Extracellular Vesicles from Airway Secretions: New Insights in Lung Diseases
Abstract
:1. Introduction
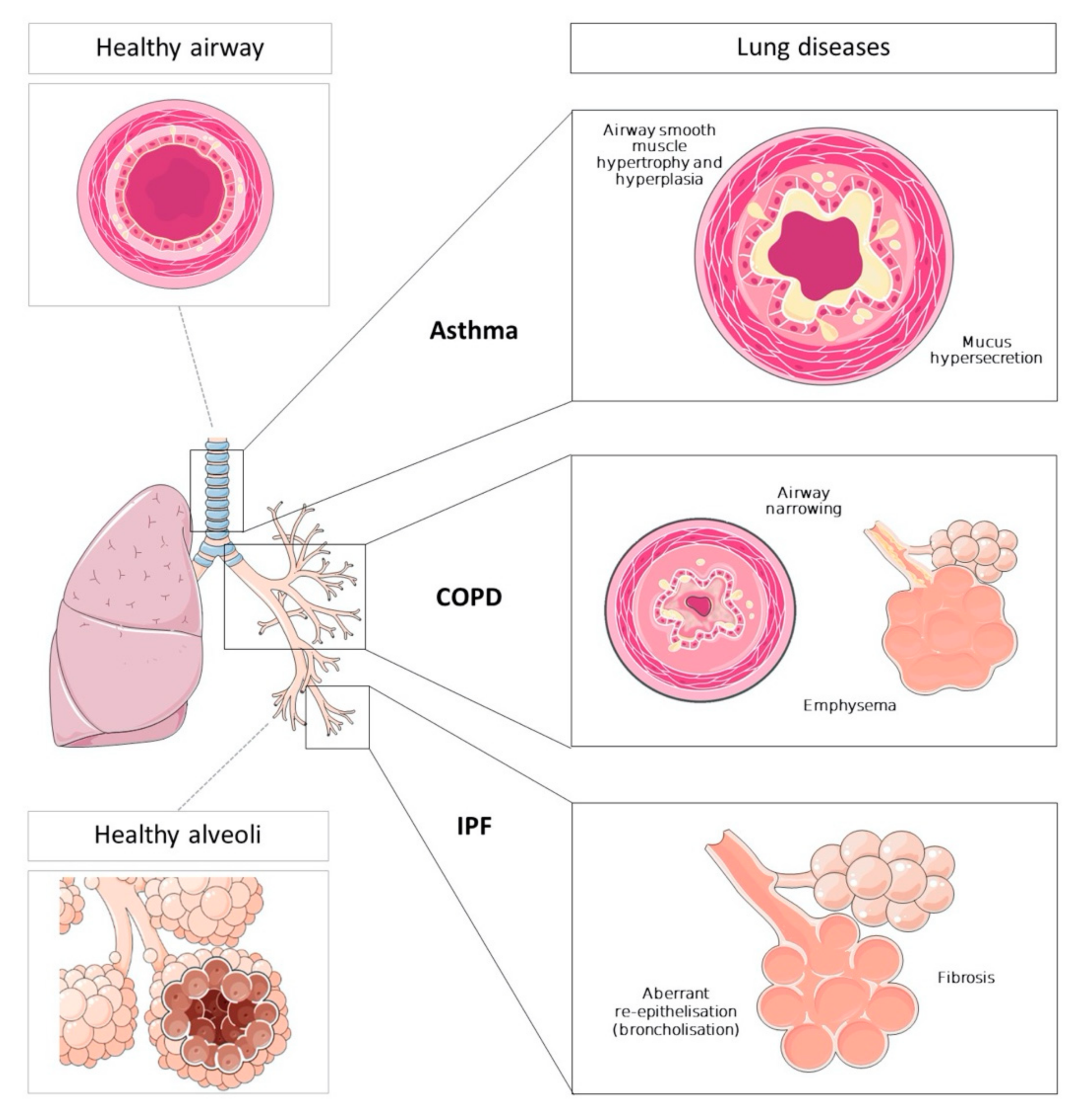
2. Lung Diseases
2.1. Asthma
2.2. Chronic Obstructive Pulmonary Disease
2.3. Idiopathic Pulmonary Fibrosis
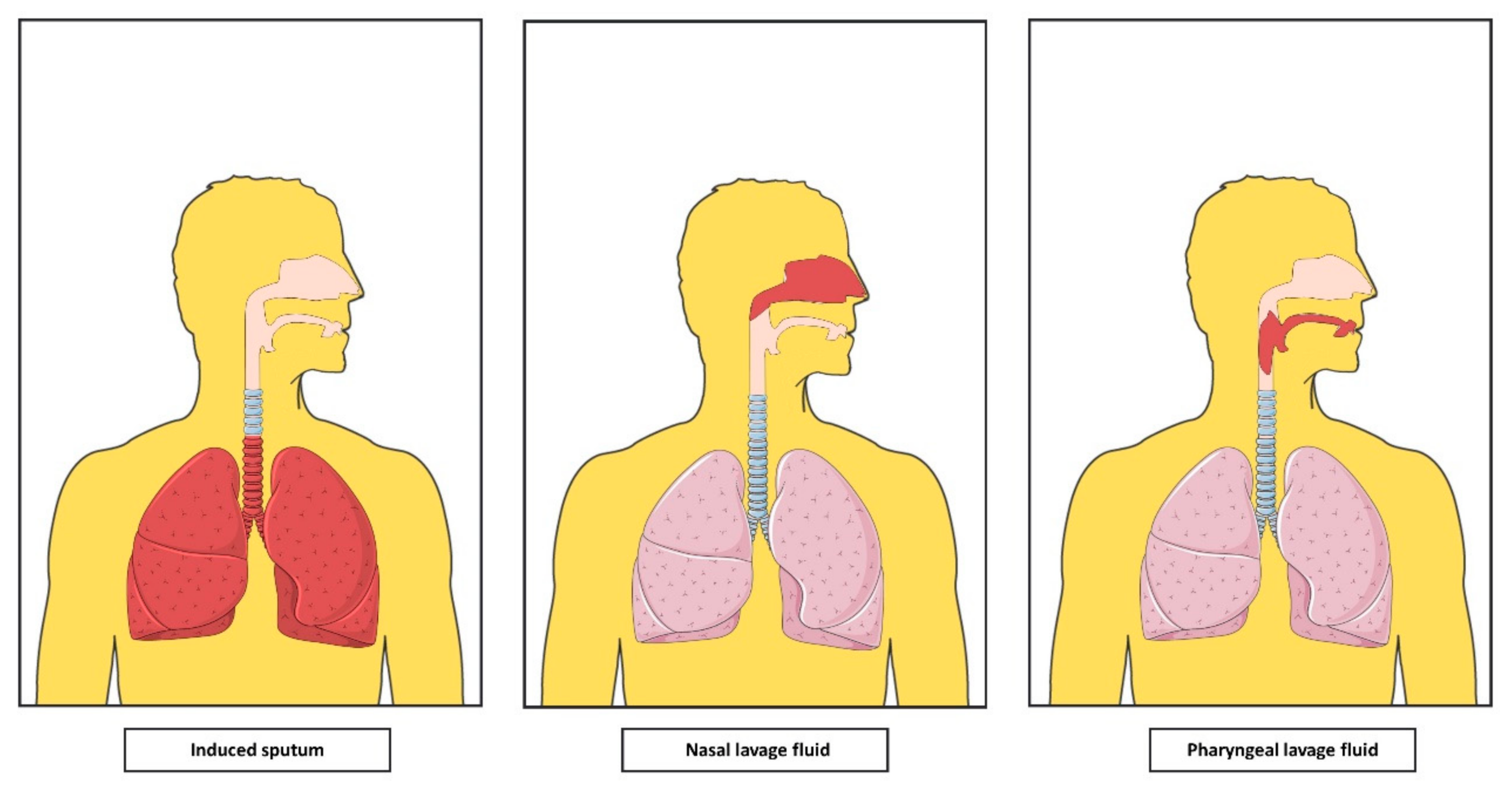
3. Airway Secretions
3.1. Bronchoalveolar Lavage Fluid
3.2. Induced Sputum
3.3. Nasal Lavage Fluid
3.4. Pharyngeal Lavage Fluid
4. Extracellular Vesicles
5. EVs in Lung Pathophysiology
5.1. EVs in Asthma
5.2. EVs in COPD
5.3. EVs in IPF
6. EVs in Airway Secretions and Potential Utility in Clinical Practice
6.1. Airway EVs in Asthma
6.2. Airway EVs in COPD
6.3. Airway EVs in IPF
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-SMA | Alpha-smooth muscle actin |
| AEC1 | Alveolar epithelial cell 1 |
| AEC2 | Alveolar epithelial cell 2 |
| BECs | Bronchial epithelial cells |
| BALF | Bronchoalveolar lavage fluid |
| COPD | Chronic obstructive pulmonary disease |
| CCN | CYR61/CTGF/NOV protein family |
| CCL | C-C motif chemokine ligand |
| CXCL | C-X-C motif chemokine ligand |
| CS | Cigarette smoke |
| EV | Extracellular vesicle |
| FEV1 | Forced expiratory volume in one second |
| FVC | Forced vital capacity |
| IPF | Idiopathic pulmonary fibrosis |
| IS | Induced sputum |
| ICS | Inhaled corticosteroids |
| ICAM-1 | Intercellular adhesion molecule 1 |
| LD | Lung Diseases |
| MMP | Matrix metallopeptidase |
| MSC | Mesenchymal stem cell |
| MCP-1 | Monocyte chemoattractant protein 1 |
| mRNA | Messenger RNA |
| miRNA | MicroRNA |
| MVBs | Multivesicular bodies |
| NL | Nasal lavage fluid |
| NO | Nitric oxide |
| NF-κβ | Nuclear factor kappa beta |
| OSA | Obstructive sleep apnea |
| PHAL | Pharyngeal lavage fluid |
| PGE2 | Prostaglandin E2 |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| SOCS | Suppressor of cytokines signaling |
| TNF-α | Tumoral necrosis factor alpha |
| TGF- β | Transforming growth factor beta |
| WNT | Wingless-type mouse mammary tumor virus integration site family |
References
- European Respiratory Society. The Global Impact of Respiratory Disease, 2nd ed.; European Respiratory Society: Lausanne, Switzerland, 2017. [Google Scholar]
- Shaykhiev, R.; Otaki, F.; Bonsu, P.; Dang, D.T.; Teater, M.; Strulovici-Barel, Y.; Salit, J.; Harvey, B.-G.; Worgall, S. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell. Mol. Life Sci. 2011, 68, 877–892. [Google Scholar] [CrossRef] [Green Version]
- Andres, J.; Smith, L.C.; Murray, A.; Jin, Y.; Businaro, R.; Laskin, J.D.; Laskin, D.L. Role of extracellular vesicles in cell-cell communication and inflammation following exposure to pulmonary toxicants. Cytokine Growth Factor Rev. 2020, 51, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.-H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems. Chest 2019, 155, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef] [Green Version]
- Muller, L.; Hong, C.-S.; Stolz, D.B.; Watkins, S.C.; Whiteside, T.L. Isolation of biologically-active exosomes from human plasma. J. Immunol. Methods 2014, 411, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-Pertierra, E.; Oliveira-Rodríguez, M.; Rivas, M.; Oliva, P.; Villafani, J.; Navarro, A.; Blanco-López, M.C.; Cernuda-Morollón, E. Characterization of Plasma-Derived Extracellular Vesicles Isolated by Different Methods: A Comparison Study. Bioengineering 2019, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.-M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Crossland, R.E.; Norden, J.; Bibby, L.A.; Davis, J.; Dickinson, A.M. Evaluation of optimal extracellular vesicle small RNA isolation and qRT-PCR normalisation for serum and urine. J. Immunol. Methods 2016, 429, 39–49. [Google Scholar] [CrossRef]
