Marracino, F.; Amico, E.; Del Vecchio, L.; Giova, S.; Jeong, S.K.; Park, B.M.; Park, B.D.; et al. Treatment with K6PC-5, a selective stimulator of SPHK1, ameliorates intestinal homeostasis in an animal model of Huntington’s disease. Neurobiol. Dis. 2020, 143, 105009. [Google Scholar] [CrossRef] [PubMed]
- Di Pardo, A.; Pepe, G.; Castaldo, S.; Marracino, F.; Capocci, L.; Amico, E.; Madonna, M.; Giova, S.; Jeong, S.K.; Park, B.M.; et al. Stimulation of Sphingosine Kinase 1 (SPHK1) Is Beneficial in a Huntington’s Disease Pre-clinical Model. Front. Mol. Neurosci. 2019, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Di Pardo, A.; Castaldo, S.; Amico, E.; Pepe, G.; Marracino, F.; Capocci, L.; Giovannelli, A.; Madonna, M.; van Bergeijk, J.; Buttari, F.; et al. Stimulation of S1PR5 with A-971432, a selective agonist, preserves blood-brain barrier integrity and exerts therapeutic effect in an animal model of Huntington’s disease. Hum. Mol. Genet. 2018, 27, 2490–2501. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, K.P.; Hong, E.J.; Carney, K.M.; D’Alecy, L.G. Potential adaptations to acute hypoxia: Hct, stress proteins, and set point for temperature regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 266, R1615–R1622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Haddad, G.G.; Xia, Y. δ-, but not μ-and κ-, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res. 2000, 885, 143–153. [Google Scholar] [CrossRef]
- Zhu, M.; Li, M.; Yang, F.; Ou, X.; Ren, Q.; Gao, H.; Zhu, C.; Guo, J. Mitochondrial ERK plays a key role in delta-opioid receptor neuroprotection against acute mitochondrial dysfunction. Neurochem. Int. 2011, 59, 739–748. [Google Scholar] [CrossRef]
- Zhu, M.; Li, M.-W.; Tian, X.-S.; Ou, X.-M.; Zhu, C.-Q.; Guo, J.-C. Neuroprotective role of δ-opioid receptors against mitochondrial respiratory chain injury. Brain Res. 2009, 1252, 183–191. [Google Scholar] [CrossRef]
- Burtscher, J.; Bean, C.; Zangrandi, L.; Kmiec, I.; Agostinho, A.; Scorrano, L.; Gnaiger, E.; Schwarzer, C. Proenkephalin Derived Peptides are Involved in the Modulation of Mitochondrial Respiratory Control During Epileptogenesis. Front. Mol. Neurosci. 2018, 11, 351. [Google Scholar] [CrossRef]
- Burtscher, J.; Schwarzer, C. The opioid system in temporal lobe epilepsy: Functional role and therapeutic potential. Front. Mol. Neurosci. 2017, 10, 245. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Chao, D.; Sandhu, H.K.; Liao, X.; Zhao, J.; Wen, G.; Xia, Y. δ-Opioid receptor activation reduces α-synuclein overexpression and oligomer formation induced by MPP+ and/or hypoxia. Exp. Neurol. 2014, 255, 127–136. [Google Scholar] [CrossRef]
- Duque-Díaz, E.; Alvarez-Ojeda, O.; Coveñas, R. Enkephalins and ACTH in the mammalian nervous system. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2019; Volume 111, pp. 147–193. [Google Scholar]
- McLaughlin, P.J. Chapter 219-Proenkephalin-Derived Peptides. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 1602–1609. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, X.-M.; Li, Y.-H.; Lu, G.-W.; Chen, B. Effect of repeated acute hypoxic treatment on the expression of alpha-synuclein in the mouse brain cortex. Acta Physiol. Sin. Chin. Ed. 2004, 56, 263–268. [Google Scholar]
- Hilditch-Maguire, P.; Trettel, F.; Passani, L.A.; Auerbach, A.; Persichetti, F.; MacDonald, M.E. Huntingtin: An iron-regulated protein essential for normal nuclear and perinuclear organelles. Hum. Mol. Genet. 2000, 9, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.-C.; Qian, H.; Ghassemi, F.; Zhao, P.; Xia, Y. Oxygen-sensitive δ-opioid receptor-regulated survival and death signals novel insights into neuronal preconditioning and protection. J. Biol. Chem. 2005, 280, 16208–16218. [Google Scholar] [CrossRef]
