Molecular Aspects of Senescence and Organismal Ageing—DNA Damage Response, Telomeres, Inflammation and Chromatin
Abstract
:1. Introduction
2. Cellular Ageing
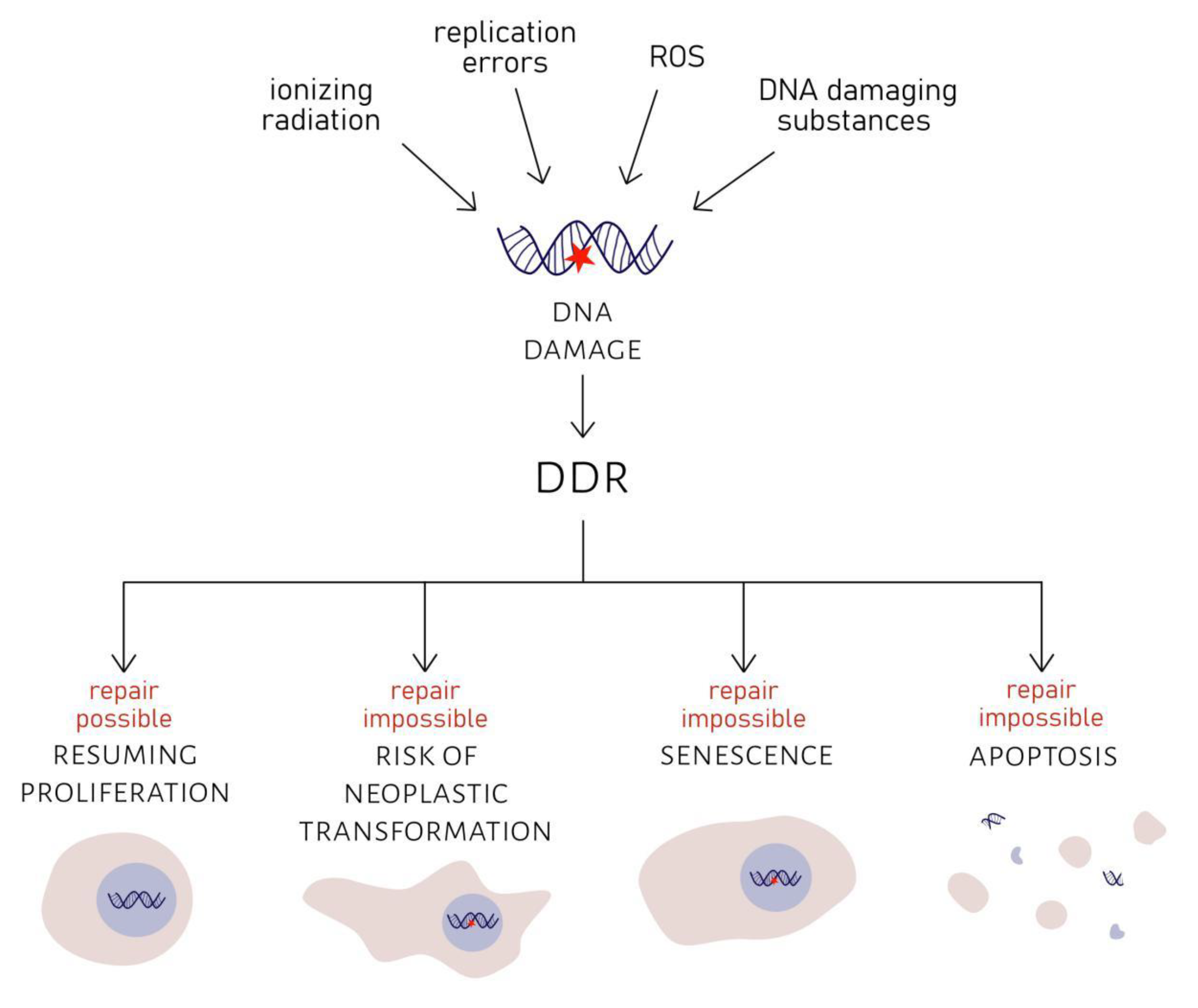
2.1. DNA Damage Response in Ageing Cells
2.2. Role of Telomere Damage in Senescence
- (1)
- If double-strand breaks that appear on telomeres due to random factors cannot be repaired, DDR proteins remain permanently attached to the damage site and the cell enters the state of senescence. These sites of DNA damage are referred to as telomere associated foci (TAF). This is one of the causes of ageing of cells that are not proliferating (differentiated or quiescent). DNA damage repair within telomeres is suppressed by TRF2. This mechanism is indispensable to the cell as it prevents telomere fusion due to NHEJ. However, TRF2 together with Rap1, do not allow regular DSBs that can appear within telomeres due to DNA damaging factors to be repaired [5,28]. In mammalian cells, ectopic insertion of TRF2 in the vicinity of DSB leads to the inability of repairing them and to persistent DDR activation [28]. The presence of TAF is independent of telomere length and telomerase activity; however, it does depend on the age of the organism. The number of TAF in mice hepatocytes and enterocytes increased exponentially with age [37].