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Vidaurre, S.; Eldh, M.; Larssen, P.; Daham, K.; Martinez-Bravo, M.-J.; Dahlén, S.-E.; Dahlén, B.; Van Hage, M.; Gabrielsson, S. RNA-containing exosomes in induced sputum of asthmatic patients. J. Allergy Clin. Immunol. 2017, 140, 1459–1461.e2. [Google Scholar] [CrossRef] [Green Version]
- Admyre, C.; Grunewald, J.; Thyberg, J.; Gripenbäck, S.; Tornling, G.; Eklund, A.; Scheynius, A.; Gabrielsson, S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur. Respir. J. 2003, 22, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zhang, D.; Zhu, Z.; Cruz, C.S.D.; Jin, Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci. Rep. 2016, 6, 35250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.-G.; Cao, Y.; Yang, J.; Lee, J.H.; Choi, H.S.; Jin, Y. Lung epithelial cell-derived extracellular vesicles activate macrophage-mediated inflammatory responses via ROCK1 pathway. Cell Death Dis. 2015, 6, e2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stremersch, S.; De Smedt, S.C.; Raemdonck, K. Therapeutic and diagnostic applications of extracellular vesicles. J. Control. Release 2016, 244, 167–183. [Google Scholar] [CrossRef] [Green Version]
- Clemmens, H.; Lambert, D.W. Extracellular vesicles: Translational challenges and opportunities. Biochem. Soc. Trans. 2018, 46, 1073–1082. [Google Scholar] [CrossRef]
- Global Asthma Network. The Global Asthma Report; Global Asthma Network: Auckland, New Zeeland, 2018. [Google Scholar]
- Ray, A.; Oriss, T.B.; Wenzel, S.E. Emerging molecular phenotypes of asthma. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L130–L140. [Google Scholar] [CrossRef]
- Wenzel, S.E. Emergence of Biomolecular Pathways to Define Novel Asthma Phenotypes. Type-2 Immunity and Beyond. Am. J. Respir. Cell Mol. Biol. 2016, 55, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Upham, J.W.; Xi, Y. Dendritic Cells in Human Lung Disease: Recent Advances. Chest 2017, 151, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Takhar, P.; Corrigan, C.J.; Smurthwaite, L.; O’Connor, B.J.; Durham, S.R.; Lee, T.H.; Gould, H.J. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J. Allergy Clin. Immunol. 2007, 119, 213–218. [Google Scholar] [CrossRef]
- Bousquet, J.; Chanez, P.; Lacoste, J.Y.; Barnéon, G.; Ghavanian, N.; Enander, I.; Venge, P.; Ahlstedt, S.; Simony-Lafontaine, J.; Godard, P.; et al. Eosinophilic Inflammation in Asthma. N. Engl. J. Med. 1990, 323, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Al-Ramli, W.; Préfontaine, D.; Chouiali, F.; Martin, J.G.; Olivenstein, R.; Lemiere, C.; Hamid, Q. TH17-associated cytokines (IL-17A and IL-17F) in severe asthma. J. Allergy Clin. Immunol. 2009, 123, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Boczkowski, J.; Jeannotte, L.; Delacourt, C. Cellular and molecular mechanisms of goblet cell metaplasia in the respiratory airways. Exp. Lung Res. 2013, 39, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Black, J.L.; Panettieri, R.A.; Banerjee, A.; Berger, P. Airway Smooth Muscle in Asthma. Just a Target for Bronchodilation? Clin. Chest Med. 2012, 33, 543–558. [Google Scholar] [CrossRef] [Green Version]
- Burney, P.G.; Patel, J.; Newson, R.; Minelli, C.; Naghavi, M. Global and regional trends in COPD mortality, 1990–2010. Eur. Respir. J. 2015, 45, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Global Initiative for Chronic Obstructive Lung Disease (GOLD), USA. 2020. Available online: www.goldcopd.org (accessed on 14 April 2020).