- Gao, C.-J.; Niu, L.; Ren, P.-C.; Wang, W.; Zhu, C.; Li, Y.-Q.; Chai, W.; Sun, X.-D. Hypoxic preconditioning attenuates global cerebral ischemic injury following asphyxial cardiac arrest through regulation of delta opioid receptor system. Neuroscience 2012, 202, 352–362. [Google Scholar] [CrossRef]
- Schoos, A.; Gabriel, C.; Knab, V.M.; Fux, D.A. Activation of HIF-1α by δ-opioid receptors induces COX-2 expression in breast cancer cells and leads to paracrine activation of vascular endothelial cells. J. Pharmacol. Exp. Ther. 2019, 370, 480–489. [Google Scholar] [CrossRef]
- Bissonnette, S.; Vaillancourt, M.; Hébert, S.S.; Drolet, G.; Samadi, P. Striatal pre-enkephalin overexpression improves Huntington’s disease symptoms in the R6/2 mouse model of Huntington’s disease. PLoS ONE 2013, 8, e75099. [Google Scholar] [CrossRef]
- Cull-Candy, S.; Brickley, S.; Farrant, M. NMDA receptor subunits: Diversity, development and disease. Curr. Opin. Neurobiol. 2001, 11, 327–335. [Google Scholar] [CrossRef]
- Hardingham, G.E. Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem. Soc. Trans. 2009, 37, 1147–1160. [Google Scholar] [CrossRef]
- Gonda, X. Basic pharmacology of NMDA receptors. Curr. Pharm. Des. 2012, 18, 1558–1567. [Google Scholar] [CrossRef]
- Schwarcz, R.; Meldrum, B. Excitatory aminoacid antagonists provide a therapeutic approach to neurological disorders. Lancet 1985, 326, 140–143. [Google Scholar] [CrossRef]
- Greenamyre, J.T. The role of glutamate in neurotransmission and in neurologic disease. Arch. Neurol. 1986, 43, 1058–1063. [Google Scholar] [CrossRef]
- Vizi, E.S.; Kisfali, M.; Lőrincz, T. Role of nonsynaptic GluN2B-containing NMDA receptors in excitotoxicity: Evidence that fluoxetine selectively inhibits these receptors and may have neuroprotective effects. Brain Res. Bull. 2013, 93, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Bading, H. Therapeutic targeting of the pathological triad of extrasynaptic NMDA receptor signaling in neurodegenerations. J. Exp. Med. 2017, 214, 569–578. [Google Scholar] [CrossRef]
- Fernandes, H.B.; Raymond, L.A. NMDA receptors and Huntington’s disease. In Biology of the NMDA Receptor; Van Dongen, A.M., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; pp. 17–40. [Google Scholar]
- Rothman, S.M.; Olney, J.W. Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child. Neurol. Soc. 1986, 19, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Goldberg, M.P.; Choi, D.W. Do NMDA antagonists prevent neuronal injury? Yes. Arch. Neurol. 1992, 49, 418–420. [Google Scholar] [CrossRef]
- Choi, D.W. Calcium: Still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995, 18, 58–60. [Google Scholar] [CrossRef]
- Pellegrini-Giampietro, D.E.; Peruginelli, F.; Meli, E.; Cozzi, A.; Albani-Torregrossa, S.; Pellicciari, R.; Moroni, F. Protection with metabotropic glutamate 1 receptor antagonists in models of ischemic neuronal death: Time-course and mechanisms. Neuropharmacology 1999, 38, 1607–1619. [Google Scholar] [CrossRef]
- Kobayashi, S.; Harris, V.A.; Welsh, F.A. Spreading depression induces tolerance of cortical neurons to ischemia in rat brain. J. Cereb. Blood Flow Metab. 1995, 15, 721–727. [Google Scholar] [CrossRef]
- Matsushima, K.; Hogan, M.J.; Hakim, A.M. Cortical spreading depression protects against subsequent focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 1996, 16, 221–226. [Google Scholar] [CrossRef]
