The Influence of the LINC00961/SPAAR Locus Loss on Murine Development, Myocardial Dynamics, and Cardiac Response to Myocardial Infarction
Abstract
1. Introduction
2. Results
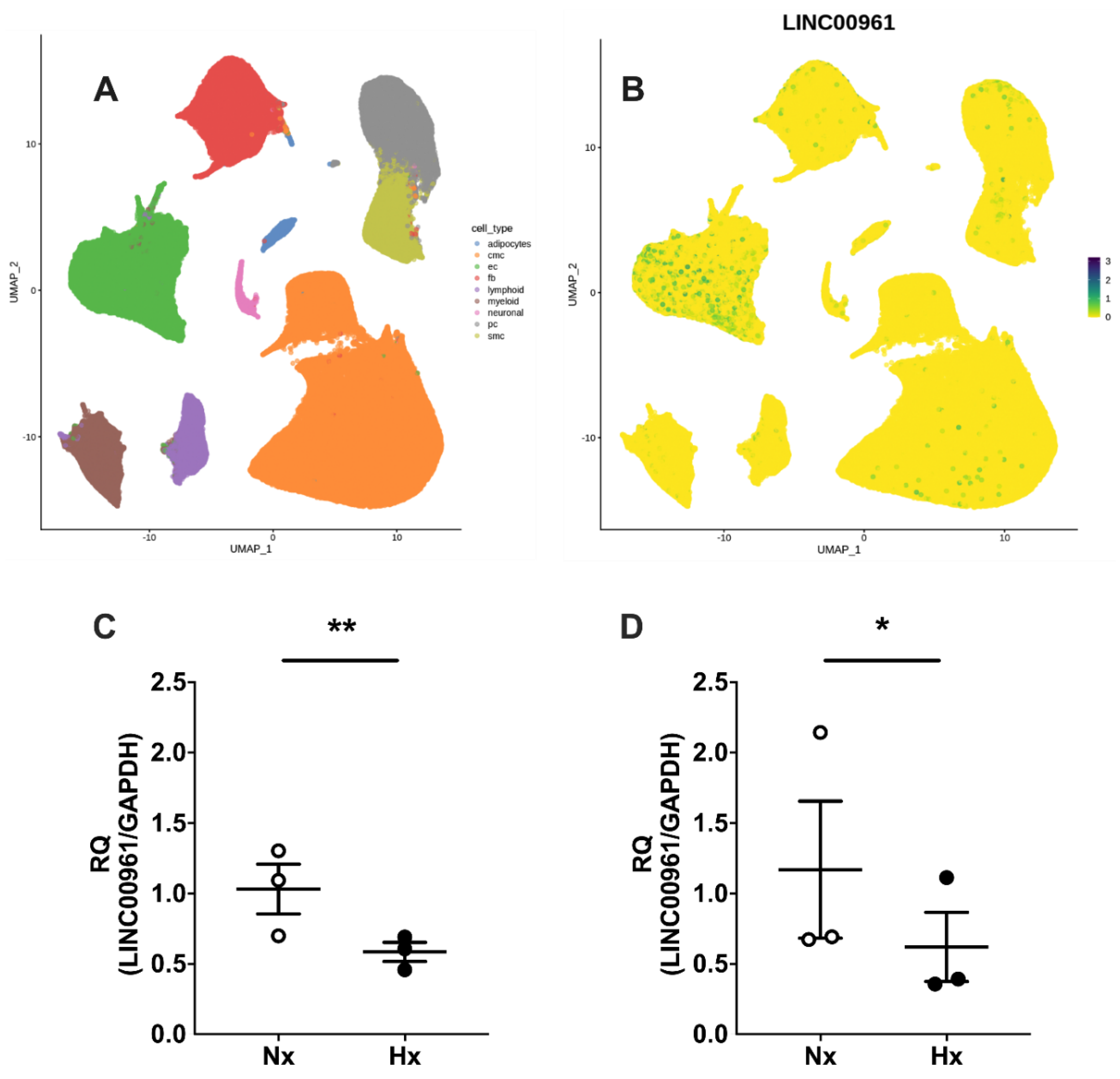
2.1. Human Cardiac SPAAR Expression and the Endothelial Cell LINC00961 Response to Hypoxia
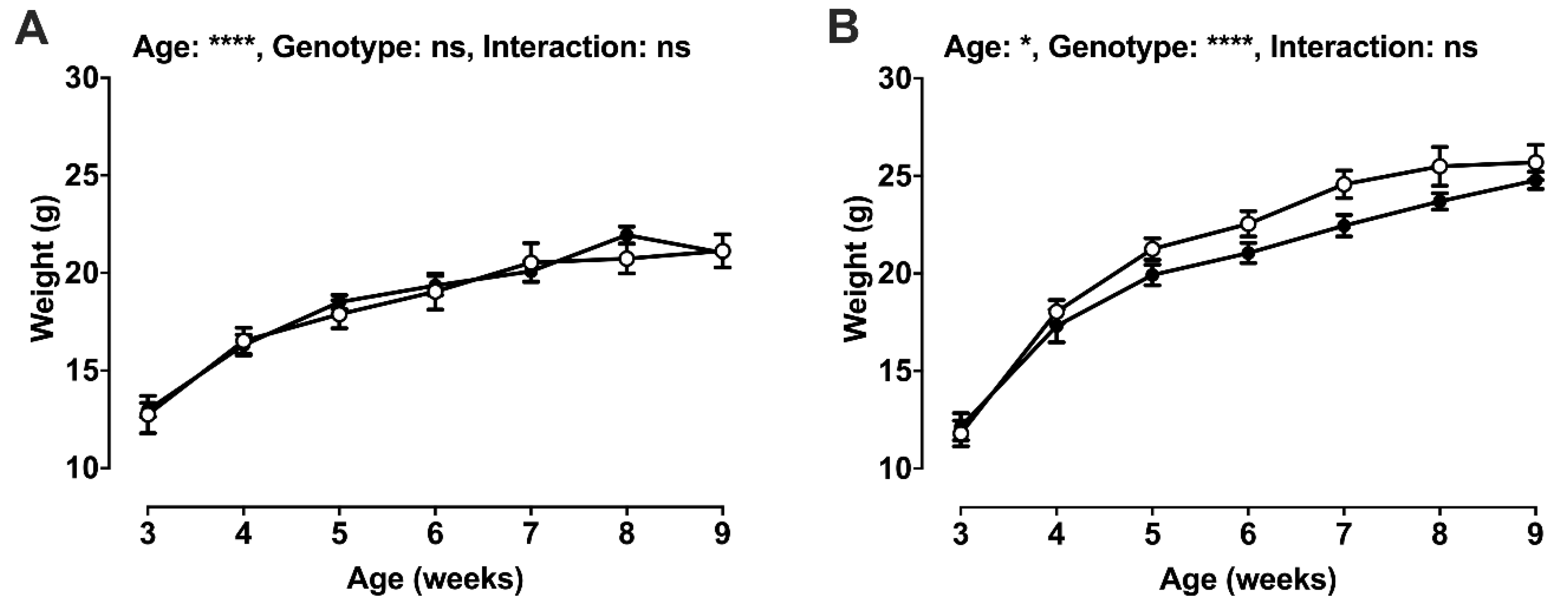
2.2. Post-Weaning Growth and Development
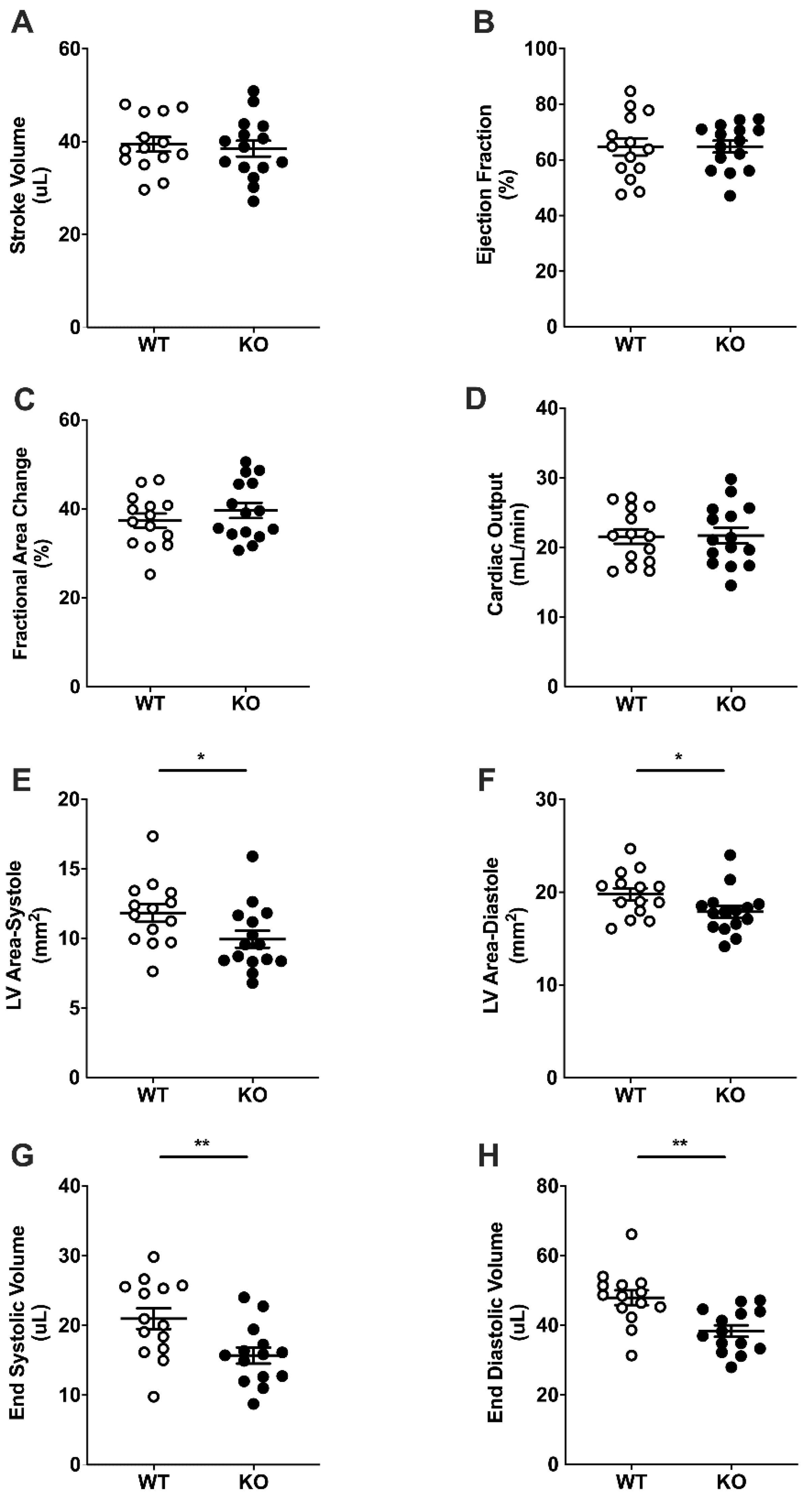
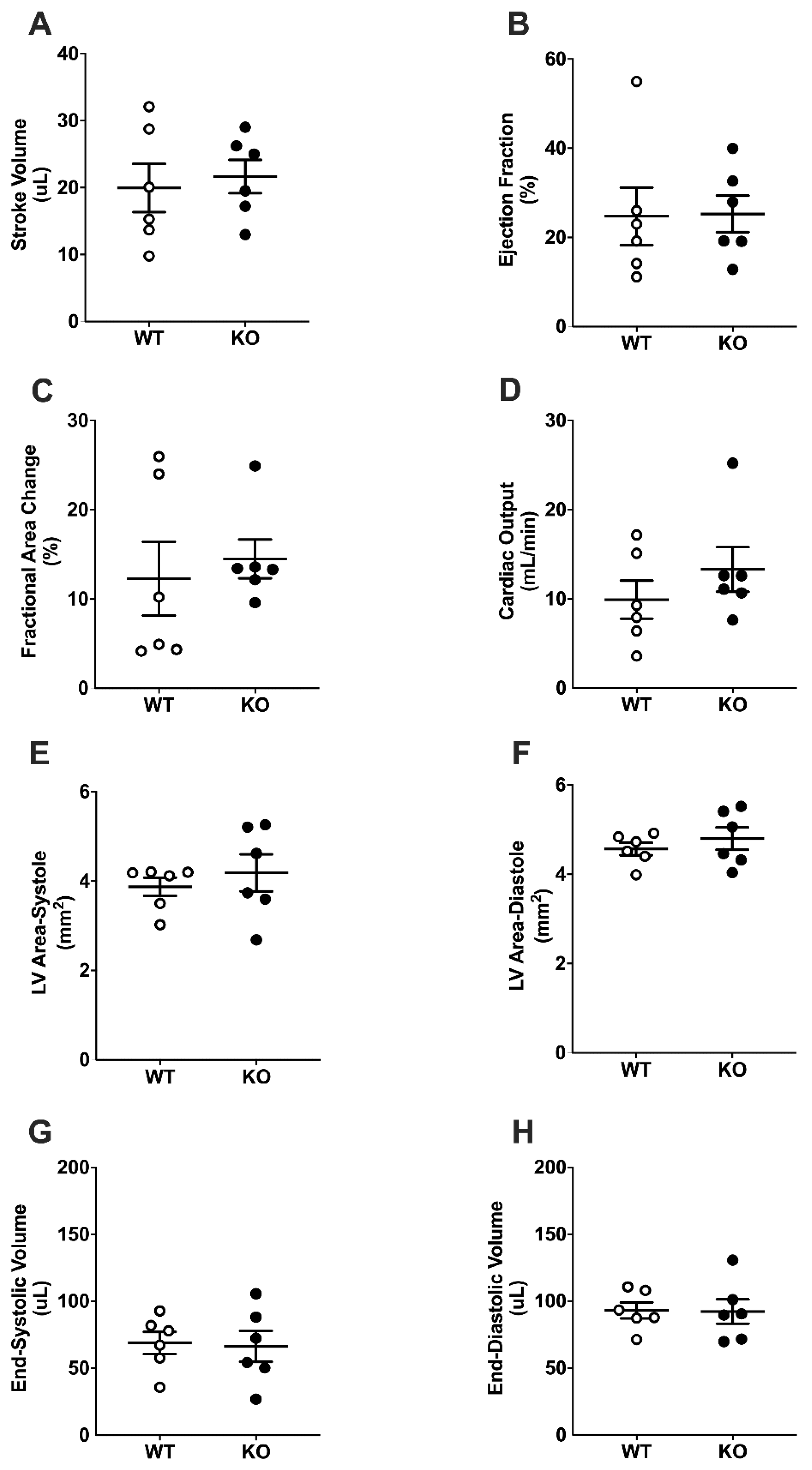