- Gan, W.Q.; Man, S.F.P.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Mercado, N.; Ito, K.; Barnes, P.J. Accelerated ageing of the lung in COPD: New concepts. Thorax 2015, 70, 482–489. [Google Scholar] [CrossRef] [Green Version]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-Airway Obstruction and Emphysema in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. Cellular and Molecular Mechanisms of Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2014, 35, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Caramori, G.; Romagnoli, M.; Casolari, P.; Bellettato, C.; Casoni, G.; Boschetto, P.; Chung, K.F.; Barnes, P.J.; Adcock, I.M.; Ciaccia, A.; et al. Nuclear localisation of p65 in sputum macrophages but not in sputum neutrophils during COPD exacerbations. Thorax 2003, 58, 348–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, R.; Thorley, A.; Culpitt, S.V.; Dodd, S.; E Donnelly, L.; DeMattos, C.; Fitzgerald, M.; Barnes, P.J. Alveolar macrophage-mediated elastolysis: Roles of matrix metalloproteinases, cysteine, and serine proteases. Am. J. Physiol. Cell. Mol. Physiol. 2002, 283, L867–L873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, S.; Hodge, G.; Scicchitano, R.; Reynolds, P.N.; Holmes, M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 2003, 81, 289–296. [Google Scholar] [CrossRef]
- Bafadhel, M.; Saha, S.K.; Siva, R.; McCormick, M.; Monteiro, W.; Rugman, P.; Dodson, P.; Pavord, I.D.; Newbold, P.; Brightling, C.E. Sputum IL-5 Concentration Is Associated with a Sputum Eosinophilia and Attenuated by Corticosteroid Therapy in COPD. Respiration 2009, 78, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, J.P.; Fogarty, A.W.; Hubbard, R.B.; McKeever, T.M. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 2015, 46, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Raghu, G.; Weycker, D.; Edelsberg, J.; Bradford, W.Z.; Oster, G. Incidence and Prevalence of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 810–816. [Google Scholar] [CrossRef]
- Ley, B.; Collard, H.R.; King, T.E. Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431–440. [Google Scholar] [CrossRef]
- Kasper, M.; Haroske, G. Alterations in the alveolar epithelium after injury leading to pulmonary fibrosis. Histol. Histopathol. 1996, 11, 463–483. [Google Scholar]
- Liang, J.; Zhang, Y.; Xie, T.; Liu, N.; Chen, H.; Geng, Y.; Kurkciyan, A.; Mena, J.M.; Stripp, B.R.; Jiang, D.; et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat. Med. 2016, 22, 1285–1293. [Google Scholar] [CrossRef]
- Selman, M.; King, T.E.; Pardo, A. Idiopathic Pulmonary Fibrosis: Prevailing and Evolving Hypotheses about Its Pathogenesis and Implications for Therapy. Ann. Intern. Med. 2001, 134, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Barkauskas, C.E.; Cronce, M.J.; Xue, Y.; Harris, J.R.; Liang, J.; Noble, P.W.; Hogan, B.L. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. USA 2011, 108, E1475–E1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horowitz, J.C.; Thannickal, V.J. Epithelial-Mesenchymal Interactions in Pulmonary Fibrosis. Semin. Respir. Crit. Care Med. 2006, 27, 600–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.; Linn, G.; Chow, Y.-H.; Kobayashi, A.; Mittelsteadt, K.; Altemeier, W.A.; Gharib, S.A.; Schnapp, L.M.; Duffield, J.S. Role of Lung Pericytes and Resident Fibroblasts in the Pathogenesis of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2013, 188, 820–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Mih, J.D.; Shea, B.S.; Kho, A.T.; Sharif, A.S.; Tager, A.M.; Tschumperlin, D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010, 190, 693–706. [Google Scholar] [CrossRef] [Green Version]
- Boers, J.E.; Ambergen, A.W.; Thunnissen, F.B.J.M. Number and Proliferation of Basal and Parabasal Cells in Normal Human Airway Epithelium. Am. J. Respir. Crit. Care Med. 1998, 157, 2000–2006. [Google Scholar] [CrossRef]
- Hong, K.U.; Reynolds, S.D.; Watkins, S.; Fuchs, E.; Stripp, B.R. Basal Cells Are a Multipotent Progenitor Capable of Renewing the Bronchial Epithelium. Am. J. Pathol. 2004, 164, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Chilosi, M.; Poletti, V.; Murer, B.; Lestani, M.; Cancellieri, A.; Montagna, L.; Piccoli, P.; Cangi, G.; Semenzato, G.; Doglioni, C. Abnormal Re-epithelialization and Lung Remodeling in Idiopathic Pulmonary Fibrosis: The Role of ΔN-p63. Lab. Investig. 2002, 82, 1335–1345. [Google Scholar] [CrossRef]