- (2)
- DNA damage response is triggered as a result of telomere shortening in proliferating cells. A permanent loss of the ability to divide due to this reason is called replicative ageing. The process of replicative ageing was described for the first time by Hayflick and Moorhead in 1961 when they noticed the limit of divisions that fibroblasts were able to undergo. After going through approximately sixty divisions, cells stopped proliferation and entered a state that was called senescence. The limit of divisions that cells were capable of due to telomere attrition was called the Hayflick limit. Telomere shortening as a result of replicative ageing causes uncapping of a single-stranded fragment on the end of a telomere. DDR factors begin to localise to the uncapped ends, and the cell enters senescence or undergoes apoptosis [38,39]. When the telomere reaches its critical length (under 6000–8000 base pairs), shelterin that protected it is lost [40]. DDR factors such as 53BP1, γ-H2AX, RAD17, ATM and Mre11 localise at these unprotected telomeres [39]. Along with consecutive cell divisions, the number of γ-H2AX foci on telomeres rises from 10% to 72% [40]. These changes are distinctive for double-strand breaks. This kind of telomere damage cannot be repaired. Thus, DDR foci persist, and cell enters the state of senescence [38].
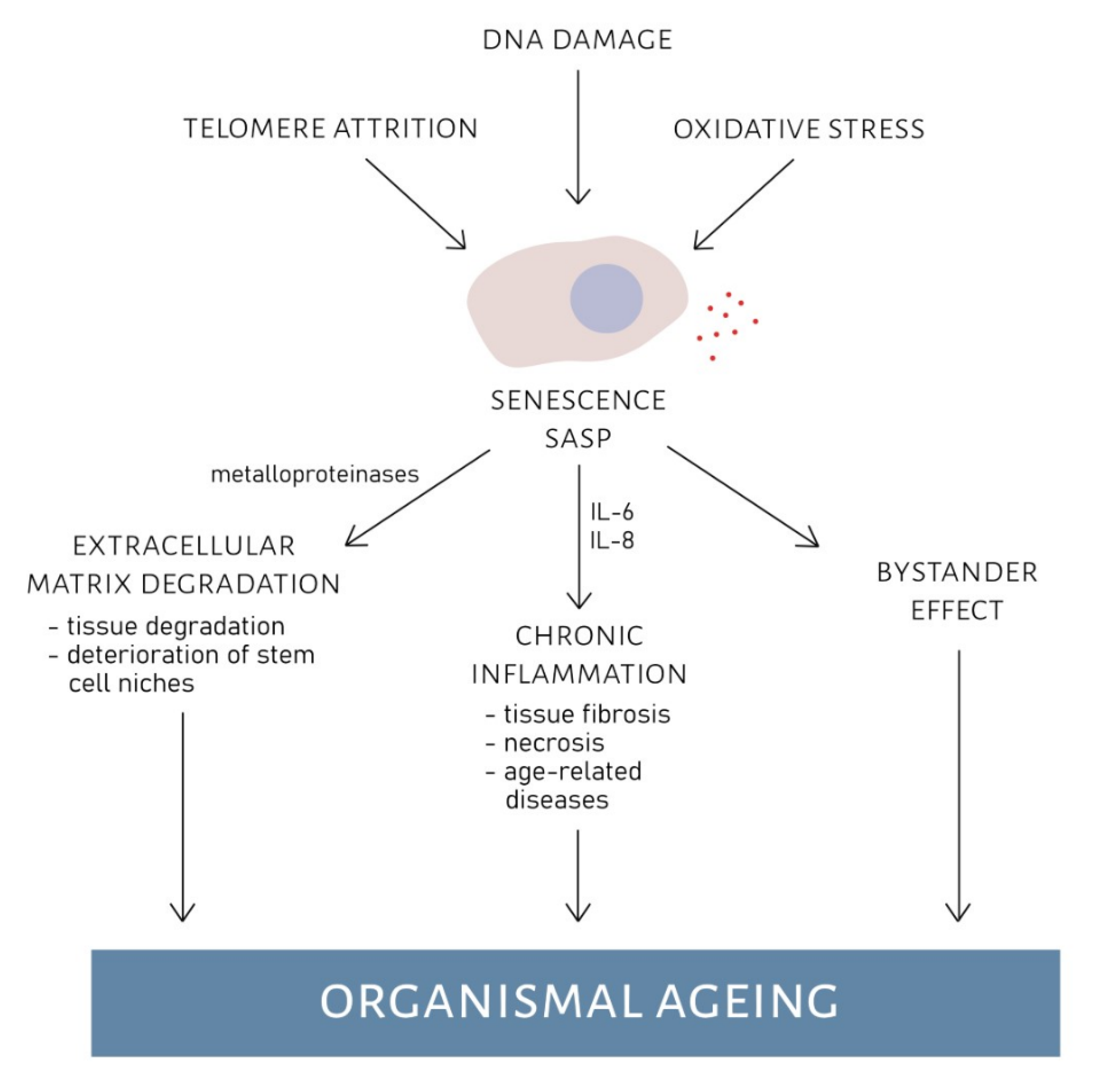
2.3. Senescence-Associated Secretory Phenotype
- Chemokines and cytokines which cause inflammation and regulate immune response (e.g., IL-6, IL-8 and CCL);
- Growth factors (e.g., GRO, HGF and IGFBP);
- Metalloproteinases which degrade the extracellular matrix;
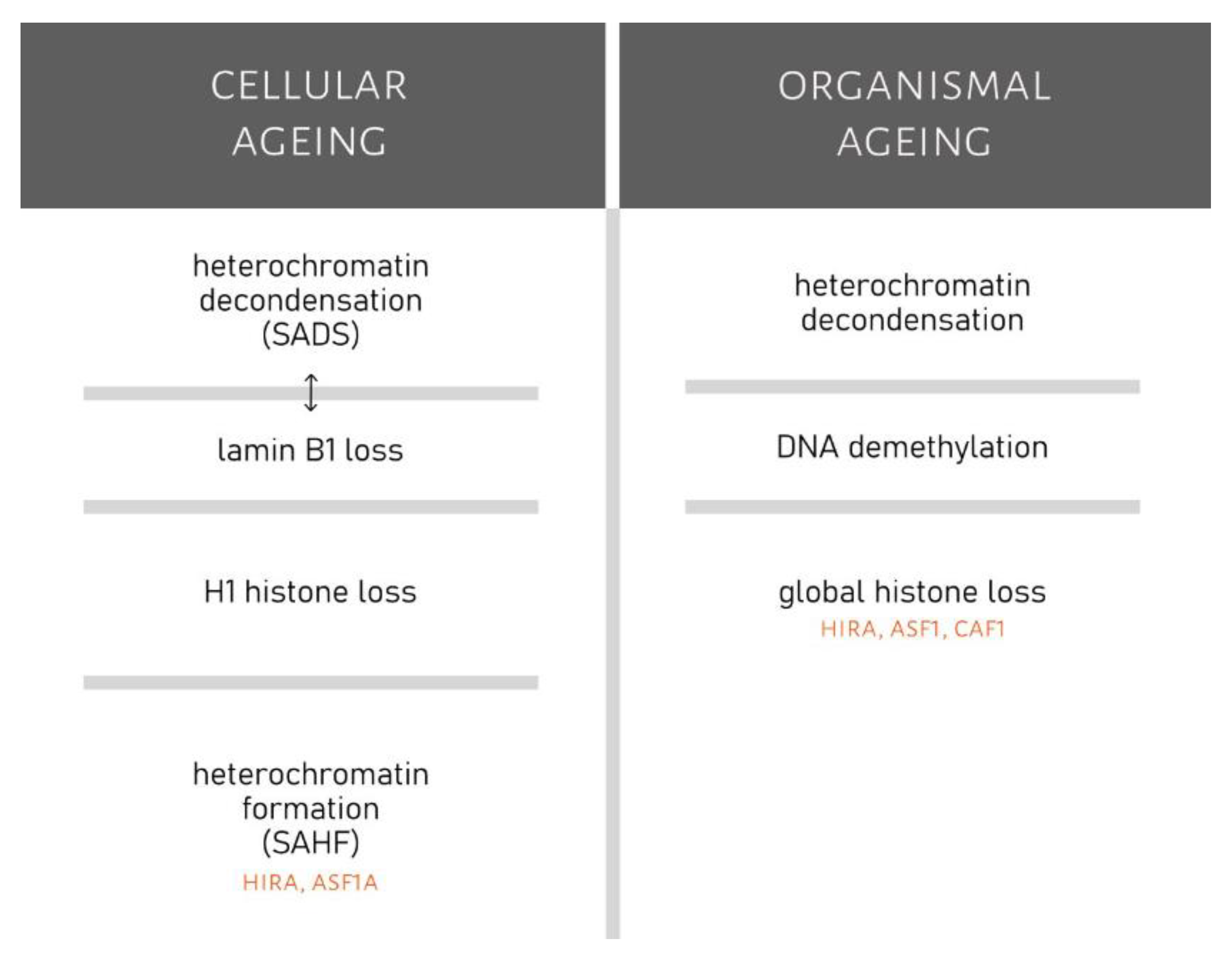
2.4. Changes in the Chromatin Structure of Senescent Cells
3. Organismal Ageing
3.1. The Role of Telomeres in Organismal Ageing
3.2. Accumulation of Senescent Cells and Organismal Ageing
3.3. Changes in Chromatin Structure and Organismal Ageing
4. Conclusions
- Telomeres became shorter with age, which correlated with the formation of persistent DDR foci.
- Impossible to repair persistent DDR foci can cause the cell to go into a state of senescence.
- Telomere associated persistent DDR foci are one of the causes of ageing of differentiated or quiescent cells.
- Shortening of telomeres to a critical length under 6000–8000 base pairs results in disconnection of the telomere protective shelterin and formation of multiple non-repairable double-stranded breaks leading to senescence.