2.3. Basal Cardiac Dimensions and Function
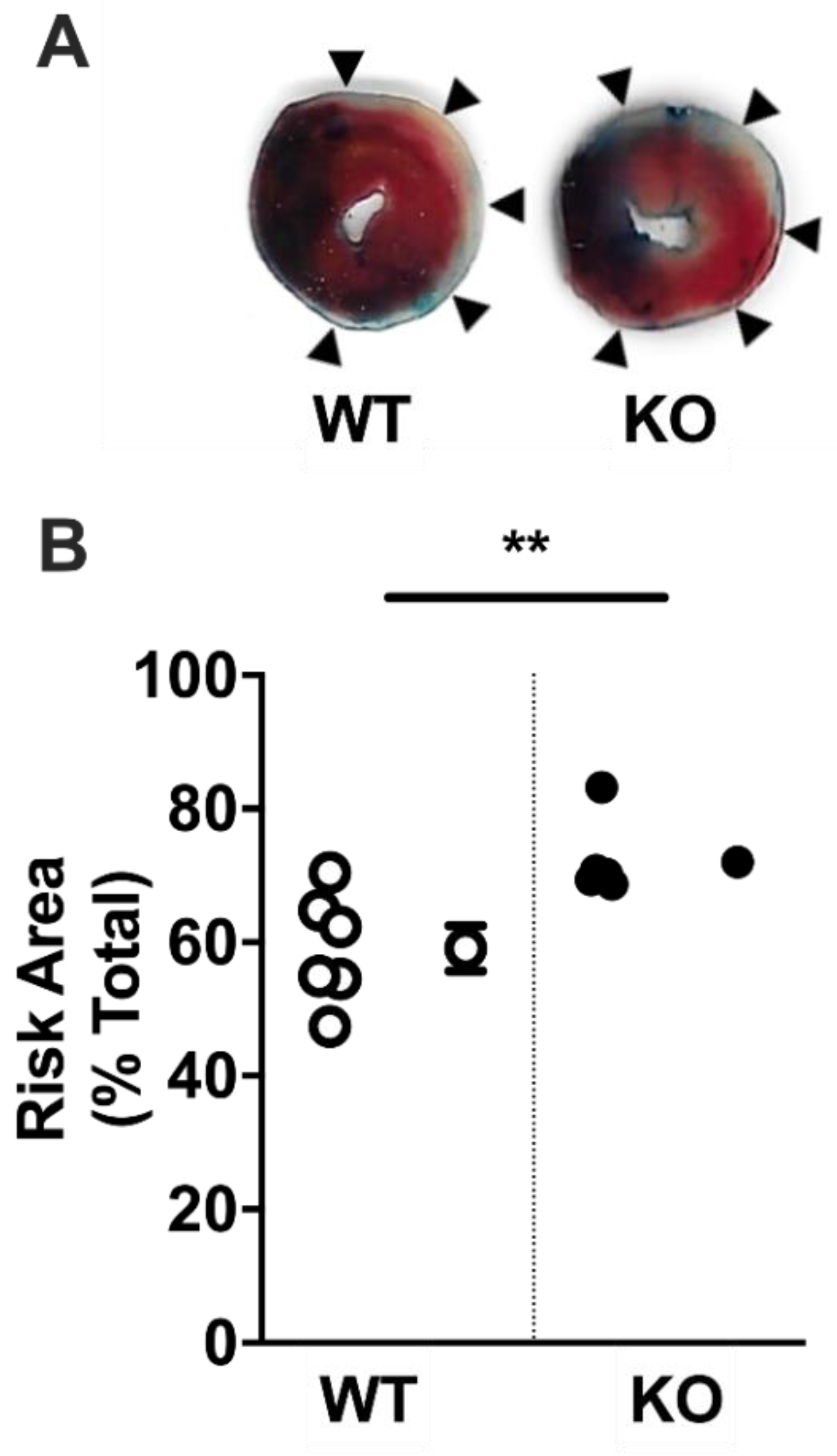
2.4. Acute Myocardial Risk Area
2.5. Cardiac Function Following Surgically Induced Myocardial Infarction
3. Discussion and Conclusions
3.1. Discussion
3.2. Conclusions
4. Materials and Methods
4.1. Ethical Approval
4.2. Single Cell RNASeq Data Mining
4.3. In Vitro Endothelial Cell Response to Hypoxia
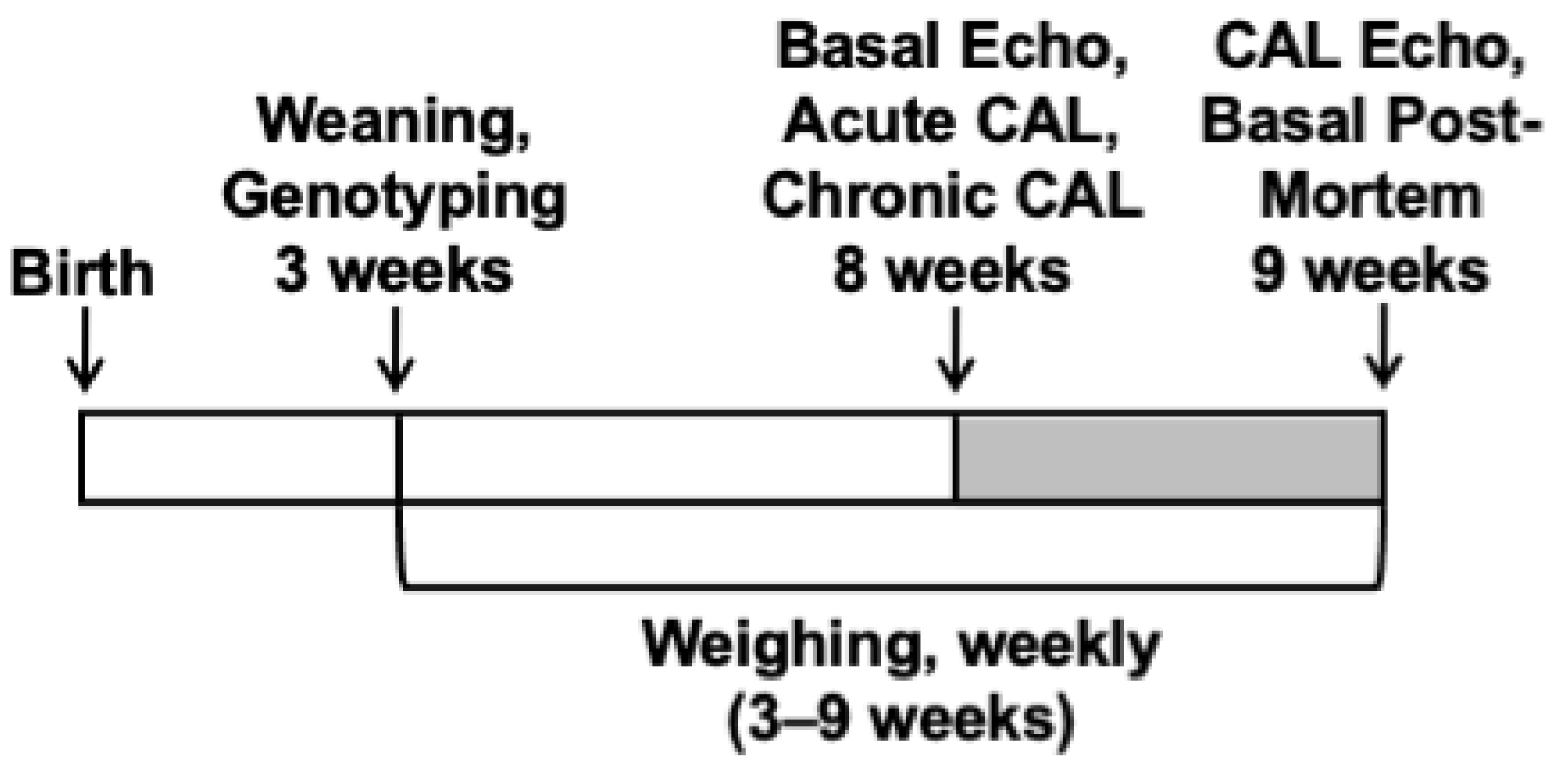