- Costabel, U.; Danel, C.; Haslam, P.; Higgenbottam, T.; Klech, H.; Pohl, W.; Semenzato, G. Technical Recommendations and Guidelines for Bronchoalveolar Lavage (BAL). Report of the European Society of Pneumology Task Group. Eur. Respir. J. 1989, 2, 561–585. [Google Scholar]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; Du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An Official American Thoracic Society Clinical Practice Guideline: The Clinical Utility of Bronchoalveolar Lavage Cellular Analysis in Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef]
- Baughman, R.P. Technical Aspects of Bronchoalveolar Lavage: Recommendations for a Standard Procedure. Semin. Respir. Crit. Care Med. 2007, 28, 475–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wattiez, R.; Hermans, C.; Cruyt, C.; Bernard, A.; Falmagne, P. Human Bronchoalveolar Lavage Fluid Protein Two-Dimensional Database: Study of Interstitial Lung Diseases. Electrophoresis 2000, 21, 2703–2712. [Google Scholar] [CrossRef]
- Conickx, G.; Cobos, F.A.; Berge, M.V.D.; Faiz, A.; Timens, W.; Hiemstra, P.S.; Joos, G.F.; Brusselle, G.; Mestdagh, P.; Bracke, K. microRNA profiling in lung tissue and bronchoalveolar lavage of cigarette smoke-exposed mice and in COPD patients: A translational approach. Sci. Rep. 2017, 7, 12871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Vliet, A.; O’Neill, C.A.; Cross, C.E.; Koostra, J.M.; Volz, W.G.; Halliwell, B.; Louie, S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol. Cell. Mol. Physiol. 1999, 276, L289–L296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radha, S.; Ravindra, N.; Afroz, T.; Prasad, S. Diagnostic utility of bronchoalveolar lavage. J. Cytol. 2014, 31, 136–138. [Google Scholar] [CrossRef]
- Meyer, K.C. Bronchoalveolar Lavage as a Diagnostic Tool. Semin. Respir. Crit. Care Med. 2007, 28, 546–560. [Google Scholar] [CrossRef] [Green Version]
- Guiot, J.; Demarche, S.F.; Henket, M.; Paulus, V.; Graff, S.; Schleich, F.; Corhay, J.-L.; Louis, R.; Moermans, C. Methodology for Sputum Induction and Laboratory Processing. J. Vis. Exp. 2017, e56612. [Google Scholar] [CrossRef]
- Paggiaro, P.L.; Chanez, P.; Holz, O.; Ind, P.W.; Djukanović, R.; Maestrelli, P.; Sterk, P.J. Sputum Induction. In European Respiratory Journal, Supplement. Eur. Respir. Soc. 2002, 20, 3s–8s. [Google Scholar] [CrossRef]
- Pizzichini, M.M.M.; Leigh, R.; Djukanovic, R.; Sterk, P.J. Safety of Sputum Induction. Eur. Respir. J. 2002, 20 (Suppl. 37), 9s–18s. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, K.M.; Alexandrakis, M.; Tzanakis, N.; Tsiligianni, I.; Tzortzaki, E.G.; Siafakas, N.M.; Bouros, D. Induced Sputum versus Bronchoalveolar Lavage Fluid in the Evaluation of Patients with Idiopathic Pulmonary Fibrosis. Respiration 2005, 72, 32–38. [Google Scholar] [CrossRef]
- Efthimiadis, A.; Spanevello, A.; Hamid, Q.; Kelly, M.M.; Linden, M.; Louis, R.; Pizzichini, M.M.M.; Pizzichini, E.; Ronchi, C.; Van Overveld, F.; et al. Methods of Sputum Processing for Cell Counts, Immunocytochemistry and in Situ Hybridisation. Eur. Respir. J. 2002, 20, 19s–23s. [Google Scholar] [CrossRef] [Green Version]
- Belda, J.; Leigh, R.; Parameswaran, K.; O’Byrne, P.M.; Sears, M.R.; Hargreave, F.E. Induced Sputum Cell Counts in Healthy Adults. Am. J. Respir. Crit. Care Med. 2000, 161, 475–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brightling, C. Clinical Applications of Induced Sputum. Chest 2006, 129, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Schleich, F.; Manise, M.; Sele, J.; Henket, M.; Seidel, L.; Louis, R. Distribution of sputum cellular phenotype in a large asthma cohort: Predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm. Med. 2013, 13, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seys, S.F. Role of sputum biomarkers in the management of asthma. Curr. Opin. Pulm. Med. 2017, 23, 34–40. [Google Scholar] [CrossRef]
- Haldar, P.; Pavord, I.D. Noneosinophilic asthma: A distinct clinical and pathologic phenotype. J. Allergy Clin. Immunol. 2007, 119, 1043–1052. [Google Scholar] [CrossRef]
- Simpson, J.L.; Scott, R.; Boyle, M.J.; Gibson, P.G. Inflammatory subtypes in asthma: Assessment and identification using induced sputum. Respirology 2006, 11, 54–61. [Google Scholar] [CrossRef]
- Global Strategy for Asthma Management and Prevention Updated 2020. Available online: www.ginasthma.org (accessed on 7 April 2020).