- Shortening of telomeres can be inverted by activating TERT and SIRT6 genes.
- Secretion of proteins by senescent cells can affect neighbouring and distant cells of the body by stimulating or inhibiting proliferation.
- Senescence-associated heterochromatin foci alter the transcriptome of the cell.
- The accumulation of senescent cells in tissues can contribute to the process of organismal ageing.
- Clearing senescent cells from tissues can have therapeutic effects on ageing organisms.
- The global demethylation of DNA is a hallmark of ageing.
- Methylation profiling is a good way to accurately estimate the biological age in humans.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| γ-H2AX | histone H2AX phosphorylated on Ser139 |
| 53BP1 | p53-binding protein 1 |
| alt-NHEJ | alternative non-homologous end-joining |
| ASF1 | anti-silencing function protein 1; histone chaperone ASF1 |
| ASF1a | antisilencing function protein 1a; histone chaperone ASF1 |
| ATM | ataxia telangiectasia mutated protein; serine-protein kinase ATM |
| ATR | ATM-and RAD-3-related kinase |
| BJ | human fibroblasts cell line |
| CAF1 | chromatin assembly factor 1 |
| CCL | chemokine (c-c motiff) ligand |
| Chk1 | serine/threonine-protein kinase 1; checkpoint kinase 1 |
| Chk2 | serine/threonine-protein kinase 2; checkpoint kinase 2 |
| c-NHEJ | canonical non-homologous end-joining |
| DDR | DNA damage response |
| DSB | DNA double-strand breaks |
| E2F | group of genes that encodes a family of transcription factors |
| FOXO4 | forkhead box protein O4 |
| GRO | growth-related oncogene |
| H2A.1 | histone H2A.1 |
| H2A.2 | histone H2A.2 |
| H2AX | variant of H3.1 histone |
| H3.3 | histone H3.3 |
| H3K4me2 | histone H3 methylated on lysine 4 |
| H3K56ac | histone H3 acetylated on lysine 56 |
| H3K9ac | histone H3 acetylated on lysine 9 |
| H3K9me | histone H3 methylated on lysine 9 |
| H3K9me3 | histone H3 triple methylated on lysine 9 |
| HGF | hepatocyte growth factor |
| HIF-1 | hypoxia inducible factor 1 |
| HIRA | histone repressor A |
| HP1 | heterochromatin protein 1 |
| HR | homologous recombination |
| IGFBP | insulin-like growth factor binding protein |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IMR90 | Institute for Medical Research-90 cell line; human fetal lung fibroblast cell line |
| Ki-67 | proliferation marker protein Ki-67 |
| LMNB1 | gene encoding lamin B1 |
| MDA231 | breast cancer cell line |
| MDC1 | mediator of DNA damage checkpoint protein 1 |
| MRC-5 | fetal lung cell line |
| MRE11 | double-strand break repair protein MRE11 |
| MRN | MRE11/RAD50/NBS1 complex |
| NAD+ | nicotinamide adenine dinucleotide |
| NFBD1 | nuclear factor with BRCT domains protein 1 |
| NF-κB | nuclear factor kappa B |
| NHEJ | non-homogenous end joining |
| p51 | p51 protein |
| p53 | p53 protein |
| PARP1 | poly (ADP-ribose) polymerase |
| POT1 | protection of telomeres 1 |
| RAD17 | cell cycle checkpoint protein RAD17 |
| RAD50 | RAD50 double strand break repair protein |
| Rap1 | repressor-activator protein 1 |
| Rb | retinoblastoma |
| ROS | reactive oxygen species |
| RPE | retinal pigment epithelium |
| SADS | senescence-associated distension of satelites |
| SAHF | senescence-associated heterochromatin foci |
| SASP | senescence-associated secretory phenotype |
| SIRT1 | sirtuin 1 |
| SIRT6 | sirtuin 6 |
| SPR-5 | probable lysine-specific histone demethylase 5 |
| TAF | telomere associated foci |
| TERC | telomerase RNA component |
| TERT | telomerase reverse transcriptase |
| TIN1 | tunicamycin induced protein 1 |
| TPP1 | tripeptidyl peptidase 1 |
| TRF2 | telomere repeat binding factor 2 |
| WI-38 | human diploid lung fibroblasts cell line |
References
- Jeyapalan, J.C.; Ferreira, M.; Sedivy, J.M.; Herbig, U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Age. Dev. 2007, 128, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Salama, R.; Sadaie, M.; Hoare, M.; Narita, M. Cellular senescence and its effector programs. Genes Dev. 2014, 28, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polo, S.E.; Jackson, S.P. Dynamics of DNA damage response proteins at DNA breaks: A focus on protein modifications. Genes Dev. 2011, 25, 409–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossiello, F.; Herbig, U.; Longhese, M.P.; Fumagalli, M.; d’Adda di Fagagna, F. Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr. Opin. Genet. Dev. 2014, 26, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, R.R.; Milholland, B.; de Bruin, A.; Curran, S.; Laberge, R.M.; van Steeg, H.; Campisi, J.; Maslov, A.Y.; Vijg, J. Controlled induction of DNA double-strand breaks in the mouse liver induces features of tissue ageing. Nat. Commun. 2015, 6, 6790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, A.; Haber, J.E. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 2014, 6, a016428. [Google Scholar] [CrossRef] [Green Version]