- Kawahara, N.; Croll, S.D.; Wiegand, S.J.; Klatzo, I. Cortical spreading depression induces long-term alterations of BDNF levels in cortex and hippocampus distinct from lesion effects: Implications for ischemic tolerance. Neurosci. Res. 1997, 29, 37–47. [Google Scholar] [CrossRef]
- Plumier, J.-C.; Krueger, A.; Currie, R.; Kontoyiannis, D.; Kollias, G.; Pagoulatos, G. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperon. 1997, 2, 162. [Google Scholar] [CrossRef]
- Kato, H.; Liu, Y.; Araki, T.; Kogure, K. MK-801, but not anisomycin, inhibits the induction of tolerance to ischemia in the gerbil hippocampus. Neurosci. Lett. 1992, 139, 118–121. [Google Scholar] [CrossRef]
- Chauhan, N.K.; Young, A.M.; Gibson, C.L.; Davidson, C. Inhibition of pre-ischeamic conditioning in the mouse caudate brain slice by NMDA-or adenosine A1 receptor antagonists. Eur. J. Pharmacol. 2013, 698, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Pringle, A.; Iannotti, F.; Wilde, G.; Chad, J.; Seeley, P.; Sundstrom, L. Neuroprotection by both NMDA and non-NMDA receptor antagonists in in vitro ischemia. Brain Res. 1997, 755, 36–46. [Google Scholar] [CrossRef]
- Chen, M.; Lu, T.-J.; Chen, X.-J.; Zhou, Y.; Chen, Q.; Feng, X.-Y.; Xu, L.; Duan, W.-H.; Xiong, Z.-Q. Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke 2008, 39, 3042–3048. [Google Scholar] [CrossRef] [PubMed]
- Wroge, C.M.; Hogins, J.; Eisenman, L.; Mennerick, S. Synaptic NMDA receptors mediate hypoxic excitotoxic death. J. Neurosci. 2012, 32, 6732–6742. [Google Scholar] [CrossRef]
- Yan, J.; Bengtson, C.P.; Buchthal, B.; Hagenston, A.M.; Bading, H. Coupling of NMDA receptors and TRPM4 guides discovery of unconventional neuroprotectants. Science 2020, 370, eaay3302. [Google Scholar] [CrossRef]
- Cho, C.-H.; Kim, E.; Lee, Y.-S.; Yarishkin, O.; Yoo, J.C.; Park, J.-Y.; Hong, S.-G.; Hwang, E.M. Depletion of 14-3-3γ reduces the surface expression of Transient Receptor Potential Melastatin 4b (TRPM4b) Channels and attenuates TRPM4b-mediated glutamate-induced neuronal cell death. Mol. Brain 2014, 7, 52. [Google Scholar] [CrossRef]
- Sun, H.-S.; Jackson, M.F.; Martin, L.J.; Jansen, K.; Teves, L.; Cui, H.; Kiyonaka, S.; Mori, Y.; Jones, M.; Forder, J.P. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat. Neurosci. 2009, 12, 1300–1307. [Google Scholar] [CrossRef]
- Aarts, M.; Iihara, K.; Wei, W.-L.; Xiong, Z.-G.; Arundine, M.; Cerwinski, W.; MacDonald, J.F.; Tymianski, M. A key role for TRPM7 channels in anoxic neuronal death. Cell 2003, 115, 863–877. [Google Scholar] [CrossRef]
- Mattson, M.P.; Goodman, Y.; Luo, H.; Fu, W.; Furukawa, K. Activation of NF-κB protects hippocampal neurons against oxidative stress-induced apoptosis: Evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J. Neurosci. Res. 1997, 49, 681–697. [Google Scholar] [CrossRef]
- Hu, B.; Fux, C.; Martone, M.; Zivin, J.; Ellisman, M. Persistent phosphorylation of cyclic AMP responsive element-binding protein and activating transcription factor-2 transcription factors following transient cerebral ischemia in rat brain. Neuroscience 1999, 89, 437–452. [Google Scholar] [CrossRef]
- Soriano, F.X.; Papadia, S.; Hofmann, F.; Hardingham, N.R.; Bading, H.; Hardingham, G.E. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J. Neurosci. 2006, 26, 4509–4518. [Google Scholar] [CrossRef] [PubMed]
- Steiger, H.-J.; Hänggi, D. Ischaemic preconditioning of the brain, mechanisms and applications. Acta Neurochir. 2007, 149, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Iwabuchi, S.; Miyazaki, H.; Okuma, Y.; Inanami, O.; Kuwabara, M.; Nomura, Y.; Kawahara, K. Relationship between the activation of cyclic AMP responsive element binding protein and ischemic tolerance in the penumbra region of rat cerebral cortex. Neurosci. Lett. 2002, 331, 13–16. [Google Scholar] [CrossRef]