- Vogelmeier, C.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- D’Silva, L.; Cook, R.J.; Allen, C.J.; Hargreave, F.E.; Parameswaran, K. Changing pattern of sputum cell counts during successive exacerbations of airway disease. Respir. Med. 2007, 101, 2217–2220. [Google Scholar] [CrossRef] [Green Version]
- Sobiecka, M.; Kus, J.; Demkow, U.; Filewska, M.; Jozwik, A.; Radwan-Rohrenschef, P.; Chorostowska-Wynimko, J. Induced sputum in patients with interstitial lung disease: A non-invasive surrogate for certain parameters in bronchoalveolar lavage fluid. J. Physiol. Pharmacol. 2008, 59 (Suppl. 6), 645–657. [Google Scholar]
- Belda, J.; Parameswaran, K.; Keith, P.K.; Hargreave, F.E. Repeatability and validity of cell and fluid-phase measurements in nasal fluid: A comparison of two methods of nasal lavage. Clin. Exp. Allergy 2001, 31, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Greiff, L.; Pipkorn, U.; Alkner, U.; Persson, C.G.A. The’nasal pool’device applies controlled concentrations of solutes on human nasal airway mucosa and samples its surface exudations/secretions. Clin. Exp. Allergy 1990, 20, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Grünberg, K.; Timmers, M.C.; Smits, H.H.; de Klerk, E.P.; Dick, E.C.; Spaan, W.J.; Hiemstra, P.S.; Sterk, P.J. Effect of Experimental Rhinovirus 16 Colds on Airway Hyperresponsiveness to Histamine and Interleukin-8 in Nasal Lavage in Asthmatic Subjects In Vivo. Clin. Exp. Allergy 1997, 27, 36–45. [Google Scholar] [CrossRef]
- Prat, J.; Xaubet, A.; Mullol, J.; Plaza, V.; Masó, M.; Lleonart, R.; Picado, C. Immunocytologic analysis of nasal cells obtained by nasal lavage: A comparative study with a standard method of cell identification. Allergy 1993, 48, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Ji, J.F.; Wang, Z.Y.; Wu, M.H.; Cheng, Y.; Jiang, M.J.; Wang, Q.P.; Chen, R.J. The significance of eosinophils in the correlation of upper and lower airway inflammation in patients with chronic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2019, 54, 450–455. [Google Scholar]
- Simpson, J.L.; Bafadhel, M. Alternatives to induced sputum for identifying inflammatory subtypes of asthma. Respirology 2017, 22, 624–625. [Google Scholar] [CrossRef] [Green Version]
- De Farias, C.F.; Amorim, M.M.; Dracoulakis, M.; Caetano, L.B.; Santoro, I.L.; Fernandes, A.L.G. Nasal lavage, blood or sputum: Which is best for phenotyping asthma? Respirology 2016, 22, 671–677. [Google Scholar] [CrossRef] [Green Version]
- Celik, H.; Akpinar, S.; Karabulut, H.; Oktar, P.; Dursun, B.; Erguden, H.C.; Gunay, S.; Sipit, T. Evaluation of IL-8 Nasal Lavage Levels and the Effects of Nasal Involvement on Disease Severity in Patients with Stable Chronic Obstructive Pulmonary Disease. Inflammation 2014, 38, 616–622. [Google Scholar] [CrossRef]
- Hauber, H.P.; Rüller, S.; Müller Peter, E.; Peter, Z. Comparison of Differential Cell Counts in Pharyngeal Lavage from Patients with Obstructive Sleep Apnea and from Healthy Control Persons. Atemwegs- und Lungenkrankheiten 2009, 35, 373–378. [Google Scholar] [CrossRef]
- Vicente, E.; Marin, J.M.; Carrizo, S.J.; Osuna, C.S.; González, R.; Marin-Oto, M.; Forner, M.; Vicente, P.; Cubero, P.; Gil, A.V.; et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur. Respir. J. 2016, 48, 1108–1117. [Google Scholar] [CrossRef] [Green Version]
- I Baess, A.; E Mohamed, E.; Eldowik, Y.M. Study of upper airway inflammation in patients with obstructive sleep apnea–hypopnea syndrome. Egypt. J. Bronchol. 2019, 13, 754. [Google Scholar] [CrossRef]
- Hauber, H.-P.; Rüller, S.; Müller, E.; Hansen, E.; Zabel, P. Pharyngeal Lavage Lymphocytosis in Patients with Obstructive Sleep Apnea: A Preliminary Observation. PLoS ONE 2011, 6, e16277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Heijnen, H.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived From Exocytosis of Multivesicular Bodies and -Granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkin-Smith, G.K.; Tixeira, R.; Paone, S.; Mathivanan, S.; Collins, C.; Liem, M.; Goodall, K.J.; Ravichandran, K.S.; Hulett, M.D.; Poon, I.K.H. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat. Commun. 2015, 6, 7439. [Google Scholar] [CrossRef] [PubMed]
- Wubbolts, R.; Leckie, R.S.; Veenhuizen, P.T.M.; Schwarzmann, G.; Möbius, W.; Hoernschemeyer, J.; Slot, J.W.; Geuze, H.J.; Stoorvogel, W. Proteomic and Biochemical Analyses of Human B Cell-Derived Exosomes: Potential Implications for Their Function and Multivesicular Body Formation. J. Biol. Chem. 2003, 278, 10963–10972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lotvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Samuel, M.; Kumar, S.; Mathivanan, S. Ticket to a bubble ride: Cargo sorting into exosomes and extracellular vesicles. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2019, 1867, 140203. [Google Scholar] [CrossRef] [PubMed]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef]
- Vallhov, H.; Gutzeit, C.; Hultenby, K.; Valenta, R.; Gronlund, H.; Scheynius, A. Dendritic cell-derived exosomes carry the major cat allergen Fel d 1 and induce an allergic immune response. Allergy 2015, 70, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, N.; Lankar, D.; Faure, F.; Regnault, A.; Dumont, C.; Raposo, G.; Hivroz, C. TCR Activation of Human T Cells Induces the Production of Exosomes Bearing the TCR/CD3/ζ Complex. J. Immunol. 2002, 168, 3235–3241. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Mazzeo, C.; Cañas, J.A.; Zafra, M.P.; Mar, F.-N.; Fernández-Nieto, M.; Sanz, V.; Mittelbrunn, M.; Izquierdo, M.; Baixaulli, F.; Sastre, J.; et al. Exosome secretion by eosinophils: A possible role in asthma pathogenesis. J. Allergy Clin. Immunol. 2015, 135, 1603–1613. [Google Scholar] [CrossRef]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res. Ther. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Riancho, J.; Sánchez-Juan, P. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 2179–2181. [Google Scholar] [CrossRef]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging Roles of Exosomes in Normal and Pathological Conditions: New Insights for Diagnosis and Therapeutic Applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimen, M.; Evryviadou, A.; Box, C.L.; Harrison, M.J.; Hazeldine, J.; Dib, L.H.; Kuravi, S.J.; Payne, H.; Price, J.M.; Kavanagh, D.; et al. Appropriation of GPIbα from platelet-derived extracellular vesicles supports monocyte recruitment in systemic inflammation. Haematologica 2019, 105, 1248–1261. [Google Scholar] [CrossRef]