4.4. Generation of Experimental Groups
4.5. Cardiac Ultrasound Echocardiography
4.6. Acute Coronary Artery Ligation
4.7. Chronic Coronary Artery Ligation
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| αSMA | Alpha-smooth muscle actin |
| bEnd.3 | Brain microvascular endothelial-like cell line |
| CAD | Coronary artery disease |
| CAL | Coronary artery ligation |
| CMC | Cardiomyocyte |
| CO | Cardiac output |
| CVD | Cardiovascular disease |
| EC | Endothelial cell |
| ECM | Extracellular matrix |
| EDV | End-systolic volume |
| EF | Ejection fraction |
| ESV | End-systolic volume |
| FB | Fibroblast |
| FAC | Fractional area change |
| FGR | Foetal growth restriction |
| GSK3B | Glycogen synthase kinase-3B |
| LAD | Left anterior descending coronary artery |
| lincRNA | Long intergenic non-coding RNA |
| lncRNAs | Long non-coding RNAs |
| LV | Left ventricle |
| MI | Myocardial infarction |
| MTORC1 | Mammalian target of rapamycin complex 1 |
| ncRNA | Non-coding RNA |
| PC | Pericyte |
| PI3K | Phosphorylation of phosphoinositide 3-kinase |
| SMC | Smooth muscle cell |
| SPAAR | Small regulatory polypeptide of amino acid response |
| STAT1 | Signal transducer and activator of transcription 1 |
| SV | Stroke volume |
| TTC | Triphenyltetrazolium chloride |
References
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 1 June 2020).