- Meller, R.; Minami, M.; Cameron, J.A.; Impey, S.; Chen, D.; Lan, J.-Q.; Henshall, D.C.; Simon, R.P. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J. Cereb. Blood Flow Metab. 2005, 25, 234–246. [Google Scholar] [CrossRef]
- Zhang, Q.-G.; Wang, R.-M.; Han, D.; Yang, L.-C.; Li, J.; Brann, D.W. Preconditioning neuroprotection in global cerebral ischemia involves NMDA receptor-mediated ERK-JNK3 crosstalk. Neurosci. Res. 2009, 63, 205–212. [Google Scholar] [CrossRef]
- Granado, N.; Ares-Santos, S.; O’Shea, E.; Vicario-Abejón, C.; Colado, M.I.; Moratalla, R. Selective vulnerability in striosomes and in the nigrostriatal dopaminergic pathway after methamphetamine administration. Neurotox. Res. 2010, 18, 48–58. [Google Scholar] [CrossRef]
- Mallet, R.T.; Burtscher, J.; Manukhina, E.B.; Downey, H.F.; Glazachev, O.S.; Serebrovskaya, T.V.; Burtscher, M. Hypoxic–hyperoxic conditioning and dementia. In Diagnosis and Management in Dementia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 745–760. [Google Scholar]
- Lautenschläger, J.; Wagner-Valladolid, S.; Stephens, A.D.; Fernández-Villegas, A.; Hockings, C.; Mishra, A.; Manton, J.D.; Fantham, M.J.; Lu, M.; Rees, E.J.; et al. Intramitochondrial proteostasis is directly coupled to α-synuclein and amyloid β 1-42 pathologies. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Klapstein, G.J.; Levine, M.S. Age-dependent biphasic changes in ischemic sensitivity in the striatum of Huntington’s disease R6/2 transgenic mice. J. Neurophysiol. 2005, 93, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Centonze, D.; Gubellini, P.; Marfia, G.A.; Pisani, A.; Sancesario, G.; Bernardi, G. Synaptic transmission in the striatum: From plasticity to neurodegeneration. Prog. Neurobiol. 2000, 61, 231–265. [Google Scholar] [CrossRef]
- Calabresi, P.; Centonze, D.; Bernardi, G. Cellular factors controlling neuronal vulnerability in the brain: A lesson from the striatum. Neurology 2000, 55, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factor 1: Regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim. Biophys. Acta BBA Bioenerg. 2011, 1813, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Tello, D.; Balsa, E.; Acosta-Iborra, B.; Fuertes-Yebra, E.; Elorza, A.; Ordóñez, Á.; Corral-Escariz, M.; Soro, I.; López-Bernardo, E.; Perales-Clemente, E. Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting Complex I activity. Cell Metab. 2011, 14, 768–779. [Google Scholar] [CrossRef]
- Lukyanova, L.D.; Kirova, Y.I. Mitochondria-controlled signaling mechanisms of brain protection in hypoxia. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Hayashi, T.; Asano, Y.; Shintani, Y.; Aoyama, H.; Kioka, H.; Tsukamoto, O.; Hikita, M.; Shinzawa-Itoh, K.; Takafuji, K.; Higo, S. Higd1a is a positive regulator of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2015, 112, 1553–1558. [Google Scholar] [CrossRef]
- Fukuda, R.; Zhang, H.; Kim, J.-W.; Shimoda, L.; Dang, C.V.; Semenza, G.L. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 2007, 129, 111–122. [Google Scholar] [CrossRef]
- Li, H.-S.; Zhou, Y.-N.; Li, L.; Li, S.-F.; Long, D.; Chen, X.-L.; Zhang, J.-B.; Feng, L.; Li, Y.-P. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019, 25, 101109. [Google Scholar] [CrossRef]
- Browne, S.E. Mitochondria and Huntington’s disease pathogenesis: Insight from genetic and chemical models. Ann. N. Y. Acad. Sci. 2008, 1147, 358–382. [Google Scholar] [CrossRef]