- Zulueta, A.; Peli, V.; Cas, M.D.; Colombo, M.; Paroni, R.; Falleni, M.; Baisi, A.; Bollati, V.; Chiaramonte, R.; Del Favero, E.; et al. Inflammatory role of extracellular sphingolipids in Cystic Fibrosis. Int. J. Biochem. Cell Biol. 2019, 116, 105622. [Google Scholar] [CrossRef]
- Wadey, R.M.; Connolly, K.D.; Mathew, D.; Walters, G.; Rees, D.A.; E James, P. Inflammatory adipocyte-derived extracellular vesicles promote leukocyte attachment to vascular endothelial cells. Atherosclerosis 2019, 283, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Goetzl, E.J.; Schwartz, J.B.; Mustapic, M.; Lobach, I.V.; Daneman, R.; Abner, E.L.; Jicha, G.A. Altered cargo proteins of human plasma endothelial cell–derived exosomes in atherosclerotic cerebrovascular disease. FASEB J. 2017, 31, 3689–3694. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.; Wang, J. Exosomes and Their Noncoding RNA Cargo Are Emerging as New Modulators for Diabetes Mellitus. Cells 2019, 8, 853. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, H.; Jin, X.; Zhang, Y.; Kang, X.; Zhang, Z.; Xu, M.; Qian, Z.; Ma, Z.; Gao, X.; et al. Adipose-specific knockdown of Sirt1 results in obesity and insulin resistance by promoting exosomes release. Cell Cycle 2019, 18, 2067–2082. [Google Scholar] [CrossRef]
- Bell, E.; A Taylor, M. Functional Roles for Exosomal MicroRNAs in the Tumour Microenvironment. Comput. Struct. Biotechnol. J. 2017, 15, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Steinbichler, T.B.; Dudás, J.; Riechelmann, H.; Skvortsova, I.-I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef]
- Chen, J.; Hu, C.; Pan, P. Extracellular Vesicle MicroRNA Transfer in Lung Diseases. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Kubo, H. Extracellular Vesicles in Lung Disease. Chest 2018, 153, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lässer, C.; Jang, S.C.; Lötvall, J. Subpopulations of extracellular vesicles and their therapeutic potential. Mol. Asp. Med. 2018, 60, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Albero, M.; Rubio-Ruiz, B.; Pérez-López, A.M.; Sebastian, V.; Martín-Duque, P.; Arruebo, M.; Santamaria, J.; Unciti-Broceta, A. Cancer-derived exosomes loaded with ultrathin palladium nanosheets for targeted bioorthogonal catalysis. Nat. Catal. 2019, 2, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.; Hariyadi, D.M.; Grainge, C.; Knight, D.; Bartlett, N.W.; Liang, M. Airway epithelial-targeted nanoparticles for asthma therapy. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L500–L509. [Google Scholar] [CrossRef]
- Sanz-Rubio, D.; Martín-Burriel, I.; Gil, A.; Cubero, P.; Forner, M.; Khalyfa, A.; Marin, J.M. Stability of Circulating Exosomal miRNAs in Healthy Subjects. Sci. Rep. 2018, 8, 10306. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.D.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef] [Green Version]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, A.; Ahmad, T.; Agrawal, A.; Ghosh, B. Proinflammatory role of epithelial cell–derived exosomes in allergic airway inflammation. J. Allergy Clin. Immunol. 2013, 131, 1194–1203.e14. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Radicioni, G.; Abdelwahab, S.; Dang, H.; Carpenter, J.; Chua, M.; Mieczkowski, P.A.; Sheridan, J.T.; Randell, S.H.; Kesimer, M. Intercellular Communication between Airway Epithelial Cells Is Mediated by Exosome-Like Vesicles. Am. J. Respir. Cell Mol. Biol. 2019, 60, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Kesimer, M.; Scull, M.; Brighton, B.; DeMaria, G.; Burns, K.; O’Neal, W.; Pickles, R.J.; Sheehan, J.K. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: A possible role in innate defense. FASEB J. 2009, 23, 1858–1868. [Google Scholar] [CrossRef] [Green Version]
- Bourdonnay, E.; Zasłona, Z.; Penke, L.R.K.; Speth, J.M.; Schneider, D.J.; Przybranowski, S.; Swanson, J.A.; Mancuso, P.; Freeman, C.M.; Curtis, J.L.; et al. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J. Exp. Med. 2015, 212, 729–742. [Google Scholar] [CrossRef]
- Admyre, C.; Bohle, B.; Johansson, S.M.; Focke-Tejkl, M.; Valenta, R.; Scheynius, A.; Gabrielsson, S. B cell–derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J. Allergy Clin. Immunol. 2007, 120, 1418–1424. [Google Scholar] [CrossRef]
- Lockett, A.D.; Brown, M.B.; Santos-Falcon, N.; Rush, N.I.; Oueini, H.; Oberle, A.J.; Bolanis, E.; Fragoso, M.A.; Petrusca, D.N.; Serban, K.A.; et al. Active Trafficking of Alpha 1 Antitrypsin across the Lung Endothelium. PLoS ONE 2014, 9, e93979. [Google Scholar] [CrossRef] [Green Version]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Roda, M.A.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e15. [Google Scholar] [CrossRef] [Green Version]
- Porro, C.; Lacedonia, D.; Carpagnano, G.E.; Trotta, T.; Palladino, G.P.; Panaro, M.A.; Zoppo, L.D.; Foschino, M.P. Microparticles in sputum of COPD patients: A potential biomarker of the disease? Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Novelli, F.; Neri, T.; Tavanti, L.; Armani, C.; Noce, C.; Falaschi, F.; Bartoli, M.L.; Martino, F.; Palla, A.; Celi, A.; et al. Procoagulant, Tissue Factor-Bearing Microparticles in Bronchoalveolar Lavage of Interstitial Lung Disease Patients: An Observational Study. PLoS ONE 2014, 9, e95013. [Google Scholar] [CrossRef]
- Martin-Medina, A.; Lehmann, M.; Burgy, O.; Hermann, S.; Baarsma, H.A.; Wagner, D.E.; De Santis, M.M.; Ciolek, F.; Hofer, T.P.; Frankenberger, M.; et al. Increased Extracellular Vesicles Mediate WNT5A Signaling in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 198, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, R.; Tameh, A.T.; Parent, C.A. Exosomes Mediate LTB4 Release during Neutrophil Chemotaxis. PLoS Biol. 2016, 14, e1002336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacy, S.H.; Woeller, C.F.; Thatcher, T.H.; Pollock, S.J.; Small, E.M.; Sime, P.J.; Phipps, R.P. Activated Human Lung Fibroblasts Produce Extracellular Vesicles with Antifibrotic Prostaglandins. Am. J. Respir. Cell Mol. Biol. 2019, 60, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Pua, H.H.; Happ, H.C.; Gray, C.J.; Mar, D.J.; Chiou, N.-T.; Hesse, L.E.; Ansel, K.M. Increased Hematopoietic Extracellular RNAs and Vesicles in the Lung during Allergic Airway Responses. Cell Rep. 2019, 26, 933–944.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levänen, B.; Bhakta, N.R.; Paredes, P.T.; Barbeau, R.; Hiltbrunner, S.; Pollack, J.L.; Sköld, C.M.; Svartengren, M.; Grunewald, J.; Gabrielsson, S.; et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J. Allergy Clin. Immunol. 2013, 131, 894–903.e8. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Araya, J.; Ito, S.; Kobayashi, K.; Kosaka, N.; Yoshioka, Y.; Kadota, T.; Hara, H.; Kuwano, K.; Ochiya, T. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J. Extracell. Vesicles 2015, 4, 28388. [Google Scholar] [CrossRef]