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Matsumura, Y.; Zhu, Y.; Jiang, H.; D’Amore, A.; Luketich, S.K.; Charwat, V.; Yoshizumi, T.; Sato, H.; Yang, B.; Uchibori, T.; et al. Intramyocardial injection of a fully synthetic hydrogel attenuates left ventricular remodeling post myocardial infarction. Biomaterials 2019, 217, 119289. [Google Scholar] [CrossRef]
- Yoon, J.H.; Abdelmohsen, K.; Gorospe, M. Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 2014, 34, 9–14. [Google Scholar] [CrossRef]
- Ballantyne, M.D.; McDonald, R.A.; Baker, A.H. lncRNA/MicroRNA interactions in the vasculature. Clin. Pharmacol. Ther. 2016, 99, 494–501. [Google Scholar] [CrossRef]
- Boulberdaa, M.; Scott, E.; Ballantyne, M.; Garcia, R.; Descamps, B.; Angelini, G.D.; Brittan, M.; Hunter, A.; McBride, M.; McClure, J.; et al. A Role for the Long Noncoding RNA SENCR in Commitment and Function of Endothelial Cells. Mol. Ther. 2016, 24, 978–990. [Google Scholar] [CrossRef]
- Kurian, L.; Aguirre, A.; Sancho-Martinez, I.; Benner, C.; Hishida, T.; Nguyen, T.B.; Reddy, P.; Nivet, E.; Krause, M.N.; Nelles, D.A.; et al. Identification of novel long noncoding RNAs underlying vertebrate cardiovascular development. Circulation 2015, 131, 1278–1290. [Google Scholar] [CrossRef]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zörnig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef]
- Ounzain, S.; Burdet, F.; Ibberson, M.; Pedrazzini, T. Discovery and functional characterization of cardiovascular long noncoding RNAs. J. Mol. Cell Cardiol. 2015, 89, 17–26. [Google Scholar] [CrossRef]
- Wang, K.; Liu, F.; Zhou, L.Y.; Long, B.; Yuan, S.M.; Wang, Y.; Liu, C.Y.; Sun, T.; Zhang, X.J.; Li, P.F. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ. Res. 2014, 114, 1377–1388. [Google Scholar] [CrossRef]
- Matsumoto, A.; Pasut, A.; Matsumoto, M.; Yamashita, R.; Fung, J.; Monteleone, E.; Saghatelian, A.; Nakayama, K.I.; Clohessy, J.G.; Pandolfi, P.P. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature 2017, 541, 228–232. [Google Scholar] [CrossRef]
- Di Salvo, T.G.; Guo, Y.; Su, Y.R.; Clark, T.; Brittain, E.; Absi, T.; Maltais, S.; Hemnes, A. Right ventricular long noncoding RNA expression in human heart failure. Pulm. Circ. 2015, 5, 135–161. [Google Scholar] [CrossRef]
- Wu, C.T.; Liu, S.; Tang, M. Downregulation of linc00961 contributes to promote proliferation and inhibit apoptosis of vascular smooth muscle cell by sponging miR-367 in patients with coronary heart disease. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8540–8550. [Google Scholar] [CrossRef]
- Liu, S.; He, Y.; Shi, J.; Liu, L.; Ma, H.; He, L.; Guo, Y. STAT1-avtiviated LINC00961 regulates myocardial infarction by the PI3K/AKT/GSK3β signaling pathway. J. Cell Biochem. 2019, 120, 13226–13236. [Google Scholar] [CrossRef]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef]
- Spencer, H.L.; Sanders, R.; Boulberdaa, M.; Meloni, M.; Cochrane, A.; Spiroski, A.M.; Mountford, J.; Emanueli, C.; Caporali, A.; Brittan, M.; et al. The LINC00961 transcript and its encoded micropeptide SPAAR regulate endothelial cell function. Cardiovasc. Res. 2020, 116, 1981–1994. [Google Scholar] [CrossRef] [PubMed]
- Kuzan, A. Thymosin β as an Actin-binding Protein with a Variety of Functions. Adv. Clin. Exp. Med. 2016, 25, 1331–1336. [Google Scholar] [CrossRef]
- Zhou, C.; Rao, L.; Shanahan, C.M.; Zhang, Q. Nesprin-1/2: Roles in nuclear envelope organisation, myogenesis and muscle disease. Biochem. Soc. Trans. 2018, 46, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020. [Google Scholar] [CrossRef]
- Goodwill, A.G.; Dick, G.M.; Kiel, A.M.; Tune, J.D. Regulation of Coronary Blood Flow. Compr. Physiol. 2017, 7, 321–382. [Google Scholar] [CrossRef]