- Beal, M.F.; Brouillet, E.; Jenkins, B.G.; Ferrante, R.J.; Kowall, N.W.; Miller, J.; Storey, E.; Srivastava, R.; Rosen, B.; Hyman, B. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J. Neurosci. 1993, 13, 4181–4192. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Gould, D. Nature and distribution of brain lesions in rats intoxicated with 3-nitropropionic acid: A type of hypoxic (energy deficient) brain damage. Acta Neuropathol. 1987, 72, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, F.; Liao, W.; Busch, C.; Castell, S.; Knapp, F.; Lindauer, U.; Megow, D.; Meisel, A.; Redetzky, A.; Ruscher, K.; et al. Respiratory chain inhibition induces tolerance to focal cerebral ischemia. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1999, 19, 1229–1237. [Google Scholar] [CrossRef]
- Pang, Z.; Geddes, J.W. Mechanisms of cell death induced by the mitochondrial toxin 3-nitropropionic acid: Acute excitotoxic necrosis and delayed apoptosis. J. Neurosci. 1997, 17, 3064–3073. [Google Scholar] [CrossRef] [PubMed]
- Damiano, M.; Diguet, E.; Malgorn, C.; D’Aurelio, M.; Galvan, L.; Petit, F.; Benhaim, L.; Guillermier, M.; Houitte, D.; Dufour, N.; et al. A role of mitochondrial complex II defects in genetic models of Huntington’s disease expressing N-terminal fragments of mutant huntingtin. Hum. Mol. Genet. 2013, 22, 3869–3882. [Google Scholar] [CrossRef]
- Song, W.; Chen, J.; Petrilli, A.; Liot, G.; Klinglmayr, E.; Zhou, Y.; Poquiz, P.; Tjong, J.; Pouladi, M.A.; Hayden, M.R. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat. Med. 2011, 17, 377–382. [Google Scholar] [CrossRef]
- Riguet, N.; Mahul-Mellier, A.-L.; Maharjan, N.; Burtscher, J.; Patin, A.; Croisier, M.; Knott, G.W.; Reiterer, V.; Farhan, H.; Lashuel, H. Disentangling the sequence, cellular and ultrastructural determinants of Huntingtin nuclear and cytoplasmic inclusion formation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, X.; Shen, K.; Wang, J.; Liu, K.; Wu, G.; Li, Y.; Luo, L.; Zheng, Z.; Hu, D. Hypoxic preconditioning combined with curcumin promotes cell survival and mitochondrial quality of bone marrow mesenchymal stem cells, and accelerates cutaneous wound healing via PGC-1α/SIRT3/HIF-1α signaling. Free Radic. Biol. Med. 2020, 159, 164–176. [Google Scholar] [CrossRef]
- Cui, L.; Jeong, H.; Borovecki, F.; Parkhurst, C.N.; Tanese, N.; Krainc, D. Transcriptional repression of PGC-1α by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 2006, 127, 59–69. [Google Scholar] [CrossRef]
- Cherubini, M.; Lopez-Molina, L.; Gines, S. Mitochondrial fission in Huntington’s disease mouse striatum disrupts ER-mitochondria contacts leading to disturbances in Ca2+ efflux and Reactive Oxygen Species (ROS) homeostasis. Neurobiol. Dis. 2020, 136, 104741. [Google Scholar] [CrossRef]
- Naseri, N.N.; Bonica, J.; Xu, H.; Park, L.C.; Arjomand, J.; Chen, Z.; Gibson, G.E. Novel metabolic abnormalities in the tricarboxylic acid cycle in peripheral cells from Huntington’s disease patients. PLoS ONE 2016, 11, e0160384. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.E.; Ferrante, R.J.; Beal, M.F. Oxidative stress in Huntington’s disease. Brain Pathol. 1999, 9, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Winderickx, J.; Franssens, V.; Liu, B. A mitochondria-associated oxidative stress perspective on Huntington’s Disease. Front. Mol. Neurosci. 2018, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Sazontova, T.; Glazachev, O.; Bolotova, A.; Dudnik, E.; Striapko, N.; Bedareva, I.; Anchishkina, N.; Arkhipenko, I. Adaptation to hypoxia and hyperoxia improves physical endurance: The role of reactive oxygen species and redox-signaling. Ross. Fiziol. Zhurnal Im. IM Sechenova 2012, 98, 793–807. [Google Scholar]