- Xu, H.; Ling, M.; Xue, J.; Dai, X.; Sun, Q.; Chen, C.; Liu, Y.; Zhou, L.; Liu, J.; Luo, F.; et al. Exosomal microRNA-21 derived from bronchial epithelial cells is involved in aberrant epithelium-fibroblast cross-talk in COPD induced by cigarette smoking. Theranostics 2018, 8, 5419–5433. [Google Scholar] [CrossRef]
- Héliot, A.; Landkocz, Y.; Saint-Georges, F.R.; Gosset, P.; Billet, S.; Shirali, P.; Courcot, D.; Martin, P.J. Smoker extracellular vesicles influence status of human bronchial epithelial cells. Int. J. Hyg. Environ. Heal. 2017, 220, 445–454. [Google Scholar] [CrossRef]
- Makiguchi, T.; Yamada, M.; Yoshioka, Y.; Sugiura, H.; Koarai, A.; Chiba, S.; Fujino, N.; Tojo, Y.; Ota, C.; Kubo, H.; et al. Serum extracellular vesicular miR-21-5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.-Y.; Zhang, W.-H.; Ma, W.-T.; Liu, Q.-H.; Xing, L.-H.; Zhao, G.-F. microRNA-328 in exosomes derived from M2 macrophages exerts a promotive effect on the progression of pulmonary fibrosis via FAM13A in a rat model. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Njock, M.-S.; Guiot, J.; A Henket, M.; Nivelles, O.; Thiry, M.; Dequiedt, F.; Corhay, J.-L.; E Louis, R.; Struman, I. Sputum exosomes: Promising biomarkers for idiopathic pulmonary fibrosis. Thorax 2018, 74, 309–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-A.; Sharif, A.S.; Tschumperlin, D.J.; Lau, L.; Limbrey, R.; Howarth, P.; Drazen, J.M. Tissue factor–bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo. J. Allergy Clin. Immunol. 2012, 130, 1375–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najrana, T.; Mahadeo, A.; Abu-Eid, R.; Kreienberg, E.; Schulte, V.; Uzun, A.; Schorl, C.; Goldberg, L.; Quesenberry, P.; Sanchez-Esteban, J. Mechanical stretch regulates the expression of specific miRNA in extracellular vesicles released from lung epithelial cells. J. Cell. Physiol. 2020, 235, 8210–8223. [Google Scholar] [CrossRef] [PubMed]
- Draijer, C.; Speth, J.M.; Penke, L.R.K.; Zaslona, Z.; Bazzill, J.D.; Lugogo, N.; Huang, Y.J.; Moon, J.J.; Peters-Golden, M. Resident alveolar macrophage-derived vesicular SOCS3 dampens allergic airway inflammation. FASEB J. 2020, 34, 4718–4731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañas, J.A.; Sastre, B.; Mazzeo, C.; Fernández-Nieto, M.; Muñoz, J.M.R.; González-Guerra, A.; Izquierdo, M.; Barranco, P.; Quirce, S.; Sastre, J.; et al. Exosomes from eosinophils autoregulate and promote eosinophil functions. J. Leukoc. Biol. 2017, 101, 1191–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañas, J.A.; Sastre, B.; Rodrigo-Muñoz, J.M.; Fernández-Nieto, M.; Barranco, P.; Quirce, S.; Sastre, J.; Del Pozo, V. Eosinophil-derived exosomes contribute to asthma remodelling by activating structural lung cells. Clin. Exp. Allergy 2018, 48, 1173–1185. [Google Scholar] [CrossRef]
- Rossaint, J.; Kühne, K.; Skupski, J.; Van Aken, H.; Looney, M.R.; Hidalgo, A.; Zarbock, A. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat. Commun. 2016, 7, 13464. [Google Scholar] [CrossRef]
- Cañas, J.A.; Sastre, B.; Muñoz, J.M.R.; Del Pozo, V. Exosomes: A new approach to asthma pathology. Clin. Chim. Acta 2019, 495, 139–147. [Google Scholar] [CrossRef]
- Lu, T.X.; Munitz, A.; Rothenberg, M.E. MicroRNA-21 Is Up-Regulated in Allergic Airway Inflammation and Regulates IL-12p35 Expression. J. Immunol. 2009, 182, 4994–5002. [Google Scholar] [CrossRef] [Green Version]
- Elbehidy, R.M.; Youssef, D.M.; El-Shal, A.S.; Shalaby, S.M.; Sherbiny, H.S.; Sherief, L.M.; Akeel, N.E. MicroRNA–21 as a novel biomarker in diagnosis and response to therapy in asthmatic children. Mol. Immunol. 2016, 71, 107–114. [Google Scholar] [CrossRef]
- Gon, Y.; Maruoka, S.; Inoue, T.; Kuroda, K.; Yamagishi, K.; Kozu, Y.; Shikano, S.; Soda, K.; Lötvall, J.; Hashimoto, S. Selective release of miRNAs via extracellular vesicles is associated with house-dust mite allergen-induced airway inflammation. Clin. Exp. Allergy 2017, 47, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-G.; Kim, S.-H.; Gao, J.; Quan, T.; Qin, Z.; Osorio, J.C.; Rosas, I.O.; Wu, M.; Tesfaigzi, Y.; Jin, Y. CCN1 secretion and cleavage regulate the lung epithelial cell functions after cigarette smoke. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L326–L337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiomi, T.; Okada, Y.; Foronjy, R.; Schiltz, J.; Jaenish, R.; Krane, S.; D’Armiento, J. Emphysematous Changes Are Caused by Degradation of Type III Collagen in Transgenic Mice Expressing MMP-1. Exp. Lung Res. 2003, 29, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y.; Tuder, R.M.; Taraseviciene-Stewart, L.; Le Cras, T.D.; Abman, S.; Hirth, P.K.; Waltenberger, J.; Voelkel, N.F. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J. Clin. Investig. 2000, 106, 1311–1319. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-J.; Liu, Y.; Chen, Y.; Yu, D.; Williams, K.J.; Liu, M.-L. Novel Proteolytic Microvesicles Released from Human Macrophages after Exposure to Tobacco Smoke. Am. J. Pathol. 2013, 182, 1552–1562. [Google Scholar] [CrossRef] [Green Version]
- Cordazzo, C.; Petrini, S.; Neri, T.; Lombardi, S.; Carmazzi, Y.; Pedrinelli, R.; Paggiaro, P.; Celi, A. Rapid shedding of proinflammatory microparticles by human mononuclear cells exposed to cigarette smoke is dependent on Ca2+ mobilization. Inflamm. Res. 2014, 63, 539–547. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, K.; Maremanda, K.; Li, D.; Chand, H.S.; Rahman, I. Differential Plasma Exosomal Long Non-Coding RNAs Expression Profiles and Their Emerging Role in E-Cigarette Users, Cigarette, Waterpipe, and Dual Smokers. Plos One. 2020, 15, e0243065. [Google Scholar] [CrossRef]