- Watts, F.Z. Repair of DNA Double-Strand Breaks in Heterochromatin. Biomolecules 2016, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Chiruvella, K.K.; Liang, Z.; Wilson, T.E. Repair of double-strand breaks by end joining. Cold Spring Harb. Perspect. Biol. 2013, 5, a012757. [Google Scholar] [CrossRef]
- Doksani, Y. The Response to DNA Damage at Telomeric Repeats and Its Consequences for Telomere Function. Genes 2019, 10, 318. [Google Scholar] [CrossRef] [Green Version]
- Mateos-Gomez, P.A.; Gong, F.; Nair, N.; Miller, K.M.; Lazzerini-Denchi, E.; Sfeir, A. Mammalian polymerase theta promotes alternative NHEJ and suppresses recombination. Nature 2015, 518, 254–257. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Jurk, D.; Maddick, M.; Nelson, G.; Martin-Ruiz, C.; von Zglinicki, T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009, 8, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Rodier, F.; Munoz, D.P.; Teachenor, R.; Chu, V.; Le, O.; Bhaumik, D.; Coppe, J.P.; Campeau, E.; Beausejour, C.M.; Kim, S.H.; et al. DNA-SCARS: Distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J. Cell Sci. 2011, 124, 68–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fumagalli, M.; Rossiello, F.; Mondello, C.; d’Adda di Fagagna, F. Stable cellular senescence is associated with persistent DDR activation. PLoS ONE 2014, 9, e110969. [Google Scholar] [CrossRef]
- Blackburn, E.H. Telomeres. Trends Biochem. Sci. 1991, 16, 378–381. [Google Scholar] [CrossRef]
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef]
- Meyne, J.; Ratliff, R.L.; Moyzis, R.K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc. Natl. Acad. Sci. USA 1989, 86, 7049–7053. [Google Scholar] [CrossRef] [Green Version]
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 1988, 85, 6622–6626. [Google Scholar] [CrossRef] [Green Version]
- Makarov, V.L.; Hirose, Y.; Langmore, J.P. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 1997, 88, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Griffith, J.D.; Comeau, L.; Rosenfield, S.; Stansel, R.M.; Bianchi, A.; Moss, H.; de Lange, T. Mammalian telomeres end in a large duplex loop. Cell 1999, 97, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Benarroch-Popivker, D.; Pisano, S.; Mendez-Bermudez, A.; Lototska, L.; Kaur, P.; Bauwens, S.; Djerbi, N.; Latrick, C.M.; Fraisier, V.; Pei, B.; et al. TRF2-mediated control of telomere DNA topology as a mechanism for chromosome-end protection. Mol. Cell 2016, 61, 274–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lange, T. Shelterin-mediated telomere protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef]
- Broccoli, D.; Smogorzewska, A.; Chong, L.; de Lange, T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat. Genet. 1997, 17, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Rai, R.; Huang, C.; Broton, C.; Long, J.; Xu, Y.; Xue, J.; Lei, M.; Chang, S.; Chen, Y. Structural and functional analyses of the mammalian TIN2-TPP1-TRF2 telomeric complex. Cell Res. 2017, 27, 1485–1502. [Google Scholar] [CrossRef]
- Lei, M.; Podell, E.R.; Cech, T.R. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat. Struct. Mol. Biol. 2004, 11, 1223–1229. [Google Scholar] [CrossRef]
- Veverka, P.; Janovic, T.; Hofr, C. Quantitative biology of human shelterin and telomerase: Searching for the weakest point. Int. J. Mol. Sci. 2019, 20, 3186. [Google Scholar] [CrossRef] [Green Version]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Smogorzewska, A.; de Lange, T. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 2002, 21, 4338–4348. [Google Scholar] [CrossRef] [Green Version]
- Gomes, N.M.; Ryder, O.A.; Houck, M.L.; Charter, S.J.; Walker, W.; Forsyth, N.R.; Austad, S.N.; Venditti, C.; Pagel, M.; Shay, J.W.; et al. Comparative biology of mammalian telomeres: Hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell 2011, 10, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Gardner, J.P.; Kimura, M.; Chai, W.; Durrani, J.F.; Tchakmakjian, L.; Cao, X.; Lu, X.; Li, G.; Peppas, A.P.; Skurnick, J.; et al. Telomere dynamics in macaques and humans. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, P.M.; Verwoerd, N.P.; van de Rijke, F.M.; Dragowska, V.; Little, M.T.; Dirks, R.W.; Raap, A.K.; Tanke, H.J. Heterogeneity in telomere length of human chromosomes. Hum. Mol. Genet. 1996, 5, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, M.F.; Winkler, D.W.; O’Reilly, K.M.; Huntington, C.E.; Nisbet, I.C.; Vleck, C.M. Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones. Proc. Biol. Sci. 2003, 270, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Karlseder, J.; Smogorzewska, A.; de Lange, T. Senescence induced by altered telomere state, not telomere loss. Science 2002, 295, 2446–2449. [Google Scholar] [CrossRef] [Green Version]