- Okamoto, A.; Sumi, C.; Tanaka, H.; Kusunoki, M.; Iwai, T.; Nishi, K.; Matsuo, Y.; Harada, H.; Takenaga, K.; Bono, H.; et al. HIF-1-mediated suppression of mitochondria electron transport chain function confers resistance to lidocaine-induced cell death. Sci. Rep. 2017, 7, 3816. [Google Scholar] [CrossRef]
- Lee, H.; Fenster, R.J.; Pineda, S.S.; Gibbs, W.S.; Mohammadi, S.; Davila-Velderrain, J.; Garcia, F.J.; Therrien, M.; Novis, H.S.; Gao, F.; et al. Cell Type-Specific Transcriptomics Reveals that Mutant Huntingtin Leads to Mitochondrial RNA Release and Neuronal Innate Immune Activation. Neuron 2020. [Google Scholar] [CrossRef]
- Dhir, A.; Dhir, S.; Borowski, L.S.; Jimenez, L.; Teitell, M.; Rötig, A.; Crow, Y.J.; Rice, G.I.; Duffy, D.; Tamby, C. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 2018, 560, 238–242. [Google Scholar] [CrossRef]
- Cummins, E.P.; Keogh, C.E.; Crean, D.; Taylor, C.T. The role of HIF in immunity and inflammation. Mol. Asp. Med. 2016, 47–48, 24–34. [Google Scholar] [CrossRef]
- Thompson, A.R.; Dickinson, R.S.; Murphy, F.; Thomson, J.P.; Marriott, H.M.; Tavares, A.; Willson, J.; Williams, L.; Lewis, A.; Mirchandani, A. Hypoxia determines survival outcomes of bacterial infection through HIF-1alpha dependent re-programming of leukocyte metabolism. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef]
- Chen, Y.-M.; He, X.-Z.; Wang, S.-M.; Xia, Y. δ-Opioid Receptors, microRNAs, and Neuroinflammation in Cerebral Ischemia/Hypoxia. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Talhi, O.; Jang, D.; Cerella, C.; Gaigneaux, A.; Kim, K.-W.; Lee, J.W.; Dicato, M.; Bachari, K.; Han, B.W.; et al. Cytostatic hydroxycoumarin OT52 induces ER/Golgi stress and STAT3 inhibition triggering non-canonical cell death and synergy with BH3 mimetics in lung cancer. Cancer Lett. 2018, 416, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Holt-Martyn, J.P.; Schofield, C.J.; Ratcliffe, P.J. Pharmacological targeting of the HIF hydroxylases—A new field in medicine development. Mol. Asp. Med. 2016, 47–48, 54–75. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, S.; Sarchielli, E.; Comeglio, P.; Porfirio, B.; Gallina, P.; Morelli, A.; Vannelli, G.B. Fibroblast growth factor and endothelin-1 receptors mediate the response of human striatal precursor cells to hypoxia. Neuroscience 2015, 289, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Niatsetskaya, Z.; Basso, M.; Speer, R.E.; McConoughey, S.J.; Coppola, G.; Ma, T.C.; Ratan, R.R. HIF prolyl hydroxylase inhibitors prevent neuronal death induced by mitochondrial toxins: Therapeutic implications for Huntington’s disease and Alzheimer’s disease. Antioxid. Redox Signal. 2010, 12, 435–443. [Google Scholar] [CrossRef]
- Yang, Y.T.; Ju, T.C.; Yang, D.I. Induction of hypoxia inducible factor-1 attenuates metabolic insults induced by 3-nitropropionic acid in rat C6 glioma cells. J. Neurochem. 2005, 93, 513–525. [Google Scholar] [CrossRef]
- Mehta, R.; Steinkraus, K.A.; Sutphin, G.L.; Ramos, F.J.; Shamieh, L.S.; Huh, A.; Davis, C.; Chandler-Brown, D.; Kaeberlein, M. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science 2009, 324, 1196–1198. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Sun, S. Increased neuronal hypoxic tolerance induced by repetitive chemical hypoxia. J. Huazhong Univ. Sci. Technol. Med. Sci. 2002, 22, 132–134. [Google Scholar] [CrossRef]