- Hemberger, M.; Hanna, C.W.; Dean, W. Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 2020, 21, 27–43. [Google Scholar] [CrossRef]
- Swanson, A.M.; David, A.L. Animal models of fetal growth restriction: Considerations for translational medicine. Placenta 2015, 36, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A.; Camm, E.J.; Niu, Y.; Richter, H.G.; Blanco, C.E.; Gottschalk, R.; Blake, E.Z.; Horder, K.A.; Thakor, A.S.; Hansell, J.A.; et al. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS ONE 2012, 7, e31017. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Morton, J.S.; Shah, A.; Do, V.; Sergi, C.; Serrano-Lomelin, J.; Davidge, S.T.; Beker, D.; Levasseur, J.; Hornberger, L.K. Intrauterine exposure to chronic hypoxia in the rat leads to progressive diastolic function and increased aortic stiffness from early postnatal developmental stages. Physiol. Rep. 2020, 8, e14327. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.D.; Herrera, E.A.; Camm, E.J.; Giussani, D.A. Vitamin C prevents intrauterine programming of in vivo cardiovascular dysfunction in the rat. Circ. J. 2013, 77, 2604–2611. [Google Scholar] [CrossRef]
- Lindgren, I.; Altimiras, J. Prenatal hypoxia programs changes in β-adrenergic signaling and postnatal cardiac contractile dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1093–R1101. [Google Scholar] [CrossRef]
- Hauton, D.; Ousley, V. Prenatal hypoxia induces increased cardiac contractility on a background of decreased capillary density. BMC Cardiovasc. Disord. 2009, 9, 1–14. [Google Scholar] [CrossRef]
- Niu, Y.; Kane, A.D.; Lusby, C.M.; Allison, B.J.; Chua, Y.Y.; Kaandorp, J.J.; Nevin-Dolan, R.; Ashmore, T.J.; Blackmore, H.L.; Derks, J.B.; et al. Maternal allopurinol prevents cardiac dysfunction in adult male offspring programmed by chronic hypoxia during pregnancy. Hypertension 2018, 72, 971–978. [Google Scholar] [CrossRef]
- Pries, A.R.; Reglin, B. Coronary microcirculatory pathophysiology: Can we afford it to remain a black box? Eur. Heart J. 2017, 38, 478–488. [Google Scholar] [CrossRef]
- Charytan, D.M.; Skali, H.; Shah, N.R.; Veeranna, V.; Cheezum, M.K.; Taqueti, V.R.; Kato, T.; Bibbo, C.R.; Hainer, J.; Dorbala, S.; et al. Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int. 2018, 93, 501–509. [Google Scholar] [CrossRef]
- Toborek, M.; Kaiser, S. Endothelial cell functions. Relationship to atherogenesis. Basic Res. Cardiol. 1999, 94, 295–314. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Harvey, N.; Noh, Y.H.; Schacht, V.; Hirakawa, S.; Detmar, M.; Oliver, G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 2002, 225, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Chen, Y.X.; Kinukawa, N.; Sueishi, K. Distributions of diffuse intimal thickening in human arteries: Preferential expression in atherosclerosis-prone arteries from an early age. Virchows Arch. 2002, 441, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.J. Letter by Campbell Regarding Article, “Coronary Microvascular Rarefaction and Myocardial Fibrosis in Heart Failure with Preserved Ejection Fraction”. Circulation 2015, 132, e205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiao, Y.; Liu, Y.; Liu, J.; Kang, Y.J. The association between myocardial fibrosis and depressed capillary density in rat model of left ventricular hypertrophy. Cardiovasc. Toxicol. 2018, 18, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Kaijser, L.; Grubbström, J.; Berglund, B. Coronary circulation in acute hypoxia. Clin. Physiol. 1990, 10, 259–263. [Google Scholar] [CrossRef]