- Palm, W.; de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008, 42, 301–334. [Google Scholar] [CrossRef] [Green Version]
- Bar, C.; Blasco, M.A. Telomeres and telomerase as therapeutic targets to prevent and treat age-related diseases. F1000Research 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, G.; Jurk, D.; Marques, F.D.; Correia-Melo, C.; Hardy, T.; Gackowska, A.; Anderson, R.; Taschuk, M.; Mann, J.; Passos, J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012, 3, 708. [Google Scholar] [CrossRef]
- D’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef]
- Takai, H.; Smogorzewska, A.; de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003, 13, 1549–1556. [Google Scholar] [CrossRef] [Green Version]
- Herbig, U.; Jobling, W.A.; Chen, B.P.; Chen, D.J.; Sedivy, J.M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol. Cell 2004, 14, 501–513. [Google Scholar] [CrossRef]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Shelton, D.N.; Chang, E.; Whittier, P.S.; Choi, D.; Funk, W.D. Microarray analysis of replicative senescence. Curr. Biol. 1999, 9, 939–945. [Google Scholar] [CrossRef] [Green Version]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.Y.; Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Ren, Y.; Dai, Z.J.; Wu, C.J.; Ji, Y.H.; Xu, J. IL-6, IL-8 and TNF-alpha levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017, 26, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Rodier, F.; Coppe, J.P.; Patil, C.K.; Hoeijmakers, W.A.; Munoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef]
- Hayakawa, T.; Iwai, M.; Aoki, S.; Takimoto, K.; Maruyama, M.; Maruyama, W.; Motoyama, N. SIRT1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation. PLoS ONE 2015, 10, e0116480. [Google Scholar] [CrossRef]
- Oberdoerffer, P.; Michan, S.; McVay, M.; Mostoslavsky, R.; Vann, J.; Park, S.K.; Hartlerode, A.; Stegmuller, J.; Hafner, A.; Loerch, P.; et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 2008, 135, 907–918. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Narita, M.; Nunez, S.; Heard, E.; Narita, M.; Lin, A.W.; Hearn, S.A.; Spector, D.L.; Hannon, G.J.; Lowe, S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003, 113, 703–716. [Google Scholar] [CrossRef] [Green Version]
- Funayama, R.; Saito, M.; Tanobe, H.; Ishikawa, F. Loss of linker histone H1 in cellular senescence. J. Cell Biol. 2006, 175, 869–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Chen, W.; Adams, P.D. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol. Cell Biol. 2007, 27, 2343–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Poustovoitov, M.V.; Ye, X.; Santos, H.A.; Chen, W.; Daganzo, S.M.; Erzberger, J.P.; Serebriiskii, I.G.; Canutescu, A.A.; Dunbrack, R.L.; et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 2005, 8, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Freund, A.; Laberge, R.M.; Demaria, M.; Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell 2012, 23, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Donahue, G.; Otte, G.L.; Capell, B.C.; Nelson, D.M.; Cao, K.; Aggarwala, V.; Cruickshanks, H.A.; Rai, T.S.; McBryan, T.; et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013, 27, 1787–1799. [Google Scholar] [CrossRef] [Green Version]
- Shimi, T.; Pfleghaar, K.; Kojima, S.; Pack, C.G.; Solovei, I.; Goldman, A.E.; Adam, S.A.; Shumaker, D.K.; Kinjo, M.; Cremer, T.; et al. The A- and B-type nuclear lamin networks: Microdomains involved in chromatin organization and transcription. Genes Dev. 2008, 22, 3409–3421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimi, T.; Butin-Israeli, V.; Adam, S.A.; Hamanaka, R.B.; Goldman, A.E.; Lucas, C.A.; Shumaker, D.K.; Kosak, S.T.; Chandel, N.S.; Goldman, R.D. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev. 2011, 25, 2579–2593. [Google Scholar] [CrossRef] [Green Version]
- Swanson, E.C.; Manning, B.; Zhang, H.; Lawrence, J.B. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. J. Cell Biol. 2013, 203, 929–942. [Google Scholar] [CrossRef]
- Honig, L.S.; Kang, M.S.; Schupf, N.; Lee, J.H.; Mayeux, R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch. Neurol. 2012, 69, 1332–1339. [Google Scholar] [CrossRef] [Green Version]