- He, S.; Chen, D.; Hu, M.; Zhang, L.; Liu, C.; Traini, D.; Grau, G.E.; Zeng, Z.; Lu, J.; Zhou, G.; et al. Bronchial epithelial cell extracellular vesicles ameliorate epithelial-mesenchymal transition in COPD pathogenesis by alleviating M2 macrophage polarization. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 259–271. [Google Scholar] [CrossRef]
- Serban, K.A.; Rezania, S.; Petrusca, D.N.; Poirier, C.; Cao, D.; Justice, M.J.; Patel, M.; Tsvetkova, I.; Kamocki, K.; Mikosz, A.; et al. Structural and functional characterization of endothelial microparticles released by cigarette smoke. Sci. Rep. 2016, 6, 31596. [Google Scholar] [CrossRef]
- Gordon, C.; Gudi, K.; Krause, A.; Sackrowitz, R.; Harvey, B.-G.; Strulovici-Barel, Y.; Mezey, J.G.; Crystal, R.G. Circulating Endothelial Microparticles as a Measure of Early Lung Destruction in Cigarette Smokers. Am. J. Respir. Crit. Care Med. 2011, 184, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E.H. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010, 207, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.T.; Melo, S.A.; Özdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Ii, V.H.G.; LeBleu, V.S.; Kalluri, R. TGF-β1–Containing Exosomes from Injured Epithelial Cells Activate Fibroblasts to Initiate Tissue Regenerative Responses and Fibrosis. J. Am. Soc. Nephrol. 2012, 24, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Szul, T.; Bratcher, P.E.; Fraser, K.B.; Kong, M.; Tirouvanziam, R.; Ingersoll, S.; Sztul, E.; Rangarajan, S.; Blalock, J.E.; Xu, X.; et al. Toll-Like Receptor 4 Engagement Mediates Prolyl Endopeptidase Release from Airway Epithelia via Exosomes. Am. J. Respir. Cell Mol. Biol. 2016, 54, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Hough, K.P.; Wilson, L.S.; Trevor, J.L.; Strenkowski, J.G.; Maina, N.; Kim, Y.-I.; Spell, M.L.; Wang, Y.; Chanda, D.; Dager, J.R.; et al. Unique Lipid Signatures of Extracellular Vesicles from the Airways of Asthmatics. Sci. Rep. 2018, 8, 10340. [Google Scholar] [CrossRef] [PubMed]
- Lässer, C.; E O’Neil, S.; Ekerljung, L.; Ekström, K.; Sjöstrand, M.; Lötvall, J. RNA-containing Exosomes in Human Nasal Secretions. Am. J. Rhinol. Allergy 2011, 25, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Lässer, C.; O’Neil, S.E.; Shelke, G.V.; Sihlbom, C.; Hansson, S.F.; Gho, Y.S.; Lundbäck, B.; Lötvall, J. Exosomes in the nose induce immune cell trafficking and harbour an altered protein cargo in chronic airway inflammation. J. Transl. Med. 2016, 14, 181. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, J.; Cheng, L.; Ni, H.; You, B.; Shan, Y.; Bao, L.; Wu, D.; Zhang, T.; Yue, H.; et al. A disintegrin and metalloprotease 10-containing exosomes derived from nasal polyps promote angiogenesis and vascular permeability. Mol. Med. Rep. 2018, 17, 5921–5927. [Google Scholar] [CrossRef] [Green Version]
- Cruz, F.F.; Borg, Z.D.; Goodwin, M.; Sokocevic, D.; Wagner, D.E.; Coffey, A.; Antunes, M.; Robinson, K.L.; Mitsialis, S.A.; Kourembanas, S.; et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles AmelioratesAspergillusHyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem Cells Transl. Med. 2015, 4, 1302–1316. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Zhang, H.; Wang, C.; He, B.; Liu, X.; Meng, X.; Peng, Y.; Xu, Z.; Fan, X.; Wu, Z.; et al. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J. Extracell. Vesicles 2020, 9, 1723260. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.-M.; Zhuansun, Y.-X.; Chen, R.; Lin, L.; Lin, Y.; Li, J.-G.; Yu-Mo, D.; Yong-Xun, Z.; Rui, C.; Ying, L.; et al. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp. Cell Res. 2018, 363, 114–120. [Google Scholar] [CrossRef]
- Burke, H.; Freeman, A.; Ostridge, K.; Staples, K.J.; Spalluto, M.; Wilkinson, T. Lung exosomal miRNAs discriminate between healthy ex-smokers and COPD. In Airway Cell Biology and Immunopathology; European Respiratory Society (ERS): Lausanne, Switzerland, 2019; Volume 5, p. 212. [Google Scholar]
- Mohamed, A.; Pekoz, A.Y.; Ross, K.; Hutcheon, G.A.; Saleem, I.Y. Pulmonary delivery of Nanocomposite Microparticles (NCMPs) incorporating miR-146a for treatment of COPD. Int. J. Pharm. 2019, 569, 118524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, H.; Wang, X.; Rai, A.; Groot, M.; Jin, Y. Exosome-Mediated Small RNA Delivery: A Novel Therapeutic Approach for Inflammatory Lung Responses. Mol. Ther. 2018, 26, 2119–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Jiang, M.; Meng, J.; Tao, L. Exosomes: Carriers of Pro-Fibrotic Signals and Therapeutic Targets in Fibrosis. Curr. Pharm. Des. 2020, 25, 4496–4509. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, N.; Willis, G.R.; Fernandez-Gonzalez, A.; Reis, M.; Nassiri, S.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shentu, T.-P.; Huang, T.-S.; Cernelc-Kohan, M.; Chan, J.; Wong, S.S.; Espinoza, C.R.; Tan, C.; Gramaglia, I.; Van Der Heyde, H.; Chien, S.; et al. Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic differentiation. Sci. Rep. 2017, 7, 18052. [Google Scholar] [CrossRef] [Green Version]

| EV Cargo | Asthma | COPD | IPF |
|---|---|---|---|
| Proteins | ADAM10 [11], MHC-II [101,103,130], HLA-DR (IS) [11] | α1-antitrypsin [131], CD66b [132,133], CD31, NE [132] | Tissue factor [134], WNT5A [135] |
| Lipids | Leukotriene B4 [136] | PGE2 [137] | |
| miRNAs | miR-223 and miR-142a [138], let-7, miR-200 families, miR-21 [139] | miR-210 [140], miR-21 [141], Let-7e, let-7g, miR-26b [142] | miR-21 [143], miR-328 [144], miR-33a, miR-142 and let-7d [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor, L.; Vera, E.; Marin, J.M.; Sanz-Rubio, D. Extracellular Vesicles from Airway Secretions: New Insights in Lung Diseases. Int. J. Mol. Sci. 2021, 22, 583. https://doi.org/10.3390/ijms22020583
Pastor L, Vera E, Marin JM, Sanz-Rubio D. Extracellular Vesicles from Airway Secretions: New Insights in Lung Diseases. International Journal of Molecular Sciences. 2021; 22(2):583. https://doi.org/10.3390/ijms22020583
Chicago/Turabian StylePastor, Laura, Elisabeth Vera, Jose M. Marin, and David Sanz-Rubio. 2021. "Extracellular Vesicles from Airway Secretions: New Insights in Lung Diseases" International Journal of Molecular Sciences 22, no. 2: 583. https://doi.org/10.3390/ijms22020583
APA StylePastor, L., Vera, E., Marin, J. M., & Sanz-Rubio, D. (2021). Extracellular Vesicles from Airway Secretions: New Insights in Lung Diseases. International Journal of Molecular Sciences, 22(2), 583. https://doi.org/10.3390/ijms22020583