- van Heesch, S.; Witte, F.; Schneider-Lunitz, V.; Schulz, J.F.; Adami, E.; Faber, A.B.; Kirchner, M.; Maatz, H.; Blachut, S.; Sandmann, C.-L.; et al. The Translational Landscape of the Human Heart. Cell 2019, 178, 242–260. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Pell, V.R.; Spiroski, A.M.; Mulvey, J.; Burger, N.; Costa, A.S.H.; Logan, A.; Gruszczyk, A.V.; Rosa, T.; James, A.M.; Frezza, C.; et al. Ischemic preconditioning protects against cardiac ischemia reperfusion injury without affecting succinate accumulation or oxidation. J. Mol. Cell Cardiol. 2018, 123, 88–91. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
| Female | Male | Significance (p) | |||||
|---|---|---|---|---|---|---|---|
| WT n = 9 | KO n = 9 | WT n = 10 | KO n = 9 | Sex | Genotype | Interaction | |
| Weight (g) | 20.8 ± 0.9 | 20.9 ± 0.5 | 27.1 ± 0.6 | 23.7 ± 0.9 | 0.01 | 0.03 | 0.02 |
| Brain (mg) | 443.9 ± 8.8 | 428.4 ± 10.6 | 450.7 ± 5.3 | 435.5 ± 12.5 | ns | ns | ns |
| Brain:Body weight (mg/g) | 21.5 ± 0.5 | 20.5 ± 0.5 | 16.7 ± 0.4 | 18.5 ± 0.5 | 0.01 | ns | 0.009 |
| Heart (mg) | 124.2 ± 7.2 | 125.7 ± 9.6 | 137.0 ± 6.3 | 115.2 ± 6.6 | ns | ns | ns |
| Heart:Body weight (mg/g) | 6.0 ± 0.4 | 6.1 ± 0.4 | 5.0 ± 0.3 | 4.9 ± 0.3 | 0.03 | ns | ns |
| Lungs, total (mg) | 167.7 ± 16.5 | 147.1 ± 7.4 | 148.0 ± 3.6 | 139.9 ± 4.9 | ns | ns | ns |
| Lungs:Body weight (mg/g) | 7.5 ± 0.3 | 7.1 ± 0.3 | 5.5 ± 0.2 | 5.9 ± 0.3 | 0.01 | ns | ns |
| Liver (mg) | 1212 ± 64 | 1104 ± 34 | 1111 ± 71 | 1174 ± 69 | ns | 0.004 | ns |
| Liver:Body weight (mg/g) | 58.4 ± 2.5 | 53.2 ± 2.5 | 53.5 ± 2.4 | 49.6 ± 2.5 | ns | ns | ns |
| Spleen, total (mg) | 88.3 ± 5.8 | 88.0 ± 3.8 | 84.9 ± 4.3 | 80.3 ± 8.6 | ns | ns | ns |
| Spleen:Body weight (mg/g) | 4.2 ± 0.2 | 4.2 ± 0.2 | 3.1 ± 0.2 | 3.4 ± 0.2 | 0.009 | ns | ns |
| Soleus, total (mg) | 6.4 ± 0.4 | 6.4 ± 0.6 | 8.6 ± 0.6 | 8.1 ± 0.8 | ns | ns | ns |
| Soleus:Body weight (mg/g) | 0.31 ± 0.02 | 0.31 ± 0.03 | 0.32 ± 0.02 | 0.34 ± 0.03 | ns | ns | ns |
| WT n = 5 | KO n = 8 | WT n = 10 | KO n = 8 | Sex | Genotype | Interaction | |
| Nose to anus (mm) | 79.5 ± 1.5 | 79.7 ± 1.1 | 82.3 ± 1.1 | 79.0 ± 0.8 | ns | ns | ns |
| Head length (mm) | 25.0 ± 0.6 | 26.7 ± 0.3 | 26.4 ± 0.2 | 26.5 ± 0.6 | ns | 0.006 | ns |
| Biparietal diameter (mm) | 11.6 ± 0.2 | 11.2 ± 0.2 | 11.8 ± 0.1 | 11.7 ± 0.2 | ns | ns | ns |
| Tibia length (mm) | 15.4 ± 1.1 | 18.4 ± 0.7 | 17.5 ± 0.5 | 17.0 ± 0.4 | ns | 0.05 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiroski, A.-M.; Sanders, R.; Meloni, M.; McCracken, I.R.; Thomson, A.; Brittan, M.; Gray, G.A.; Baker, A.H. The Influence of the LINC00961/SPAAR Locus Loss on Murine Development, Myocardial Dynamics, and Cardiac Response to Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 969. https://doi.org/10.3390/ijms22020969
Spiroski A-M, Sanders R, Meloni M, McCracken IR, Thomson A, Brittan M, Gray GA, Baker AH. The Influence of the LINC00961/SPAAR Locus Loss on Murine Development, Myocardial Dynamics, and Cardiac Response to Myocardial Infarction. International Journal of Molecular Sciences. 2021; 22(2):969. https://doi.org/10.3390/ijms22020969
Chicago/Turabian StyleSpiroski, Ana-Mishel, Rachel Sanders, Marco Meloni, Ian R. McCracken, Adrian Thomson, Mairi Brittan, Gillian A. Gray, and Andrew H. Baker. 2021. "The Influence of the LINC00961/SPAAR Locus Loss on Murine Development, Myocardial Dynamics, and Cardiac Response to Myocardial Infarction" International Journal of Molecular Sciences 22, no. 2: 969. https://doi.org/10.3390/ijms22020969
APA StyleSpiroski, A.-M., Sanders, R., Meloni, M., McCracken, I. R., Thomson, A., Brittan, M., Gray, G. A., & Baker, A. H. (2021). The Influence of the LINC00961/SPAAR Locus Loss on Murine Development, Myocardial Dynamics, and Cardiac Response to Myocardial Infarction. International Journal of Molecular Sciences, 22(2), 969. https://doi.org/10.3390/ijms22020969