- Vera, E.; Bernardes de Jesus, B.; Foronda, M.; Flores, J.M.; Blasco, M.A. Telomerase reverse transcriptase synergizes with calorie restriction to increase health span and extend mouse longevity. PLoS ONE 2013, 8, e53760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.A. Genomes, 2nd ed.; Wiley-Liss: Oxford, UK, 2002. [Google Scholar]
- Carneiro, M.C.; Henriques, C.M.; Nabais, J.; Ferreira, T.; Carvalho, T.; Ferreira, M.G. Short telomeres in key tissues initiate local and systemic aging in Zebrafish. PLoS Genet. 2016, 12, e1005798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Shen, J.; Kugan, N.; Furth, E.E.; Lombard, D.B.; Cheung, C.; Pak, S.; Luo, G.; Pignolo, R.J.; DePinho, R.A.; et al. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol. Cell Biol. 2004, 24, 8437–8446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertuch, A.A. The molecular genetics of the telomere biology disorders. RNA Biol. 2016, 13, 696–706. [Google Scholar] [CrossRef] [Green Version]
- Grill, S.; Nandakumar, J. Molecular mechanisms of telomere biology disorders. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Dokal, I. Dyskeratosis congenita. Hematol. Am. Soc. Hematol. Educ. Prog. 2011, 2011, 480–486. [Google Scholar] [CrossRef] [Green Version]
- Armanios, M.; Blackburn, E.H. The telomere syndromes. Nat. Rev. Genet. 2012, 13, 693–704. [Google Scholar] [CrossRef]
- Niewisch, M.R.; Savage, S.A. An update on the biology and management of dyskeratosis congenita and related telomere biology disorders. Exp. Rev. Hematol. 2019, 12, 1037–1052. [Google Scholar] [CrossRef]
- Birch, J.; Anderson, R.K.; Correia-Melo, C.; Jurk, D.; Hewitt, G.; Marques, F.M.; Green, N.J.; Moisey, E.; Birrell, M.A.; Belvisi, M.G.; et al. DNA damage response at telomeres contributes to lung aging and chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1124–L1137. [Google Scholar] [CrossRef] [Green Version]
- Jaskelioff, M.; Muller, F.L.; Paik, J.H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadinanos, J.; et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011, 469, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Li, C.; Li, C.; Huang, Z.; Zeng, J.; Han, L.; Wang, Q. SIRT6 participates in the quality control of aged oocytes via modulating telomere function. Aging 2019, 11, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Wordsworth, J.; Wang, C.; Jurk, D.; Lawless, C.; Martin-Ruiz, C.; von Zglinicki, T. A senescent cell bystander effect: Senescence-induced senescence. Aging Cell 2012, 11, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Enkhmaa, B.; Anuurad, E.; Zhang, W.; Kim, K.; Berglund, L. Diverging trajectory patterns of systemic versus vascular inflammation over age in healthy Caucasians and African-Americans. Atherosclerosis 2015, 239, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Arai, Y.; Martin-Ruiz, C.M.; Takayama, M.; Abe, Y.; Takebayashi, T.; Koyasu, S.; Suematsu, M.; Hirose, N.; von Zglinicki, T. Inflammation, but not telomere length, predicts successful ageing at extreme old age: A longitudinal study of semi-supercentenarians. EBio Med. 2015, 2, 1549–1558. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef] [Green Version]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Stabile, H.; Scarno, G.; Fionda, C.; Gismondi, A.; Santoni, A.; Gadina, M.; Sciume, G. JAK/STAT signaling in regulation of innate lymphoid cells: The gods before the guardians. Immunol. Rev. 2018, 286, 148–159. [Google Scholar] [CrossRef]
- Owen, K.L.; Brockwell, N.K.; Parker, B.S. JAK-STAT signaling: A double-edged sword of immune regulation and cancer progression. Cancers 2019, 11, 2002. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, C.E.; Tritsaris, K.; Reibel, J.; Lauritzen, M.; Mortensen, E.L.; Osler, M.; Pedersen, A.M. Elevated p16ink4a Expression in Human Labial Salivary Glands as a Potential Correlate of Cognitive Aging in Late Midlife. PLoS ONE 2016, 11, e0152612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I.; et al. Aging of mice is associated with p16(Ink4a)- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging 2016, 8, 1294–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A.; et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 2017, 169, 132–147 e116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greer, E.L.; Becker, B.; Latza, C.; Antebi, A.; Shi, Y. Mutation of C. elegans demethylase spr-5 extends transgenerational longevity. Cell Res. 2016, 26, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Sidler, C.; Kovalchuk, O.; Kovalchuk, I. Epigenetic regulation of cellular senescence and aging. Front. Genet. 2017, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Larson, K.; Yan, S.J.; Tsurumi, A.; Liu, J.; Zhou, J.; Gaur, K.; Guo, D.; Eickbush, T.H.; Li, W.X. Heterochromatin formation promotes longevity and represses ribosomal RNA synthesis. PLoS Genet. 2012, 8, e1002473. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Ehrlich, M.; Wang, R.Y. 5-Methylcytosine in eukaryotic DNA. Science 1981, 212, 1350–1357. [Google Scholar] [CrossRef]