- Skillings, E.A.; Morton, A.J. Delayed Onset and Reduced Cognitive Deficits through Pre-Conditioning with 3-Nitropropionic Acid is Dependent on Sex and CAG Repeat Length in the R6/2 Mouse Model of Huntington’s Disease. J. Huntingt. Dis. 2016, 5, 19–32. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, R. Effects of Brain Ischemic Preconditioning on Cognitive Decline and Motor Incoordination in 3-Nitropropionic Acid-Intoxicated Rats: Probable Mechanisms of Action. Neurophysiology 2019, 51, 160–170. [Google Scholar] [CrossRef]
- Baillieul, S.; Chacaroun, S.; Doutreleau, S.; Detante, O.; Pépin, J.L.; Verges, S. Hypoxic conditioning and the central nervous system: A new therapeutic opportunity for brain and spinal cord injuries? Exp. Biol. Med. 2017, 242, 1198–1206. [Google Scholar] [CrossRef]
- Bono-Yagüe, J.; Gómez-Escribano, A.P.; Millán, J.M.; Vázquez-Manrique, R.P. Reactive Species in Huntington Disease: Are They Really the Radicals You Want to Catch? Antioxidants 2020, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.S.; Dorsey, E.R.; Hayden, M.R. Therapeutic approaches to Huntington disease: From the bench to the clinic. Nat. Rev. Drug Discov. 2018, 17, 729–750. [Google Scholar] [CrossRef] [PubMed]
- Katschinski, D.M. Is there a molecular connection between hypoxia and aging? Exp. Gerontol. 2006, 41, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Motori, E.; Atanassov, I.; Kochan, S.M.V.; Folz-Donahue, K.; Sakthivelu, V.; Giavalisco, P.; Toni, N.; Puyal, J.; Larsson, N.G. Neuronal metabolic rewiring promotes resilience to neurodegeneration caused by mitochondrial dysfunction. Sci. Adv. 2020, 6, eaba8271. [Google Scholar] [CrossRef] [PubMed]

| Conditioning Agent | Outcomes | Model | Reference |
|---|---|---|---|
| HIF-1 inducer cobalt chloride (CoCl2) | Hypoxic conditions modulate proliferation and differentiation of human striatal precursor cells and increase VEGF | Human fetal striatal neuroblasts (for transplantation as therapeutic strategy for HD) | [207] |
| HIF-1 inducers cobalt chloride, mimosine and DFO | Attenuated cytotoxicity | C6 astroglial cells, 3-NP and antimycin A toxicity | [209] |
| HIF-1 inducer DFO | Attenuated cytotoxicity and increased VEGF, no rescue of complex II deficits | Mouse striatal and cortical neurons, 3-NP toxicity | [208]. |
| HIF-1 stabilization by knockdown of egl-9 or vhl-1 | Reduced paralysis and increased lifespan | Caenorhabditis elegans with mutated Htt | [210] |
| 3-NP | Increased neuronal hypoxic tolerance | Mouse hippocampal slices, hypoxia | [211] |
| 3-NP | Improvements in multiple behaviors and general health | R6/2 with PolyQ of 250 or 400 | [212] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burtscher, J.; Maglione, V.; Di Pardo, A.; Millet, G.P.; Schwarzer, C.; Zangrandi, L. A Rationale for Hypoxic and Chemical Conditioning in Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 582. https://doi.org/10.3390/ijms22020582
Burtscher J, Maglione V, Di Pardo A, Millet GP, Schwarzer C, Zangrandi L. A Rationale for Hypoxic and Chemical Conditioning in Huntington’s Disease. International Journal of Molecular Sciences. 2021; 22(2):582. https://doi.org/10.3390/ijms22020582
Chicago/Turabian StyleBurtscher, Johannes, Vittorio Maglione, Alba Di Pardo, Grégoire P. Millet, Christoph Schwarzer, and Luca Zangrandi. 2021. "A Rationale for Hypoxic and Chemical Conditioning in Huntington’s Disease" International Journal of Molecular Sciences 22, no. 2: 582. https://doi.org/10.3390/ijms22020582
APA StyleBurtscher, J., Maglione, V., Di Pardo, A., Millet, G. P., Schwarzer, C., & Zangrandi, L. (2021). A Rationale for Hypoxic and Chemical Conditioning in Huntington’s Disease. International Journal of Molecular Sciences, 22(2), 582. https://doi.org/10.3390/ijms22020582