- Ehrlich, M.; Gama-Sosa, M.A.; Huang, L.H.; Midgett, R.M.; Kuo, K.C.; McCune, R.A.; Gehrke, C. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucl. Acids Res. 1982, 10, 2709–2721. [Google Scholar] [CrossRef]
- Doerfler, W. The significance of DNA methylation patterns: Promoter inhibition by sequence-specific methylation is one functional consequence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 326, 253–265. [Google Scholar] [CrossRef]
- Saxonov, S.; Berg, P.; Brutlag, D.L. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc. Natl. Acad. Sci. USA 2006, 103, 1412–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalan, S.; Carja, O.; Fagny, M.; Patin, E.; Myrick, J.W.; McEwen, L.M.; Mah, S.M.; Kobor, M.S.; Froment, A.; Feldman, M.W.; et al. Trends in DNA methylation with age replicate across diverse human populations. Genetics 2017, 206, 1659–1674. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.L.; Smith, R.A.; Ma, S.; Cutler, R.G. Genomic 5-methyldeoxycytidine decreases with age. J. Biol. Chem. 1987, 262, 9948–9951. [Google Scholar] [CrossRef]
- Choi, H.; Joe, S.; Nam, H. Development of tissue-specific age predictors using DNA methylation data. Genes 2019, 10, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.E.; Shin, K.J.; Lee, H.Y. DNA methylation-based age prediction from various tissues and body fluids. BMB Rep. 2017, 50, 546–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.L.; Nie, J.; Ku, J.; Dougherty, U.; West-Szymanski, D.C.; Collin, F.; Ellison, C.K.; Sieh, L.; Ning, Y.; Deng, Z.; et al. A human tissue map of 5-hydroxymethylcytosines exhibits tissue specificity through gene and enhancer modulation. Nat. Commun. 2020, 11, 6161. [Google Scholar] [CrossRef]
- Cho, S.; Jung, S.E.; Hong, S.R.; Lee, E.H.; Lee, J.H.; Lee, S.D.; Lee, H.Y. Independent validation of DNA-based approaches for age prediction in blood. Forens. Sci. Int. Genet. 2017, 29, 250–256. [Google Scholar] [CrossRef]
- Hong, S.R.; Jung, S.E.; Lee, E.H.; Shin, K.J.; Yang, W.I.; Lee, H.Y. DNA methylation-based age prediction from saliva: High age predictability by combination of 7 CpG markers. Forens. Sci. Int. Genet. 2017, 29, 118–125. [Google Scholar] [CrossRef]
- Naue, J.; Hoefsloot, H.C.J.; Mook, O.R.F.; Rijlaarsdam-Hoekstra, L.; van der Zwalm, M.C.H.; Henneman, P.; Kloosterman, A.D.; Verschure, P.J. Chronological age prediction based on DNA methylation: Massive parallel sequencing and random forest regression. Forens. Sci. Int. Genet. 2017, 31, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Eipel, M.; Mayer, F.; Arent, T.; Ferreira, M.R.; Birkhofer, C.; Gerstenmaier, U.; Costa, I.G.; Ritz-Timme, S.; Wagner, W. Epigenetic age predictions based on buccal swabs are more precise in combination with cell type-specific DNA methylation signatures. Aging 2016, 8, 1034–1048. [Google Scholar] [CrossRef] [Green Version]
- Tasselli, L.; Zheng, W.; Chua, K.F. SIRT6: Novel mechanisms and links to aging and disease. Trends Endocrinol. Metab. 2017, 28, 168–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Histone Variant | Yeast | Mouse | Rat | Human |
|---|---|---|---|---|
| H3K9me | ↓ | |||
| H3K9me3 | ↓ | ↑ | ||
| H3K9ac | ↓ | ↑ | ↓ | |
| H3K56ac | ↓ | ↓ | ||
| H3.1 | ↑ | ↑ | ↑ | |
| H3.3 | ↓ | ↓ | ||
| H2A.1 | ↑ | ↑ | ↑ | |
| H2A.2 | ↓ | ↓ | ||
| γ-H2AX | ↓ | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławińska, N.; Krupa, R. Molecular Aspects of Senescence and Organismal Ageing—DNA Damage Response, Telomeres, Inflammation and Chromatin. Int. J. Mol. Sci. 2021, 22, 590. https://doi.org/10.3390/ijms22020590
Sławińska N, Krupa R. Molecular Aspects of Senescence and Organismal Ageing—DNA Damage Response, Telomeres, Inflammation and Chromatin. International Journal of Molecular Sciences. 2021; 22(2):590. https://doi.org/10.3390/ijms22020590
Chicago/Turabian StyleSławińska, Natalia, and Renata Krupa. 2021. "Molecular Aspects of Senescence and Organismal Ageing—DNA Damage Response, Telomeres, Inflammation and Chromatin" International Journal of Molecular Sciences 22, no. 2: 590. https://doi.org/10.3390/ijms22020590
APA StyleSławińska, N., & Krupa, R. (2021). Molecular Aspects of Senescence and Organismal Ageing—DNA Damage Response, Telomeres, Inflammation and Chromatin. International Journal of Molecular Sciences, 22(2), 590. https://doi.org/10.3390/ijms22020590





